
Survival nomogram and risk classification system for patients with adrenocortical carcinoma: a study based on the SEER database and external validation in China
Highlight box
Key findings
• A predictive nomogram was constructed to identify high-risk adrenocortical carcinoma (ACC) patients, while a web-based survival rate calculator was developed to provide an advantageous visualization and dynamically predict the overall survival (OS) rates of patients.
What is known and what is new?
• Previous studies have introduced predictive nomograms.
• Our study has incorporated treatment-related data, and a web-based survival rate calculator was crafted to offer advantageous visualization and dynamically predict the OS rates of patients.
What is the implication, and what should change now?
• The established prognostic risk stratification system holds promise in aiding clinicians in identifying ACC patients at high-risk, thereby potentially enhancing clinical benefits for this patient population.
Introduction
Adrenocortical carcinoma (ACC) is an extremely rare and highly invasive malignant tumor, with an annual incidence of 0.7 to 2 cases per million in the population (1). This condition affects individuals of all age groups, with two distinct incidence peaks: the first peak primarily occurring during childhood, predominantly linked to hereditary syndromes such as Li-Fraumeni and Beckwith-Wiedemann syndrome, and the second peak between the ages of 40 and 60 years (2,3). The overall prognosis for ACC is generally unfavorable, particularly when the disease is diagnosed at an advanced stage. The projected median overall survival (OS) duration from the time of diagnosis is approximately 3.21 years (4). Additionally, the 5-year survival rate falls between 15% and 30% in the majority of reported series (5). The identification of prognostic risk factors for ACC is of paramount significance for tailoring individualized treatment strategies, post-treatment monitoring, and patient management.
The Surveillance, Epidemiology, and End Results (SEER) database covers more than 28% of the United States population, providing comprehensive data on demographics, primary tumor location, tumor morphology, stage at diagnosis, treatment modalities, vital status follow-up, and causes of mortality. The vast array of information available in the SEER database renders it a highly potent instrument for understanding the intricate patterns and trends observed in diverse cancer types, as well as for formulating and assessing treatment modalities aimed at enhancing patient prognosis (6,7). The objective of this study is to utilize clinical data sourced from the SEER database to investigate prognostic factor influencing outcomes in ACC patients. Additionally, our aim is to establish and verify a nomogram model that can accurately predict OS rates within this cohort. The development of this model holds potential for aiding clinicians in identifying subgroups at higher risk, as well as in devising tailored therapeutic strategies that may benefit patient prognosis. We present this article in accordance with the TRIPOD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-571/rc).
Methods
Data collection
In this study, we collected and analyzed clinical data from patients who were diagnosed with ACC between 2004 and 2015, as recorded in the ICD-O-3 codes [8370] of the SEER program. The SEER research data were accessed using SEER*Stat 8.4.1.2 software obtained from the official website (http://seer.cancer.gov//seerstat/). Exclusion criteria were applied to ensure data quality, including the following: (I) missing tumor size information; (II) unknown SEER tumor staging; (III) missing or incomplete data on tumor staging (T stage, N stage, M stage); (IV) incomplete information on treatment modalities such as radiotherapy and chemotherapy; (V) tumors without specified laterality or bilateral tumors. The detailed process of screening the SEER database is depicted in Figure 1.
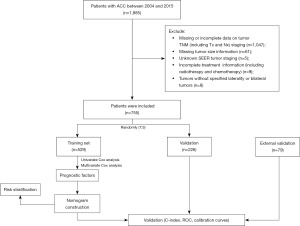
Based on the aforementioned inclusion and exclusion criteria, a total of 755 patients were recruited for this study. These patients were randomly divided into a training set (n=529) and a validation set (n=226) at a ratio of 7:3. Additionally, the clinical data used for the external validation set were collected from patients who received treatment at The First Affiliated Hospital of Naval Medical University between 2008 and 2022. Throughout the study period, data acquisition was carried out by three independent investigators. Two investigators were responsible for data extraction, while the third investigator verified the accuracy of the acquired data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional committee had waived the need for further ethical approval. Given our team’s previous research on retroperitoneal tumors, which received approval from the Medical Ethics Committee of Changhai Hospital, Shanghai, this study was an extension of our prior work. The Ethics Committee had granted approval for this research with reference to the approval document (No. CHEC2019-142). The informed consent for this retrospective analysis was waived.
Variable collection
The clinical variables collected in the present study included the following: age at diagnosis, gender, primary tumor site, tumor size, the American Joint Committee on Cancer (AJCC) 7th TNM stage, tumor stage, treatment modality (radiation and chemotherapy), survival duration, and survival status. The outcome measure utilized in this study was OS, defined as the interval from the date of diagnosis until either the occurrence of death or the most recent follow-up time.
