
Emerging strategies for the prevention of bacterial biofilm in prosthetic surgery
Introduction
Penile prostheses are an effective and widely used treatment of erectile dysfunction (ED). Recent estimates indicate that approximately 10.3 million men were diagnosed with ED in 2022 and 1.7 million of those men would be appropriate penile prosthesis candidates (Figure 1) (1). Inflatable penile implants (IPPs) have been increasing in usage with over 13,000 implants placed in 2010 (2). Advancements in antibiotic prophylaxis, antiseptic device preparation, surgical techniques, and implant designs have contributed to a notable reduction in infection rates, with infection rates currently hovering around 1–5% for initial implantation procedures (3,4).

IPPs also have a greater than 90% satisfaction rate, so many patients request revisions should they stop working (5). However, one of the feared complications of revision surgeries is infection. The infection rate for revision surgeries is 10–13%, which is substantially higher than the initial implantation (6). This heightened risk of infection, leading to further surgeries, has necessitated continued efforts to better understand and eliminate infections. A better recognition of the role that biofilms play in implant infections could help reduce this dreaded complication.
A biofilm has traditionally been described as “a structured consortium of bacteria encased in a self-producing matrix” with a distinct pattern of structure and gene expression that is often associated with adherence to a surface (7). An implanted penile prosthesis unfortunately provides an ideal environment for biofilm development (8). Indeed, Werneburg et al. identified biofilms on almost all explanted IPPs (93%) for both infectious and non-infectious/mechanical reasons (9). Biofilms present a unique infectious challenge for the prosthetic urologist as they can reduce bacterial growth rate, promote antibiotic resistance, damage local tissue and device structure, and trigger inflammation (10). As these biofilms are a major source of infection after prosthetic surgery, it is critical to understand the formation of biofilms and treatment methods in order to prevent their formation and reduce infections.
This article reviews biofilm formation, mechanisms by which biofilms contribute to infection and evade traditional antibiotic therapies, current treatments, and novel approaches for biofilm prevention and treatment.
Biofilm formation
The development of a biofilm can be separated into a four-step process: bacterial attachment, aggregation and accumulation, maturation, and detachment (Figure 2) (11,12).
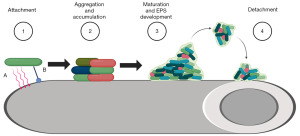
The initial step of attachment is arguably the most pivotal and can be divided into reversible attachment and irreversible adhesion (13). Reversible attachment is mediated by van der Waals and electrostatic forces between the bacteria and the implant surface (7). Bacterial attachment occurs when the net sum of attractive van der Waals forces is greater than the repulsive electrostatic forces between the bacteria and the implant. Irreversible adhesion involves interactions between specific bacterial proteins, such as autolysins and pili, and the implant surface. Autolysins are proteins associated with the bacterial cell surface through ionic or hydrophobic interactions and provide an adhesive function (7). Pili are thin filamentous protein extensions found on cell surfaces including on Escherichia coli (type I pili) and Pseudomonas aeruginosa (type IVa pili) (14).
Once bacteria have attached, bacterial aggregation and accumulation occurs and leads to the development of structural layers of bacteria within the forming biofilm. These layers make up the biomass component of a biofilm. The formation of layers triggers changes in gene expression in a phenomenon known as quorum sensing, which helps regulate the biofilm lifecycle (15). This process relies on the interaction of the layers via intercellular adhesions.
The third step of biofilm formation (maturation) consists of the development of extracellular polymeric substance (EPS), which encases the previously formed layers of bacteria. EPS is a complex matrix made of carbohydrates, lipids, proteins, and extracellular DNA (eDNA) (7). Mature biofilm colonies typically consist of primarily EPS, which can comprise up to 85% of the biofilm, with the remaining 15% from biomass (16). The EPS creates a structure with channels which allows for bulk fluid flow for bacterial nutrient transport (11).
During the fourth stage (detachment), the biofilm grows to critical density. Quorum sensing through the biofilm layers induces expression of cleavage enzymes which release bacteria from the colony. The detached bacteria are now able to enter the bloodstream, potentially causing new infections or seeding other locations.
