
Narrative review: evolution in device technology and advances in surgical techniques on AMS 800 device in the last 50 years
Introduction
The external urinary sphincter complex is important to provide and maintain urinary continence. Damage to the urinary sphincter mechanism in males can occur from pelvic (especially prostate) surgery or radiation, trauma, and neurological disorders (1-3). Once the native urinary sphincter is damaged, patients will develop stress urinary incontinence. Hence, there exists a need to develop a mechanical continence device to restore urinary incontinence.
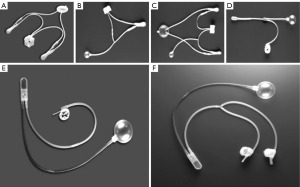
While Dr. Foley was largely credited with inventing the first useable urinary continence device using an inflatable cuff placed under the anterior urethra with an external detachable control pump to regulate compression of the mechanical cuff in 1947 (4), Dr. Scott, who designed the first modern artificial urinary sphincter (AUS) in 1972, was considered the father of modern urinary prosthetic surgery (5). The AUS is a device designed to simulate the function of the biological urinary sphincter to prevent urinary flow through mucosal coaptation, compression, and pressure transmission. The basic design of this modern AUS consists of 3 separate components namely a pressure regulating balloon (PRB), an inflatable cuff, and a control pump. The challenges in designing an AUS involve not only the mechanical operation of the artificial sphincter but also the production of a device that is effective, safe, and durable for patients in the long term (6-9). This hydraulically controlled AUS device has undergone many scientific advances in terms of device technology, materials, and designs over the past 5 decades to evolve into the current AMS 800 (Boston Scientific, previously the American Medical Systems, Minnetonka, MN, USA), which is and remains the gold standard of treatment for male stress urinary incontinence (Figure 1).
The following article provides a narrative review regarding the evolution and development of the AMS 800 devices over the years and evaluates the advances in surgical techniques relating to AMS 800 implantation. I present this article in accordance with the Narrative Review reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-10/rc).
Methods
Available literature pertaining to the AMS 800 device was reviewed from the MEDLINE and EMBASE databases were searched from 1 January 2000 to 31 December 2022 (Table 1). The search strategy incorporated the following keywords namely “artificial urinary sphincter”, “stress incontinence”, “surgical techniques”, and “technology advances”. Emphasis is placed on key scientific publications including previous reviews and clinical guidelines relevant to AMS 800 device(s) and surgical techniques. This narrative review is not designed to provide surgical recommendations nor address specific clinical challenges faced by urologists at the time of AMS 800 implantation. The material provided in this article was made independently by the author with no direct input from the Boston Scientific company.
Table 1
| Items | Specification |
|---|---|
| Date of search | 1 December 2022 |
| Databases and other sources searched | MEDLINE and EMBASE databases |
| Search terms used | The search strategy incorporated the following keywords namely “artificial urinary sphincter”, “stress incontinence”, “surgical techniques”, and “technology advances” |
| Timeframe | 1 January 2000 to 31 December 2022 |
| Inclusion criteria | English language only |
| Selection process | All studies were screened and approved by the author. Quality assessment was performed using the risk of bias tool for RCTs and non-randomized interventions |
RCTs, randomized controlled trials.
AMS 800 device designs, innovations, and surgical techniques
Evolution in device designs and innovative technology
The very first modern AMS 800 device is known as AS 721 which denotes the year 1972 when it was designed. It consisted of 4 separate components namely a fluid reservoir, an inflation pump, a deflation pump, and an inflatable cuff with 4 unidirectional valves (5,10). Unfortunately, the complex design was associated with poor mechanical reliability and high complication rates, and a newer model, the AS 742 was introduced a year later where the PRB replaced the unidirectional valves to provide a constant pre-determined pressure within the sphincteric system to cycle the cuff. A delay-fill resistor was added to the AS 742 design to simplify the device (11,12). The AS 761 prototype was introduced in 1976 with the incorporation of a newly designed automatic cuff closure system to avoid pressure transmission from the reservoir to the urethral cuff. In 1979, the device was further simplified into a single unit of the control assembly, known as both AS 791 and AS 792 models (11-14). It was in 1982 the modern AMS 800 (Boston Scientific, previously the American Medical Systems, Minnetonka, MN, USA) was born with a new control assembly unit (valves and resistor) within the pump chamber as well as a deactivation valve poppet mechanism for delayed activation and/or locked the device in deactivation state (i.e., deflated cuff or closed position).