Identification of prognostic factors for survival
All variables were converted into categorical variables and represented as frequencies and proportions. Univariate and multivariate analyses were conducted using the Cox proportional hazard regression models to assess the hazard ratio (HR) and its corresponding 95% confidence interval (CI) for all potential risk factors.
Creation and verification of prognostic models for OS
The validation of the nomogram was conducted utilizing various statistical methods including the concordance index (C-index), receiver-operating characteristic curve (ROC), calibration curves, and decision curve analysis (DCA). The C-index was employed to assess the predictive accuracy and discrimination capability of each factor and the nomogram itself. ROC and calibration curves (using 1,000 bootstrap resamples) were depicted to verify the discrimination and calibration of the model. DCA was carried out to evaluate the clinical utility of the new nomogram. Each patient’s risk score was determined based on the nomogram and subsequently categorized into high- and low-risk groups using the median risk score as the cutoff. OS survival curve was generated using the Kaplan-Meier method, and statistical analysis was performed using the log-rank test.
Statistical analysis
All statistical analyses were conducted using the R version 4.2.1 software, and the following packages were employed: “foreign”, “survival”, “survminer”, “ggDCA”, “ggrisk”, and “rms”. The Chi-squared test or Fisher exact test was utilized to detect the differences of variables of sets. A significance level of P<0.05 was considered to be statistically significant.
Results
Demographic and clinicopathological characteristics
Based on the specified inclusion and exclusion criteria, a total of 755 patients diagnosed with ACC between 2004 and 2015 were selected from SEER database. Among them, 529 patients were assigned to the training set, while the remaining 226 patients were included in the validation set. Statistical analysis utilizing the Chi-squared test revealed no significant difference between the training set and validation set. Demographically, the average age of our cohort was 55.08±18.23 years, with a higher proportion of female patients (60.93%). The most frequently observed primary site of ACC was the left adrenal gland (53.38%). Furthermore, tumors larger than 10cm constituted the majority (50.99%) of cases. SEER staging categorized cases into local, regional, and distant stages, with local staging accounted for the highest proportion (45.56%). According to AJCC 7th TNM staging, the majority of patients were classified as T2 (52.72%), N0 (89.80%), and M0 (72.98%), respectively. Regarding treatment, a small number of patients received adjuvant therapies, including radiotherapy (14.70%) and chemotherapy (40.00%). The clinicopathological characteristics of the training and the validation set are shown in Table 1.
Table 1
| Variables | Total (n=755) | Training set (n=529) | Validation set (n=226) | P valuea |
|---|---|---|---|---|
| Age, years | >0.99 | |||
| <50 | 280 (37.09) | 196 (37.05) | 84 (37.17) | |
| ≥50 | 475 (62.91) | 333 (62.95) | 142 (62.83) | |
| Sex | >0.99 | |||
| Male | 295 (39.07) | 207 (39.13) | 88 (38.94) | |
| Female | 460 (60.93) | 322 (60.87) | 138 (61.06) | |
| Laterality | 0.0761 | |||
| Left | 403 (53.38) | 294 (55.58) | 109 (48.23) | |
| Right | 352 (46.62) | 235 (44.42) | 117 (51.77) | |
| Tumor size, cm | 0.097 | |||
| <5 | 77 (10.20) | 46 (8.70) | 31 (13.72) | |
| 5–10 | 293 (38.81) | 212 (40.08) | 81 (35.84) | |
| >10 | 385 (50.99) | 271 (51.23) | 114 (50.44) | |
| T | 0.0647 | |||
| T1 | 52 (6.89) | 29 (5.48) | 23 (10.18) | |
| T2 | 398 (52.72) | 289 (54.63) | 109 (48.23) | |
| T3 | 170 (22.52) | 114 (21.55) | 56 (24.78) | |
| T4 | 135 (17.88) | 97 (18.34) | 38 (16.81) | |
| N | 0.2323 | |||
| N0 | 678 (89.80) | 470 (88.85) | 208 (92.04) | |
| N1 | 77 (10.20) | 59 (11.15) | 18 (7.96) | |
| M | 0.6462 | |||
| M0 | 551 (72.98) | 383 (72.40) | 168 (74.34) | |
| M1 | 204 (27.02) | 146 (27.60) | 58 (25.66) | |
| Tumor stage | 0.944 | |||
| Localized | 344 (45.56) | 239 (45.18) | 105 (46.46) | |
| Regional | 192 (25.43) | 135 (25.52) | 57 (25.22) | |
| Distant | 219 (29.01) | 155 (29.30) | 64 (28.32) | |
| Radiotherapy | 0.3376 | |||
| Yes | 111 (14.70) | 73 (13.80) | 38 (16.81) | |
| No | 644 (85.30) | 456 (86.20) | 188 (83.19) | |
| Chemotherapy | 0.7334 | |||
| Yes | 302 (40.00) | 209 (39.51) | 93 (41.15) | |
| No | 453 (60.00) | 320 (60.49) | 133 (58.85) | |
Data are presented as n (%). a, Chi-squared test. ACC, adrenocortical carcinoma; SEER, Surveillance, Epidemiology, and End Results.