The bacterial profile of infected IPPs and their biofilms
Understanding the bacterial profile of infected IPPs, non-infected IPPs, and biofilms is crucial for both treating and preventing infections. Most older published data discussed that S. epidermidis, a component of common skin flora, was the predominant bacterial presence for IPP revisions for non-infectious indications (Table 1) (17-21). However, in a study of 153 culture-positive infected implants by Gross et al., the authors reported that the three most predominant organisms were E. coli (18.3%), coagulase-negative Staphylococcus species (15%), and Candida species (11.1%) (22). More current literature has posited a shift in the organisms found from surgeries on infected and non-infected IPPs. Chung et al. recently used next-generation sequencing (NGS) to demonstrate that the most common organisms from revisions was P. aeruginosa for infection and E. coli for mechanical malfunction (23). Chandrapal and colleagues further complicate the understanding of bacteria and implants with their study of 202 revision surgeries in which they found that only 22% of revisions that became infected grew the same organisms at explantation for infection compared to their original cultures obtained during the first revision for mechanical malfunction (24). Ultimately, our understanding of the bacterial profile of IPPs has evolved over time and studies have demonstrated that the bacterial profile is neither static nor as simple as once postulated. This understanding is crucial for developing targeted antibiotic therapies against biofilms.
Table 1
| Study | No. of bacterial samples | Microbial data |
|---|---|---|
| Licht, 1995 | 28 cultures grown from 28 of 65 (43%) clinically uninfected prostheses | Staphylococcal epidermidis (93%) |
| Group B Streptococcus species (3.5%) | ||
| Enterococcus species (3.5%) | ||
| Henry, 2004 | 64 cultures grown from 54 of 77 (70%) clinically uninfected prostheses | Staphylococcus epidermidis (39%) |
| Staphylococcus lugdunensis (22%) | ||
| Staphylococcus capitis (5%) | ||
| Staphylococcus hemolyticus (5%) | ||
| Streptococcus mitis (5%) | ||
| MRSA (3%) | ||
| Silverstein, 2006 | 21 cultures grown from 8 of 10 (80%) clinically uninfected prostheses | Gram-positive cocci (38%) |
| Gram-negative rods (33%) | ||
| Yeast (29%) | ||
| Abouassaly, 2006 | 50 cultures grown from 19 of 55 (35%) clinically uninfected prostheses | Coagulase-negative Staphylococcus species (92%) |
| Staphylococcus aureus (4%) | ||
| Enterococcus species (4%) | ||
| Henry, 2008 | 124 cultures grown from 97 of 148 (66%) clinically uninfected prostheses | Staphylococcus epidermidis (44%) |
| Staphylococcus lugdunensis (18.5%) | ||
| Staphylococcus hemolyticus (7%) | ||
| Staphylococcus capitis (5%) | ||
| Streptococcus mitis (3%) | ||
| MRSA (1.6%) | ||
| Other (20.2%) | ||
| Gross, 2017 | 153 cultures grown from 227 clinically infected prostheses | Escherichia coli (18.3%) |
| Coagulase-negative Staphylococcus spp. (15%) | ||
| Candida spp. (11.1%) | ||
| Chung, 2022 | 8 NGS positive samples from 8 infected revisions | Pseudomonas aeruginosa (>40%) |
| Escherichia coli (>20%) | ||
| Enterococcus faecalis (>20%) | ||
| 4 NGS positive samples from 5 erosion revisions | Staphylococcus epidermis (>60%) | |
| Corynebacterium amycolatum (>40%) | ||
| 36 NGS positive samples from 70 mechanical malfunction revisions | Escherichia coli (>60%) | |
| Cutibacterium acnes (>50%) | ||
| Corynebacterium tuberculostearicum (>40%) |
MRSA, methicillin-resistant S. aureus; NGS, next-generation sequencing.
A not-so-simple clinical presentation
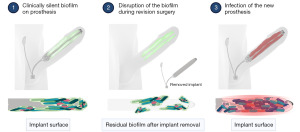
While most IPPs host biofilms, only a minority develop infections. In a multicenter study, Henry et al. revealed that the majority (70%) of patients did not exhibit symptoms of clinical infection even though their penile prostheses had culture positive bacteria and frequently had visible biofilm on their implant (18). One postulation as to how biofilms cause infections is that the biofilm formed during the initial surgery remains clinically silent but is disrupted during a revision surgery (Figure 3) (25). Henry et al. reported that 25% of implant spaces had culture positive bacteria even after antiseptic washout was performed during revision surgery for clinically non-infected prostheses (21). This could account for the higher infection rates observed after revision procedures versus virgin implants. Thus, a better appreciation of biofilms and their potential to cause infection, even in clinically asymptomatic patients, is important to reduce infection rates of revision surgeries.