The current AMS 800 system consists of 3 individual components namely a PRB, an inflatable cuff, and a control pump. The PRB has a dual function as a pressure regulator and serves as a fluid reservoir so that isotonic fluid (usually normal saline) can be transferred from the PRB to the urinary cuff and vice versa in a unidirectional fashion. When the sphincter is in its active (unlocked) state, the fluid from the PRB travels down the pressure gradient to the urinary cuff resulting in cuff occlusion and mucosal coaptation (11,12). Manual compression of the control pump draws the fluid out of the sphincter cuff and delivers fluid from the control pump back to the PRB. The delayed-fill resistor within the control pump will automatically redraw fluid from the PRB back into the cuff within a minute, causing the cuff to reinflate and compress the urethral lumen so that the patient becomes dry again (11). The addition of the locking mechanism through a button on the side of the control pump allows for the AUS device to be locked (deactivated) in an open position for delayed AUS activation postoperatively and the opportunity for nocturnal deactivation of the AUS device (11,12).
Over the last three decades, the basic design of AMS 800 has remained largely unchanged, and the principle of operation is based on hydraulics movement of fluid between the urinary cuff and PRB to provide occluding luminal pressure when continence is desired and to relieve the pressure when the patient intends to void (11,12). Further scientific advances have been made to improve mechanical reliability and durability as well as clinical outcomes. These innovations include a fluoro-silicone gel lining of the inner surface of the cuff to prevent friction between the inner and outer leaflets; a narrow-backed cuff to improve pressure transmission and decrease the risk of erosion and tissue atrophy; kink-resistant tubing to break tubing fatigue and break; colour-coded tubing to facilitate the identification of the correct tubing for connection; quick connectors to ease the connection between the tubing and minimise the risk of fluid loss at the tie-connectors sites; a Y-connector component for the addition of a second cuff or tandem cuff placement in revision AUS surgery; an InhibiZone-antibiotic (rifampicin and minocyline) coating to minimise prosthetic infection and explant risks; and a smallest 3.5 cm AUS cuff for the atrophied urethra (11,15). While the InhibiZone coated AMS 800 device is marketed in most developed Western countries, an uncoated version of the AMS 800 device is only available in many parts of the world including the Africa and Asia-Pacific regions (8,15).
Contemporary literature on AMS 800 device reports success rates of 61–100% in terms of urinary continence outcomes (no pad or one pad per day), while the risk of infection or erosion is thought to occur in 8.5% of the cases (3.3–27.8%), mechanical failure in 6.2% of cases (2.0–13.8%) and reoperation rate of 26% (14.8–44.8%) (7). A publication by a large multi-institutional cohort study with mid-term 32-month mean follow-up (16) showed that the overall dry rate and surgical revision were 58% and 30.7%, respectively. A more recent publication on long-term data on AMS 800 with a median duration of 15 (8.25–19.75) years (17) reported that only 43.8% still had their primary AUS with survival rates without AUS explantation were 87% and 80% at 10 and 20 years, while survival rates without AUS revision were 20% and 5% at 10 and 20 years. In contrast, the evidence supporting the use of AMS 800 for non-neurogenic female stress urinary incontinence (SUI) is low even though a circumferential coapting cuff invariably provides a more secured urethral occlusion (8) with a recent systematic review reported total continence rates of 42–86%, revision rates of 6–44% and mechanical failure rates of 2–41% (18).
Presently, there is very limited published data on a direct comparison between the AMS 800 device and other similar AUS-like devices in terms of clinical outcomes and cost-analysis modelling (19). Newer and novel AUS-like devices such as the Pro-ACT device (Uromedica, MN, USA) (20,21), Zephyr ZSI 375 (Mayor group, Villeurbanne, France) (22), VICTO urinary sphincters (Promedon, Cordoba, Argentina) (23), and Rigicon ContiClassic or ContiReflex (Rigicon Inc., NY, USA) (24) have been designed to overcome some of the current limitations of the AMS 800 device including a simpler design with fewer components, easier device preparation and an adjustable cuff or improved PRB. Many of these devices are not available worldwide. While these devices have shown similar continence rates, they have a unique set of complications of their own, and actual long-term efficacy and safety remain largely unknown.