We enrolled a total of 79 patients diagnosed with ACC in our hospital. In terms of patient demographics, the mean age of our cohort was 50.13±15.66 years, with a predominance of male patients (53.16%). Among the tumor characteristics, the left adrenal gland (54.43%) was the most frequently affected primary site. The majority of tumors exceeded a size of 10 cm (46.84%). Tumor staging demonstrated that most patients were categorized as having local-stage ACC (45.57%). Regarding the AJCC 7th TNM stage, the majority of patients were classified as T2 (27.85%), N0 (92.41%), and M0 (81.01%), respectively. A minority of patients underwent adjuvant therapies, including radiotherapy (18.99%) and chemotherapy (34.18%). The clinicopathological characteristics of the SEER set and external validation set of Changhai Hospital are shown in Table 2.
Table 2
| Variables | Total (n=834) | SEER set (n=755) | External validation set (n=79) | P valuea |
|---|---|---|---|---|
| Age, years | 0.7064 | |||
| <50 | 311 (37.29) | 280 (37.09) | 31 (39.24) | |
| ≥50 | 523 (62.71) | 475 (62.91) | 48 (60.76) | |
| Sex | 0.0152 | |||
| Male | 337 (40.41) | 295 (39.07) | 42 (53.16) | |
| Female | 497 (59.59) | 460 (60.93) | 37 (46.84) | |
| Laterality | 0.8583 | |||
| Left | 446 (53.48) | 403 (53.38) | 43 (54.43) | |
| Right | 388 (46.52) | 352 (46.62) | 36 (45.57) | |
| Tumor size, cm | 0.0593 | |||
| <5 | 92 (11.03) | 77 (10.2) | 15 (18.99) | |
| 5–10 | 320 (38.37) | 293 (38.81) | 27 (34.18) | |
| >10 | 422 (50.6) | 385 (50.99) | 37 (46.84) | |
| T | <0.0001 | |||
| T1 | 67 (8.03) | 52 (6.89) | 15 (18.99) | |
| T2 | 420 (50.36) | 398 (52.72) | 22 (27.85) | |
| T3 | 187 (22.42) | 170 (22.52) | 17 (21.52) | |
| T4 | 160 (19.18) | 135 (17.88) | 25 (31.65) | |
| N | 0.462 | |||
| N0 | 751 (90.05) | 678 (89.8) | 73 (92.41) | |
| N1 | 83 (9.95) | 77 (10.2) | 6 (7.59) | |
| M | 0.1227 | |||
| M0 | 615 (73.74) | 551 (72.98) | 64 (81.01) | |
| M1 | 219 (26.26) | 204 (27.02) | 15 (18.99) | |
| Tumor stage | 0.1334 | |||
| Localized | 380 (45.56) | 344 (45.56) | 36 (45.57) | |
| Regional | 219 (26.26) | 192 (25.43) | 27 (34.18) | |
| Distant | 235 (28.18) | 219 (29.01) | 16 (20.25) | |
| Radiotherapy | 0.3116 | |||
| Yes | 126 (15.11) | 111 (14.7) | 15 (18.99) | |
| No | 708 (84.89) | 644 (85.3) | 64 (81.01) | |
| Chemotherapy | 0.3137 | |||
| Yes | 329 (39.45) | 302 (40.00) | 27 (34.18) | |
| No | 505 (60.55) | 453 (60.00) | 52 (65.82) | |
Data are presented as n (%). a, Chi-squared test. ACC, adrenocortical carcinoma; SEER, Surveillance, Epidemiology, and End Results.