Challenges to treating biofilms
Physical barriers and hydrolases
While it is logical to think that biofilm removal may decrease infections in subsequent revisions, it has proved to be much more difficult in practice. Biofilms have an inherent resistance to traditional antibiotic therapy and patient immune responses (Table 2). For example, the EPS creates molecular and charge barriers that hinder the penetration of antibiotics (26). High concentrations of hydrolases, such as β-lactamases, within the biofilm’s layers further inactivate antibiotics that do manage to penetrate this matrix (27). Furthermore, the EPS prevents the distribution of chemotactic signals necessary for inducing the patient’s inflammatory response and recruiting immune cells like neutrophils (7). The human body also interferes with antibiotic activity through the formation of a fibrous capsule around implants intended to prevent the spread of foreign substances, as demonstrated by Histoplasma capsulatum and Mycobacterium tuberculosis infections (11). This capsule also reduces blood flow into the biofilm, which further impacts antibiotic delivery (28).
Table 2
| Mechanisms | Description |
|---|---|
| Physical barriers and hydrolases | The EPS hinders antibiotic penetration and distribution of chemotactic signals by the immune response. The host immune response also forms a fibrous capsule around implants that reduces blood flow and antibiotic delivery. High concentrations of hydrolases inactivate antibiotics that do penetrate |
| Sub-therapeutic antibiotic concentrations | Antibiotics reaching the biofilm have sub-therapeutic levels, potentially promoting resistance and conferring survival advantages to bacteria |
| Metabolic inactivity and genetic alterations | Metabolically inactive persister cells within biofilms resist conventional antibiotics that target growth mechanisms. Oxidative stress within a biofilm can also induce genetic alterations that enhance bacterial resistance |
EPS, extracellular polymeric substance.
Sub-therapeutic antibiotic concentrations
The antibiotics that do reach the biomass component have sub-therapeutic concentrations which may promote antibiotic resistance and confer another survival advantage to the bacteria. The exact mechanism of this advantage is not completely understood, but Hoffman et al. described the aminoglycoside response regulator (arr) gene of P. aeruginosa as a potential mechanism (29). In their study, subinhibitory concentrations of tobramycin induced arr gene expression and biofilm production in wild-type P. aeruginosa while arr knockouts were unable to respond to the tobramycin and exhibited reduced biofilm formation.
Metabolic inactivity and genetic alterations
In addition to gene expression, a reduced bacterial metabolic state is also responsible for biofilm antibiotic resistance. Persister cells are metabolically inactive bacteria found in biofilms that demonstrate resistance to traditional antibiotics (26). Many conventional antibiotics target bacterial growth mechanisms such as the disruption of cell wall production or protein synthesis. However, these mechanisms are not effective against persister cells. Furthermore, the increased oxidative stress in the biofilm environment can lead to genetic alterations to reproducing bacterial cells (30). Bacteria may exhibit increased expression of β-lactamase, activate multidrug efflux pumps, or downregulate outer membrane proteins, which all further enhance their resistance to antibiotics (26,31). Ultimately, these antibiotic defenses can allow a biofilm to withstand antibiotic concentrations 10–1,000 times greater than those required for their planktonic counterparts.
Current strategies to reduce biofilms
A prominent strategy to combat infection and biofilms has been through coating prosthetics with antibiotics. Boston Scientific (Marlborough, MA, USA) released the InhibiZone technology in 2001 for the AMS 700 inflatable IPP which contains minocycline and rifampin (32). These antibiotics were chosen due to the low incidence of allergies and their effectiveness against common skin organisms which typically infect vascular catheters as well as IPPs (33). The InhibiZone gradually releases antibiotics following implantation, primarily within the initial three-day period, and then gradually diminishes over the subsequent three weeks (34). Coloplast (Minneapolis, MN, USA) released the Titan IPP in 2002 which features a hydrophilic coating of polyvinylpyrrolidone that can absorb an antibiotic when dipped into an aqueous solution (32). This allows the prosthetic surgeon to select an antibiotic of choice for each patient. A new penile prosthetic from Rigicon (Ronkonkoma, NY, USA) features a similar hydrophilic coating (35).