Advances in surgical techniques
Surgical approaches
Trans-scrotal approach
The traditional approach to AUS implantation involves a perineal incision to access to place the cuff at the proximal bulbar urethra segment and a second inguinal incision to place the PRB (in the retroperitoneal space) and pump (in the scrotal or subdartos pouch) separately.
The trans-scrotal (or penoscrotal) approach allows for concurrent placement of all 3 AUS components through the same skin incision (8,9,25). In contrast to the high lithotomy position, the patient is placed in a supine frog-leg position to facilitate access to the perineum, genitalia, and groin, providing certain advantages and disadvantages (Table 2). While anatomically it is possible to access the proximal aspect of the bulbar urethra with new technique modifications such as better and deeper tissue retraction (26), the perineal approach offers a more proximal cuff placement with a larger cuff use, which may translate to a higher continence rate (8,27).
Table 2
| Approaches | Perineal cuff placement | Trans-scrotal cuff placement |
|---|---|---|
| Advantages | More proximal bulbar urethral cuff placement | A single incision and the ability to place all components at once |
| Potentially higher continence rate | Potentially shorter operative time | |
| Allows for other concurrent penile surgery (such as penile prosthesis) | ||
| Disadvantages | Often requires 2 incisions to place the cuff and the PRB/pump separately | Cuff placement may be more distal in bulbar urethra |
| Potentially longer operative time | Potentially lower continence rate |
PRB, pressure regulating balloon.
Laparoscopic and/or robotic AUS surgery
In recent years, there has been an increased interest in the use of laparoscopic and/or robotic surgery in complex functional reconstructive urology such as female urinary incontinence. Laparoscopic (28,29) and robotic-assisted (30,31) surgical approaches have been described and are gaining popularity for AUS cuff placement in the bladder neck since they are considered less invasive and offer patients a faster recovery rate compared to traditional open retropubic surgery. While direct comparative study between these surgical approaches is limited, one pilot study (32) demonstrated that the robot-assisted approach has lower complications and earlier recovery rates compared to open surgery. Nonetheless, the AUS surgery for female SUI remains uncommon and is often performed in a select few major tertiary hospitals.
Similarly, robotic-assisted AUS implantation in spinal cord-injured patients has been around for almost a decade now (33). However, the position of the neurologic patients can be challenging, and prolonged exposure to abdominal insulation can pose a cardiorespiratory risk (34). Furthermore, the longer-term safety and cost efficacy of this robotic technique over traditional open surgical methods in this unique population is largely unknown.
Placement of AUS cuff
Bladder neck cuff placement
A lower midline or Pfannenstiel incision will be required to access and place the cuff at the bladder neck. This retropubic approach is technically more challenging and is routinely performed in females (35-37) or paediatric patients (38) requiring an AUS device. Similarly, the neurologic patients who perform clean intermittent catheterization, the AUS cuff should be placed at the bladder neck (or peri-prostatic tissue in males) in a retropubic approach to ensure a lower rate of cuff erosion from frequent urethral instrumentations (39). For patients with neurological disorders, AUS cuff placement at the bladder neck demonstrated higher revision-free device survival and lower complication rates when compared to bulbar cuff placement (40). In a relatively small study, it was shown that patients with neurogenic aetiology reported a lower continence rate (23% vs. 41% completely dry) and that only 15% of patients have their original device in-situ without revisions (40). Nonetheless, the non-mechanical failure rate of the AUS is significantly higher in the neurogenic population (41).
For females with urodynamic SUI, a transvaginal approach is not recommended due to the potentially higher risk of cuff extrusion or erosion as well as infection from greater exposure to bacterial contamination per vagina. At the time of surgery, a cystostomy can be useful to guide the placement of the cuff around the bladder neck and exclude bladder neck or vaginal injury (42).