Identification of prognostic factors for ACC
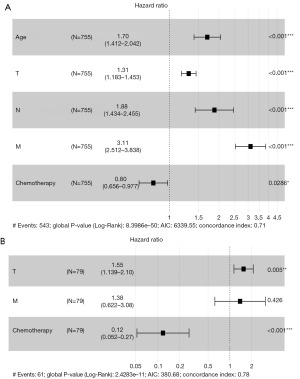
As shown in Table 3, we utilized univariate Cox regression analysis to identify prognostic factors associated with ACC. The results of the analysis, based on the SEER database, revealed that age, T, N, M, tumor stage, and chemotherapy showed a significant association with prognosis (P<0.05). Similarly, in our hospital’s database, T, M, tumor stage, and chemotherapy were found to be significantly associated with prognosis in ACC. Subsequently, a multivariable Cox regression analysis was performed based on the aforementioned results. Due to distinct perspectives on the same risk factor represented by T, N, M, and tumor staging, we individually incorporated them into a multivariable regression analysis. Based on all patient data from the SEER database, the C-index for the two models was 0.711 (95% CI: 0.690–0.732) and 0.709 (95% CI: 0.688–0.730), indicating that the model incorporating T, N, and M demonstrated greater discriminatory power in predicting OS. Multivariate analyses of ACC in SEER cohort and external validation cohort of Changhai Hospital were shown in Figure 2. The analysis demonstrated that age (HR 1.70, 95% CI: 1.412–2.042), T (HR 1.31, 95% CI: 1.183–1.453), N (HR 1.88, 95% CI: 1.434–2.455), M (HR 3.11, 95% CI: 2.512–3.838), and chemotherapy (HR 0.80, 95% CI: 0.656–0.977) were significantly associated with ACC-OS in the SEER database. However, in our institution, only T (HR 1.55, 95% CI: 1.139–2.10) and chemotherapy (HR 0.12, 95% CI: 0.052–0.27) were found to be significantly associated with ACC-OS. The aforementioned statistically significant variables were incorporated into our predictive model.
Table 3
| Variables | SEER cohort (n=755) | External validation cohort (n=79) | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age, years | |||||||
| <50 | 1 (reference) | 1 (reference) | |||||
| ≥50 | 1.72 | 1.43–2.06 | <0.001 | 1.04 | 0.60–1.82 | 0.881 | |
| Sex | |||||||
| Female | 1 (reference) | 1 (reference) | |||||
| Male | 1.02 | 0.86–1.21 | 0.853 | 1.23 | 0.73–2.07 | 0.431 | |
| Laterality | |||||||
| Left | 1 (reference) | 1 (reference) | |||||
| Right | 1.05 | 0.89–1.24 | 0.589 | 1.3 | 0.78–2.16 | 0.319 | |
| Tumor size, cm | |||||||
| <5 | 1 (reference) | 1 (reference) | |||||
| 5–10 | 1.12 | 0.83–1.52 | 0.451 | 0.78 | 0.36–1.67 | 0.523 | |
| >10 | 1.27 | 0.94–1.70 | 0.116 | 1.34 | 0.68–2.67 | 0.401 | |
| T | |||||||
| T1 | 1 (reference) | 1 (reference) | |||||
| T2 | 1.14 | 0.79–1.65 | 0.476 | 1.89 | 0.82–4.32 | 0.133 | |
| T3 | 1.94 | 1.32–2.85 | 0.001 | 2.51 | 1.05–6.00 | 0.039 | |
| T4 | 2.77 | 1.87–4.10 | <0.001 | 4.13 | 1.85–9.25 | 0.001 | |
| N | |||||||
| N0 | 1 (reference) | 1 (reference) | |||||
| N1 | 2.9 | 2.25–3.74 | <0.001 | 1.02 | 0.37–2.83 | 0.969 | |
| M | |||||||
| M0 | 1 (reference) | 1 (reference) | |||||
| M1 | 3.37 | 2.81–4.05 | <0.001 | 2.87 | 1.50–5.49 | 0.001 | |
| Tumor stage | |||||||
| Distant | 1 (reference) | 1 (reference) | |||||
| Regional | 0.44 | 0.35–0.54 | <0.001 | 0.43 | 0.21–0.86 | 0.018 | |
| Localized | 0.22 | 0.18–0.27 | <0.001 | 0.27 | 0.13–0.53 | <0.001 | |
| Radiotherapy | |||||||
| No | 1 (reference) | 1 (reference) | |||||
| Yes | 0.86 | 0.67–1.11 | 0.242 | 0.64 | 0.32–1.27 | 0.199 | |
| Chemotherapy | |||||||
| No | 1 (reference) | 1 (reference) | |||||
| Yes | 0.77 | 0.65–0.91 | 0.002 | 0.13 | 0.06–0.29 | <0.001 | |
ACC, adrenocortical carcinoma; SEER, Surveillance, Epidemiology, and End Results; HR, hazard ratio; CI, confidence interval.