The antibiotic and hydrophilic coatings have successfully reduced infection rates to approximately 1% in patients without risk factors (3,4). Despite these breakthroughs, the evolving landscape of biofilms presents with continued challenges. Wilson et al. demonstrated that the combination of minocycline and rifampin had little to no effect on more virulent bacteria, such as Pseudomonas, which was recently implicated as the most common organism on infected implants (50%) (23,36).
Advancements in surgical techniques may have also helped reduce the potential ramifications of biofilm presence. Studies have shown decreased infection rates in revision surgeries when adjunctive washouts are performed (28,37). Swords et al. also presented a case series demonstrating that a synthetic calcium sulfate spacer could be placed during a revision surgery with delayed reimplantation. This spacer provided constant delivery of antibiotics and the authors reported an infection rate of 0% after reimplantation (38). This suggests that while antibiotic coatings may prevent infections caused by planktonic bacteria during initial implantation, additional antibiotic treatments are necessary to remove latent microbes or established biofilms.
Patient preparation
In addition to advanced technology and treatments, simple patient preparation and surgical field optimization remain crucial components to limiting infection and biofilm formation.
Firstly, perioperative measures are crucial in preventing surgical site infections, as many result from contamination with surgically-mobilized skin flora (39). Comprehensive patient examination and antibiotic treatment for systemic infections, such as urine cultures, nasal swabs, and genital examination for Candida, are important measures to identify and treat existing issues that can complicate surgical outcomes (40,41). Additionally, colonization, or carrier status, of methicillin-resistant S. aureus (MRSA) in the nares is a risk for surgical site infection, and patients with polymerase chain reaction (PCR)-positive MRSA showed a 9-fold increased likelihood of infection in a cohort study of 9,863 surgical procedures including urologic surgery (42). Thus, recognizing the contribution of skin flora to surgical site infections and adopting appropriate preoperative measures, such as examining and treating existing infections, may reduce microbial reservoirs that potentially seed biofilms.
Once in the operating room, if hair removal is necessary, removal should be via hair clippers instead of shaving with a razor. A recent Cochrane review of 25 trials reported moderate-certainty level evidence of increased risk of surgical site infections for shaving with razor versus clippers [risk ratio (RR) 1.64, 95% confidence interval (CI): 1.16 to 2.33], as well as for hair removal with a razor versus no removal (RR 1.82, 95% CI: 1.05 to 3.14) (43). Additionally, the implementation of the “no touch” technique, wherein immediately prior to implantation all surgical gloves and instruments are considered contaminated and replaced, in addition to infection-retarded coatings, decreased infection rates from 2% to 0.46% in a cohort of 1,511 IPP surgeries performed by one surgeon (44). Other intraoperative infection control procedures to consider include decreased traffic, personnel, and length of time (39).
Novel methods to prevent biofilms
Biofilms continue to persist despite advancements with coatings and surgical techniques. Novel approaches to combat biofilm formation and treat existing biofilms offer promising avenues. A diverse array of strategies, including surface modification, EPS degrading enzymes, novel antibiotics, ultrasound, and smart technology, have been explored. Importantly, the majority of these methods are still in the experimental phase and primarily studied in vitro, with limited clinical trial data available.
Surface modifications
The prevention of bacterial attachment has been a primary strategy in the battle against biofilm formation. Biofilms cannot form without attachment, and therefore the survival advantages that biofilms exhibit compared to planktonic bacteria are inhibited. One approach involves altering the surface properties of implants to render the surface less attractive to microbes. For example, modifications to surface electric charges may increase repulsive electrostatic forces and inhibit the first step of biofilm formation. Uncharged hydrophilic and hydrophobic surfaces can also repel protein-rich solutions such as blood or sera that commonly carry bacteria (45). The application of heparin can alter the hydrophilicity of surfaces and has been employed for urethral catheters and stents to reduce bacterial adhesion (46,47). Awonusi et al. demonstrated that heparin coated ureteral stents generated less bacterial adhesion than non-heparin coated stents over a 28-day period and also had nearly constant release of heparin over the time period (48).