Transcorporal or interposition cuff placement
In revision surgery and high-risk candidates such as those with pelvic radiation therapy, previous urethra surgery, or trauma, the urethra is often atrophied and at high risk of cuff erosion. Patients with poorly controlled diabetes or a history of peripheral vascular disease are also considered high-risk subpopulations for urethral erosion (8,9,43). In revision AUS surgery, the placement of the urethral cuff along the distal bulbar urethra is often necessary. However, this segment of the urethra is often narrower and will accommodate a smaller cuff, which means a potentially higher risk of urethra erosion (43).
The smaller 3.5 cm cuff should be avoided since it can be associated with a higher risk of urethral atrophy and at this cuff diameter measurement, the geometry of the inflated cuff may not be circumferential in urethral coaptation (8,9). Instead, a transcorporal cuff placement or effort to place the AUS cuff in a “more robust” proximal bulbar urethra segment is advocated. For transcorporal cuff placement, it is often not necessary to repair (close) the incised tunica albuginea of the corporal cavernosal unless there is excessive bleeding (43). For males who have a normal erectile function, the corporal tunica layers should be reapproximated to minimize corporal bleeding and avoid postoperative venous-occlusive dysfunction.
The idea of using a biological graft material as an interposition graft between the cuff and urethra is novel and potentially safer since it can decrease urethral erosion in high-risk populations and avoid complexity and morbidities related to transcorporal cuff approach (43). Various interposition graft materials have been described, such as an autologous rectus fascia (44) and small intestine submucosa allograft (45).
Tandem cuff placement
For patients who report persistent or recurrent stress urinary incontinence, a tandem cuff can be placed at the revision surgery. The proposed benefits of a tandem cuff are easier operation by adding and connecting an additional cuff using the Y connector to the existing cuff, and a longer segment of urethral coaptation by having 2 occluding cuffs (43,46). The caveat of the tandem cuff is to avoid close proximity between the 2 cuffs which can cause mechanical friction or may be associated with a higher risk of an inadvertent cuff injury at the time of cuff placement.
Published literature comparing cuff downsize and tandem cuff placement showed no significant difference in the overall device survival (47) although there was a higher rate of complete continence and improvement in the urinary continence score seen in males with double-cuff compared with single-cuff devices (48,49).
Current limitations of AMS 800 and future directions
While the long-term AMS 800 efficacy, safety, and durability are well documented, it is not without its limitations and complications (8,9,11). Firstly, the patient should be mentally competent to understand the mechanics of the AUS device and have sufficient hand strength to operate this device. The patient must manually manipulate the pump to open the AUS cuff to void every time. The current specifications of the AMS 800 device such as the cuff diameter are set by the manufacturer and therefore cannot be modified. Furthermore, the PRB and pressure within the sphincteric cuff are fixed and pre-determined at the time of device implantation which may not account for or protect against a sudden increase in intra-abdominal pressure resulting in SUI when the transmitted bladder pressure exceeds the resting urethral cuff pressure. Mechanical and non-mechanical complications can occur especially in high-risk populations (for example in radiated patients) despite strict adherence to surgical principles and manufacturer’s guidelines (8,9,43). Over time, urethral tissue atrophy invariably will occur, and SUI will return. In some parts of the world, the AMS 800 device is a costly device for uninsured patients (or where hospitals do not provide this device free) and is available in an uncoated (non-InhihiZone antibiotic) version (15).
Future directions in AUS device development likely reside in the incorporation of newer and novel state-of-the-art technology (16). The introduction of nanotechnology such as piezoceramics materials, coupled with advances in electronic and remote-control systems have led to the development of an electronic control system for the AMS 800 AUS (50). This novel remote-controlled hydromechanical AUS consists of a piezoelectric micropump with a Bluetooth 2.1 microcontroller and rechargeable lithium battery, mounted in a silicon-coated acrylonitrile butadiene styrene case. Similarly, other novel prototypes such as the tape mechanical occlusive device (TMOD) (51) and shape memory alloy-based bladder actuator with capacitive sensor (52) will serve as important springboards for future urinary devices. With continued scientific advances in materials and power-harvesting technologies, these devices will hopefully become a new standard in surgical treatment for SUI in the future.