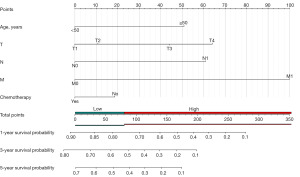
Nomogram construction and validation
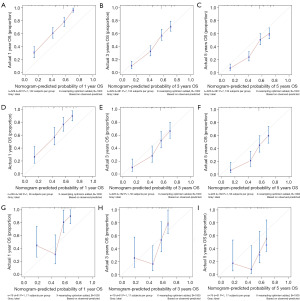
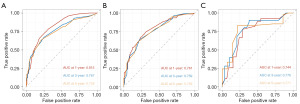
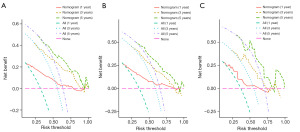
The predictive model was visually depicted through a nomogram format (Figure 3) and underwent validation processes using both the internal validation cohort and an external validation cohort. Nomograms are graphical tools based on statistical predictive models that provide predictions on probable outcomes. Each variable is assigned a risk score, and the total sum of scores corresponds to the probability of survival. Meanwhile, the C-index in the training set was 0.708 (95% CI: 0.684–0.732), in the validation set was 0.713 (95% CI: 0.672–0.753), and in the external validation set was 0.702 (95% CI: 0.623–0.780). The calibration curves (Figure 4) of the nomogram indicated a high level of consistency between predictions and observations among the training, validation, and external validation cohorts, suggesting the nomogram’s accuracy. ROC curves (Figure 5) revealed that the area under the receiver operating characteristic (AUC) values at 1, 3, and 5 years were 0.815 (95% CI: 0.778–0.852), 0.767 (95% CI: 0.727–0.808), and 0.758 (95% CI: 0.717–0.799) in the training set; 0.781 (95% CI: 0.728–0.845), 0.759 (95% CI: 0.696–0.823), and 0.759 (95% CI: 0.694–0.825) in the validation set; and 0.744 (95% CI: 0.628–0.860), 0.776 (95% CI: 0.661–0.891), and 0.771 (95% CI: 0.634–0.907) in the external validation set, respectively. Additionally, DCA (Figure 6) displayed positive net benefits across all cohorts. In conclusion, by considering the C-index, calibration curve, ROC, and DCA results, the predictive model constructed based on the aforementioned factors demonstrated significant predictive value for OS in ACC patients, with high accuracy and clinical applicability.
According to these results, we established a dynamic web-based calculator (https://zhizhouli.shinyapps.io/Adrenocortical_Carcinoma/) to predict the OS of patients with ACC according to a nomogram (Figure 7). The calculator prognosticated patients’ survival based on their clinical attributes.

Prognostic risk stratification
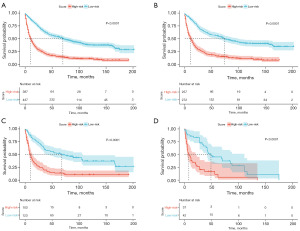
We established a prognostic risk stratification system for OS based on the total points assigned to each patient in the training cohort. Utilizing this innovative classification system, all patients were categorized into either the low-risk group (272/529, 51.42%; score ranging from 0 to 80.16) or the high-risk group (257/529, 48.58%; score ranging from 80.16 to 294.29) using the median score as the threshold (Figure 3). In addition, we visualize the risk scoring distribution in Figure 8. Subsequent Kaplan-Meier analysis revealed a significant divergence in OS rates between the two risk groups (Figure 9). Within the entire cohort of all 834 patients, the median survival time in the low-risk group was 70 months (95% CI: 57–87 months), while in the high-risk group it was 10 months (95% CI: 8–13 months).