Conclusions
From the engineering point of view, the current AMS 800 device is ingenious and has stood the test of time. Continued innovations in device design, technology, and surgical techniques have ensured that the AMS 800 device is and remains the standard of care in male SUI. However, this device is far from perfect, and other AUS-like devices aim to address the current AMS 800 limitations. Future directions in the treatment of male SUI likely reside in cellular regenerative therapy and nanotechnology to restore, replace, or simulate the damaged native urinary sphincter.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Andrology and Urology for the series “50 Years Anniversary of the Modern Artificial Urinary Sphincter”. The article has undergone external peer review.
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-10/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-10/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-10/coif). The series “50 Years Anniversary of the Modern Artificial Urinary Sphincter” was commissioned by the editorial office without any funding or sponsorship. E.C. serves as an unpaid editorial board member of Translational Andrology and Urology from August 2018 to July 2024 and served as the unpaid Guest Editor of the series. The author has no other conflicts of interest to declare.
Ethical Statement: The authors is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Petros P, Quaghebeur J, Wyndaele JJ. An anatomical pathogenesis of lower urinary tract definitions from the 2002 ICS report symptoms, conditions, syndromes, urodynamics. Neurourol Urodyn 2022;41:740-55. Erratum in: Neurourol Urodyn 2023;42:1169. [Crossref] [PubMed]
- Chung E, Katz DJ, Love C. Adult male stress and urge urinary incontinence - A review of pathophysiology and treatment strategies for voiding dysfunction in men. Aust Fam Physician 2017;46:661-6. [PubMed]
- Gacci M, Sakalis VI, Karavitakis M, et al. European Association of Urology Guidelines on Male Urinary Incontinence. Eur Urol 2022;82:387-98. [Crossref] [PubMed]
- Foley EBF. An artificial sphincter: a new device and operation for control of enuresis and urinary incontinence. J Urol 1947;58:250-9. [Crossref] [PubMed]
- Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by implantable prosthetic sphincter. Urology 1973;1:252-9. [Crossref] [PubMed]
- Chung E. Contemporary surgical devices for male stress urinary incontinence: a review of technological advances in current continence surgery. Transl Androl Urol 2017;6:S112-21. [Crossref] [PubMed]
- Van der Aa F, Drake MJ, Kasyan GR, et al. The artificial urinary sphincter after a quarter of a century: a critical systematic review of its use in male non-neurogenic incontinence. Eur Urol 2013;63:681-9. [Crossref] [PubMed]
- Chung E, Liao L, Kim JH, et al. The Asia-Pacific AMS800 artificial urinary sphincter consensus statement. Int J Urol 2023;30:128-38. [Crossref] [PubMed]
- Biardeau X, Aharony SAUS Consensus Group, et al. Artificial Urinary Sphincter: Report of the 2015 Consensus Conference. Neurourol Urodyn 2016;35:S8-24. [Crossref] [PubMed]
- Timm GW, Bradley WE, Scott FB. Experimental evaluation of an implantable externally controllable urinary sphincter. Invest Urol 1974;11:326-30. [PubMed]
- Chung E. A state-of-the-art review on the evolution of urinary sphincter devices for the treatment of post-prostatectomy urinary incontinence: past, present and future innovations. J Med Eng Technol 2014;38:328-32. [Crossref] [PubMed]
- Montague DK. Evolution of implanted devices for urinary incontinence. Cleve Clin Q 1984;51:405-9. [Crossref] [PubMed]
- Hajivassiliou CA. The development and evolution of artificial urethral sphincters. J Med Eng Technol 1998;22:154-9. [Crossref] [PubMed]
- Choe JM, Staskin DR. Artificial urinary sphincter: evolution and development. J Long Term Eff Med Implants 1997;7:75-100. [PubMed]
- Available online: https://www.bostonscientific.com/en-US/products/artificial-urinary-sphincter/ams-800- artificial-urinary-sphincter.html (last accessed 1 December 2022)
- Tutolo M, Cornu JN, Bauer RM, et al. Efficacy and safety of artificial urinary sphincter (AUS): Results of a large multi-institutional cohort of patients with mid-term follow-up. Neurourol Urodyn 2019;38:710-8. [Crossref] [PubMed]
- Léon P, Chartier-Kastler E, Rouprêt M, et al. Long-term functional outcomes after artificial urinary sphincter implantation in men with stress urinary incontinence. BJU Int 2015;115:951-7. [Crossref] [PubMed]
- Reus CR, Phé V, Dechartres A, et al. Performance and Safety of the Artificial Urinary Sphincter (AMS 800) for Non-neurogenic Women with Urinary Incontinence Secondary to Intrinsic Sphincter Deficiency: A Systematic Review. Eur Urol Focus 2020;6:327-38. [Crossref] [PubMed]
- Chung E, Ranaweera M, Cartmill R. Newer and novel artificial urinary sphincters (AUS): the development of alternatives to the current AUS device. BJU Int 2012;110:5-11. [Crossref] [PubMed]
- Available online: https://www.uromedica-inc.com/proact (last accessed 1 December 2022).
- Larson T, Jhaveri H, Yeung LL. Adjustable continence therapy (ProACT) for the treatment of male stress urinary incontinence: A systematic review and meta-analysis. Neurourol Urodyn 2019;38:2051-9. [Crossref] [PubMed]
- Available online: https://www.zsimplants.ch/en/products-en/incontinence/zsi-375-en/zsi-375-information (last accessed 1 December 2022)
- Available online: http://www.victosphincter.com/ (last accessed 1 December 2022)
- Available online: https://www.rigicon.com/artificial-urinary-sphincter-conticlassic/ (last accessed 1 December 2022)
- Wilson S, Delk J 2nd, Henry GD, et al. New surgical technique for sphincter urinary control system using upper transverse scrotal incision. J Urol 2003;169:261-4. [Crossref] [PubMed]
- Wilson SK, Aliotta PJ, Salem EA, et al. New enhancements of the scrotal one-incision technique for placement of artificial urinary sphincter allow proximal cuff placement. J Sex Med 2010;7:3510-5. [Crossref] [PubMed]
- Henry GD, Graham SM, Cornell RJ, et al. A multicenter study on the perineal versus penoscrotal approach for implantation of an artificial urinary sphincter: cuff size and control of male stress urinary incontinence. J Urol 2009;182:2404-9. [Crossref] [PubMed]
- Mandron E, Bryckaert PE, Papatsoris AG. Laparoscopic artificial urinary sphincter implantation for female stress urinary incontinence: technique and 4-year experience in 25 patients. BJU Int 2010;106:1194-8. [Crossref] [PubMed]
- Rouprêt M, Misrai V, Vaessen C, et al. Laparoscopic approach for urinary sphincter implantation in women with intrinsic sphincter deficiency incontinence: a single-centre preliminary experience. Eur Urol 2010;57:499-504. [Crossref] [PubMed]
- Biardeau X, Rizk J, Marcelli F, et al. Robot-assisted laparoscopic approach for artificial urinary sphincter implantation in 11 women with urinary stress incontinence: surgical technique and initial experience. Eur Urol 2015;67:937-42. [Crossref] [PubMed]
- Peyronnet B, Capon G, Belas O, et al. Robot-assisted AMS-800 Artificial Urinary Sphincter Bladder Neck Implantation in Female Patients with Stress Urinary Incontinence. Eur Urol 2019;75:169-75. [Crossref] [PubMed]
- Peyronnet B, Vincendeau S, Tondut L, et al. Artificial urinary sphincter implantation in women with stress urinary incontinence: preliminary comparison of robot-assisted and open approaches. Int Urogynecol J 2016;27:475-81. [Crossref] [PubMed]
- Yates DR, Phé V, Rouprêt M, et al. Robot-assisted laparoscopic artificial urinary sphincter insertion in men with neurogenic stress urinary incontinence. BJU Int 2013;111:1175-9. [Crossref] [PubMed]
- Srivastava A, Niranjan A. Secrets of safe laparoscopic surgery: Anaesthetic and surgical considerations. J Minim Access Surg 2010;6:91-4. [Crossref] [PubMed]
- Peyronnet B, O'Connor E, Khavari R, et al. AMS-800 Artificial urinary sphincter in female patients with stress urinary incontinence: A systematic review. Neurourol Urodyn 2019;38:S28-41. [Crossref] [PubMed]
- Chung E, Navaratnam A, Cartmill RA. Can artificial urinary sphincter be an effective salvage option in women following failed anti-incontinence surgery? Int Urogynecol J 2011;22:363-6. [Crossref] [PubMed]
- Chartier-Kastler E, Van Kerrebroeck P, Olianas R, et al. Artificial urinary sphincter (AMS 800) implantation for women with intrinsic sphincter deficiency: a technique for insiders? BJU Int 2011;107:1618-26. [Crossref] [PubMed]