Discussion
ACC is a rare endocrine malignancy, often associated with an unfavorable prognosis (8). The primary treatment modalities for ACC include surgical resection, chemotherapy, radiotherapy, and immunotherapy (9). Surgery is currently the main curative treatment for ACC. Even in advanced stages with metastatic ACC patients, surgery has the potential to potentially improve patient survival rates (10). For localized ACC patients, laparoscopic approaches can serve as a possible alternative to open adrenalectomy (11). Additionally, for the treatment of benign-appearing adrenal tumors with a diameter of ≤6 cm, minimally invasive adrenalectomy has become the preferred surgical approach (12-14). Despite the diverse therapeutic approaches available, ACC remains to be characterized by high recurrence and mortality rates. In many malignancies, the application of nomograms has demonstrated superior accuracy compared to conventional staging systems (15-17). For instance, the TNM staging system fails to account for several crucial risk factors, such as age and treatment response. Nomograms, in other words, offer a more precise and convenient method for clinicians to develop tailored treatment strategies and follow-up plans for individual patients (18). While there is currently a nomogram predicting OS in ACC patients, it does not take into account the influence of therapeutic factors (19). Therefore, we employed the SEER database, which contains relevant treatment details, to identify independent risk factors and develop a nomogram and risk stratification system capable of predicting patient prognosis.
Our nomogram, which incorporates age, T, N, M, and chemotherapy, was developed to estimate the probability of OS in patients with ACC. The evaluation demonstrated that our constructed nomogram exhibited substantial discriminatory power and calibration accuracy in the training set, validation set, and external validation set. The C-index and AUC values all exceeded 0.70, indicating the impressive discriminative ability of the nomogram. Further clinical utility analysis employing DCA curves indicated favorable clinical net benefits associated with the nomogram. Moreover, ACC patients could be stratified into high- and low-risk groups based on the nomogram risk scores, and there was a statistically significant difference in survival outcomes between these groups.
According to the findings of the nomogram models, it was observed that T, N, M had a significant influence on the prognosis in this study. The prognosis was found to worsen with increasing age of patients with ACC. Moreover, as the SEER tumor stage escalated, there was a progressive deterioration in tumor progression, corresponding to the trends observed in the nomogram models. In addition, it should be noted that chemotherapy plays a pivotal role in determining prognosis in these patients.
From an age perspective, previous investigations on prognostic factors in ACC have demonstrated inconsistent findings compared to our study. Some studies have reported no correlations between age and ACC outcome (20,21). However, after excluding children with ACC, significant disparities in prognosis became apparent (19,22,23). In our study, we aimed to comprehensively evaluate patients across all age groups. To achieve this, we strategically selected the age of 50 years, which is considered a peak incidence point (24), and divided the cohort into two distinct populations. And notable discrepancies were observed within the patient cohort from the SEER database. The process of aging is accompanied by changes in genomic stability, protein function, and metabolism (25). Additionally, younger patients appear to have higher tolerance for adverse treatment effects, such as chemotherapy-induced myelosuppression, due to their better physical condition compared to elderly patients. All of these factors are known to play a role in the development and progression of tumors in relation to age.
The AJCC TNM staging system is widely accepted as a prognostic tool and therapeutic guidance for patients with cancer. In our study, we excluded patients with stage TX or NX in order to ensure greater accuracy and avoid potential errors resulting from excessively detailed classifications when assessing the impact of factors on prognosis. Hence, we included T, N, M in our model, consistent with previous studies that have also confirmed a statistically significant correlation between T staging and ACC prognosis (19,26,27). Previous studies have demonstrated that patients at N0 stage exhibit superior survival rates compared to those at N1 stage, indicating a detrimental effect of lymph node metastasis on ACC patient outcomes (19,27,28). A similar trend was observed for patients at the M stage, wherein distant metastasis of ACC is associated with reduced survival rates (23). Our conclusions are in line with other studies and are also applicable to patients with SEER tumor stage (19,24,27). Therefore, higher TNM stages suggest a poorer survival prognosis for ACC patients.
In spite of the ongoing controversy surrounding the utilization of adjuvant therapy, the administration of chemotherapy can be justified in a subset of patients exhibiting positive resection margins or following the resection of localized recurrence (29). Furthermore, our research indicates that chemotherapy also plays a significant role in predicting prognosis, as displayed by our nomogram. Przytulska et al. highlighted the uncertainty surrounding the use of radiotherapy as an adjuvant treatment, while targeted radionuclide therapy shows promise as a viable alternative (30). Conversely, our study did not observe a significant improvement in survival rates among ACC patients, thus precluding its inclusion as a factor in our nomogram. Head et al. retrospectively reported on 6 cases of metastatic ACC patients treated with a combination of pembrolizumab and mitotane, with all 6 cases experiencing favorable therapeutic outcomes and noteworthy extensions in survival duration (31). Consequently, further investigation should explore the potential impact of targeted therapies, radiation therapy, and immunotherapy on the prognosis of ACC.