- Ruiz E, Puigdevall J, Moldes J, et al. 14 years of experience with the artificial urinary sphincter in children and adolescents without spina bifida. J Urol 2006;176:1821-5. [Crossref] [PubMed]
- Guillot-Tantay C, Chartier-Kastler E, Mozer P, et al. Male neurogenic stress urinary incontinence treated by artificial urinary sphincter AMS 800™ (Boston Scientific, Boston, USA): Very long-term results (>25 years). Prog Urol 2018;28:39-47. [Crossref] [PubMed]
- Khene ZE, Paret F, Perrouin-Verbe MA, et al. Artificial Urinary Sphincter in Male Patients with Spina Bifida: Comparison of Perioperative and Functional Outcomes between Bulbar Urethra and Bladder Neck Cuff Placement. J Urol 2018;199:791-7. [Crossref] [PubMed]
- Murphy S, Rea D, O'Mahony J, et al. A comparison of the functional durability of the AMS 800 artificial urinary sphincter between cases with and without an underlying neurogenic aetiology. Ir J Med Sci 2003;172:136-8. [Crossref] [PubMed]
- Chung E, Cartmill RA. 25-year experience in the outcome of artificial urinary sphincter in the treatment of female urinary incontinence. BJU Int 2010;106:1664-7. [Crossref] [PubMed]
- Chung E. Artificial urinary sphincter surgery in the special populations: neurological, revision, concurrent penile prosthesis and female stress urinary incontinence groups. Asian J Androl 2020;22:45-50. [Crossref] [PubMed]
- Gani J, Hennessey DB, Hoag N, et al. A pilot study of autologous rectus fascial wrap at the time of artificial urinary sphincter placement in patients at risk of cuff erosion. Int Urol Nephrol 2020;52:851-7. [Crossref] [PubMed]
- Trost L, Elliott D. Small intestinal submucosa urethral wrap at the time of artificial urinary sphincter placement as a salvage treatment option for patients with persistent/recurrent incontinence following multiple prior sphincter failures and erosions. Urology 2012;79:933-8. [Crossref] [PubMed]
- Chung E, Cartmill R. Diagnostic challenges in the evaluation of persistent or recurrent urinary incontinence after artificial urinary sphincter (AUS) implantation in patients after prostatectomy. BJU Int 2013;112:32-5. [Crossref] [PubMed]
- Linder BJ, Viers BR, Ziegelmann MJ, et al. Artificial urinary sphincter revision for urethral atrophy: Comparing single cuff downsizing and tandem cuff placement. Int Braz J Urol 2017;43:264-70. [Crossref] [PubMed]
- O'Connor RC, Gerber GS, Avila D, et al. Comparison of outcomes after single or DOUBLE-CUFF artificial urinary sphincter insertion. Urology 2003;62:723-6. [Crossref] [PubMed]
- Eswara JR, Chan R, Vetter JM, et al. Revision Techniques After Artificial Urinary Sphincter Failure in Men: Results From a Multicenter Study. Urology 2015;86:176-80. [Crossref] [PubMed]
- Biardeau X, Hached S, Loutochin O, et al. Montreal electronic artificial urinary sphincters: Our futuristic alternatives to the AMS800™. Can Urol Assoc J 2017;11:E396-404. [Crossref] [PubMed]
- Malaeb BS, Elliott SP, Lee J, et al. Novel artificial urinary sphincter in the canine model: the tape mechanical occlusive device. Urology 2011;77:211-6. [Crossref] [PubMed]
- Hassani F, Gammad GGL, Mogan RP, et al. Design and anchorage dependence of shape memory alloy actuators on enhanced voiding of a bladder. Adv Mater Tchnol 2017;3:1700184. [Crossref]