Our developed model exhibits sufficient accuracy to aid clinicians in identifying high-risk ACC patients. While previous studies have introduced predictive nomograms, our investigation serves as a complement to these prior works. In contrast to the aforementioned studies, our study has incorporated treatment-related data to assess the prognosis of ACC patients. Moreover, we have included ACC patients diagnosed within our institution as an external validation cohort to substantiate the results of the training set. Additionally, we have crafted a web-based survival rate calculator based on prediction nomograms for ACC. Notably, this calculator offers an advantageous visualization and dynamically predicts the OS rates of patients.
Our study still has several limitations and deficiencies that should be acknowledged. Firstly, it is important to note that our nomogram was developed based on data extracted from the SEER database; therefore, some patient information may be incomplete or missing, leading to potential selection bias. Secondly, crucial clinical details and genetic reports, including dietary habits, smoking history, alcohol consumption, hormone levels, and genetic test results, were not available in the SEER database. These factors play a significant role in the prognosis evaluation of malignant tumors. Previous studies have demonstrated the clinical relevance of markers such as cortisol secretion and Ki67 proliferation index as potential prognostic factors (32,33), which were not incorporated into our model. Additionally, information on specific chemoradiotherapy, targeted therapy, and immunotherapy regimens could not be obtained from the SEER database. Furthermore, this study solely relied on retrospective analysis; thus, a prospective study is essential to validate our findings. Lastly, our prediction model necessitates regular updates in order to enhance its accuracy.
Conclusions
In summary, a reliable nomogram was constructed in this study employing clinical variables, which have been elucidated to be correlated with survival outcomes in patients with ACC, based on the clinical data extracted from the SEER database. Both internal and external validation were conducted subsequently to confirm the accuracy and reliability of the nomogram. The established prognostic risk stratification system holds promise in aiding clinicians in identifying ACC patients at high-risk, thereby potentially enhancing clinical benefits for this patient population.
Acknowledgments
We extend our appreciation to the SEER database for granting us access to freely available data.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-571/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-571/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-571/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-571/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional committee had waived the need for further ethical approval. Given our team’s previous research on retroperitoneal tumors, which received approval from the Medical Ethics Committee of Changhai Hospital, Shanghai, this study was an extension of our prior work. The Ethics Committee had granted approval for this research with reference to the approval document (No. CHEC2019-142). The informed consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fassnacht M, Assie G, Baudin E, et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:1476-90. [Crossref] [PubMed]
- Sinclair TJ, Gillis A, Alobuia WM, et al. Surgery for adrenocortical carcinoma: When and how? Best Pract Res Clin Endocrinol Metab 2020;34:101408. [Crossref] [PubMed]
- Libé R. Adrenocortical carcinoma (ACC): diagnosis, prognosis, and treatment. Front Cell Dev Biol 2015;3:45. [Crossref] [PubMed]
- Ayala-Ramirez M, Jasim S, Feng L, et al. Adrenocortical carcinoma: clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur J Endocrinol 2013;169:891-9. [Crossref] [PubMed]
- Cherradi N. microRNAs as Potential Biomarkers in Adrenocortical Cancer: Progress and Challenges. Front Endocrinol (Lausanne) 2016;6:195. [PubMed]
- Doll KM, Rademaker A, Sosa JA. Practical Guide to Surgical Data Sets: Surveillance, Epidemiology, and End Results (SEER) Database. JAMA Surg 2018;153:588-9. [Crossref] [PubMed]
- Kratzer TB, Jemal A, Miller KD, et al. Cancer statistics for American Indian and Alaska Native individuals, 2022: Including increasing disparities in early onset colorectal cancer. CA Cancer J Clin 2023;73:120-46. [Crossref] [PubMed]
- Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev 2014;35:282-326. [Crossref] [PubMed]
- Vaidya A, Nehs M, Kilbridge K. Treatment of Adrenocortical Carcinoma. Surg Pathol Clin 2019;12:997-1006. [Crossref] [PubMed]
- Livhits M, Li N, Yeh MW, et al. Surgery is associated with improved survival for adrenocortical cancer, even in metastatic disease. Surgery 2014;156:1531-40; discussion 1540-1. [Crossref] [PubMed]
- Lombardi CP, Raffaelli M, De Crea C, et al. Open versus endoscopic adrenalectomy in the treatment of localized (stage I/II) adrenocortical carcinoma: results of a multiinstitutional Italian survey. Surgery 2012;152:1158-64. [Crossref] [PubMed]
- Conzo G, Pasquali D, Colantuoni V, et al. Current concepts of pheochromocytoma. Int J Surg 2014;12:469-74. [Crossref] [PubMed]
- Conzo G, Gambardella C, Candela G, et al. Single center experience with laparoscopic adrenalectomy on a large clinical series. BMC Surg 2018;18:2. [Crossref] [PubMed]
- Conzo G, Pasquali D, Gambardella C, et al. Long-term outcomes of laparoscopic adrenalectomy for Cushing disease. Int J Surg 2014;12:S107-11. [Crossref] [PubMed]
- Liu H, Li Z, Zhang Q, et al. Multi institutional development and validation of a nomogram to predict prognosis of early-onset gastric cancer patients. Front Immunol 2022;13:1007176. [Crossref] [PubMed]
- Wang J, Zhanghuang C, Jin L, et al. Development and validation of a nomogram to predict cancer-specific survival in elderly patients with papillary thyroid carcinoma: a population-based study. BMC Geriatr 2022;22:736. [Crossref] [PubMed]
- Fan Y, Wang Y, He L, et al. Clinical features of patients with HER2-positive breast cancer and development of a nomogram for predicting survival. ESMO Open 2021;6:100232. [Crossref] [PubMed]
- Kent DM, Steyerberg E, van Klaveren D. Personalized evidence based medicine: predictive approaches to heterogeneous treatment effects. BMJ 2018;363:k4245. [Crossref] [PubMed]
- Kong J, Zheng J, Cai J, et al. A nomogram for individualized estimation of survival among adult patients with adrenocortical carcinoma after surgery: a retrospective analysis and multicenter validation study. Cancer Commun (Lond) 2019;39:80. [Crossref] [PubMed]
- Stojadinovic A, Ghossein RA, Hoos A, et al. Adrenocortical carcinoma: clinical, morphologic, and molecular characterization. J Clin Oncol 2002;20:941-50. [Crossref] [PubMed]
- Vassilopoulou-Sellin R, Schultz PN. Adrenocortical carcinoma. Clinical outcome at the end of the 20th century. Cancer 2001;92:1113-21. [Crossref] [PubMed]
- Scollo C, Russo M, Trovato MA, et al. Prognostic Factors for Adrenocortical Carcinoma Outcomes. Front Endocrinol (Lausanne) 2016;7:99. [Crossref] [PubMed]
- Asare EA, Wang TS, Winchester DP, et al. A novel staging system for adrenocortical carcinoma better predicts survival in patients with stage I/II disease. Surgery 2014;156:1378-85; discussion 1385-6. [Crossref] [PubMed]
- Kebebew E, Reiff E, Duh QY, et al. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg 2006;30:872-8. [Crossref] [PubMed]
- Covarrubias AJ, Perrone R, Grozio A, et al. NAD(+) metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol 2021;22:119-41. [Crossref] [PubMed]
- Kim Y, Margonis GA, Prescott JD, et al. Nomograms to Predict Recurrence-Free and Overall Survival After Curative Resection of Adrenocortical Carcinoma. JAMA Surg 2016;151:365-73. [Crossref] [PubMed]
- Wang S, Chen SS, Gao WC, et al. Prognostic Factors of Adrenocortical Carcinoma: An Analysis of the Surveillance Epidemiology and End Results (SEER) Database. Asian Pac J Cancer Prev 2017;18:2817-23. [PubMed]
- Tseng J, DiPeri T, Chen Y, et al. Adrenocortical Carcinoma: The Value of Lymphadenectomy. Ann Surg Oncol 2022;29:1965-70. [Crossref] [PubMed]
- Al-Ward R, Zsembery C, Habra MA. Adjuvant therapy in adrenocortical carcinoma: prognostic factors and treatment options. Endocr Oncol 2022;2:R90-R101. [Crossref] [PubMed]
- Przytulska J, Rogala N, Bednarek-Tupikowska G. Current and emerging therapies for adrenocortical carcinoma--review. Adv Clin Exp Med 2015;24:185-93. [Crossref] [PubMed]
- Head L, Kiseljak-Vassiliades K, Clark TJ, et al. Response to Immunotherapy in Combination With Mitotane in Patients With Metastatic Adrenocortical Cancer. J Endocr Soc 2019;3:2295-304. [Crossref] [PubMed]
- Jouinot A, Bertherat J. MANAGEMENT OF ENDOCRINE DISEASE: Adrenocortical carcinoma: differentiating the good from the poor prognosis tumors. Eur J Endocrinol 2018;178:R215-30. [Crossref] [PubMed]
- de Jong MC, Khan S, Christakis I, et al. Comparative performances of nomograms and conditional survival after resection of adrenocortical cancer. BJS Open 2021;5:zraa036. [Crossref] [PubMed]