Biosurfactants
Biological approaches that involve biosurfactants from commensal bacteria to thwart adhesion and proliferation of pathologic bacteria are also under examination. This is a natural phenomenon seen with human gut flora to prevent Clostridium difficile infections (49). Velraeds et al. demonstrated that the biosurfactant, surlactin, produced by Lactobacillus acidophilus could prevent biofilm formation by uropathogens like E. coli and S. epidermidis on silicone rubber (50). The use of biosurfactants could provide antibiotic advantages such as decreased resistance and better biodegradability, but their use has been limited by high production cost as well as limited information on their toxicity in humans (51).
Disruption of fibrin binding
Another method to prevent biofilm adherence targets the fibrin deposits that bacteria can bind. Tissue plasminogen activator (tPA) demonstrated reduction of S. aureus biofilm formation on indwelling medical devices by splitting local fibrin. Kwiecinski et al. reported that after three days, tPA coated coverslips implanted in mice models demonstrated fewer S. aureus colonies than non-coated coverslips (52). Similarly, S. aureus was shown to be dependent on the coagulation pathway to produce prothrombin and fibrin (53). This suggests that direct thrombin inhibitors could be used as coating to prevent S. aureus binding and biomass accumulation. Nitric oxide (NO) has also been proposed as a compound that can alter bacterial cell membrane adhesin proteins, such as those required to bind to fibrin (54,55). Holt et al. created a NO-releasing xerogel coating that reduced bacterial colonization on orthopedic fixation pins in rat models for up to 48 days, though the majority of NO was released during the initial 5 days (56). tPA or direct thrombin inhibitor coatings and NO releasing xerogels have the potential to be used as coating for penile prostheses. The tPA and NO studies were conducted in animal models and further in vivo testing is required to demonstrate long-term efficacy.
N-acetyl cysteine (NAC) coatings
NAC is a Food and Drug Administration (FDA)-approved drug widely used for various clinical conditions, such as acetaminophen intoxication, bronchitis, and bipolar disorder (57). NAC has the potential to reduce bacterial adhesion and formation, making it a candidate for medical implant coating (58). Costa et al. recently demonstrated that NAC could be covalently immobilized onto a chitosan (Ch)-derived implant-related coating with high local concentration for bone-related implants (59). Their coating successfully prevented MRSA adherence and biofilm development without cytotoxic effects. However, stabilization of the NAC coating was only demonstrated at physiologic conditions for 7 days and future studies could test stabilization on other compounds such as silicone.
Biofilm EPS degradation
Recent research has also identified potential drugs that may be able to target the EPS of biofilms. DNase I has the potential to degrade eDNA, which is necessary for EPS, which would disrupt the structural integrity and promote antibiotic penetrance (60). DNase I has demonstrated efficacy against eDNA of several strains of bacteria, including those commonly found on penile prostheses: S. aureus, P. aeruginosa, and E. coli (60,61). However, in a study by Sharma and Pagedar Singh, when compared to individual biofilms, DNase I was found to be less effective against mixed species biofilms which are commonly found on penile prostheses (23,61). Nonetheless, DNase I has efficacy against a unique aspect of biofilms and may be valuable when used in conjunction with other antibiotic treatments.
Antimicrobial peptides (AMPs)
AMPs are a versatile class of molecules that not only exhibit bactericidal activity but also reduce mature biofilms. AMPs have the promising feature of exhibiting bactericidal effects at concentrations above the minimum inhibitory concentration (MIC) of the corresponding planktonic bacteria as well as demonstrating antibiofilm effects at concentrations lower than the MIC (62). Thus, AMPs may be a possible solution for the issue that many conventional antibiotics only reach the biomass component in sub-therapeutic concentrations. AMPs primarily act on bacterial cell membranes via disruption of their integrity and stability, which leads to cellular content leakage and bacterial cell death (62,63). de Breij et al. demonstrated that even at low concentrations, the SAAP-148 AMP effectively eliminated S. aureus biofilms and persister cells that were resistant to high rifampicin levels (64). Similarly, a combination of temporin 1TB, an AMP derived from frog skin, and ethylenediaminetetraacetic acid (EDTA) demonstrated bactericidal effects against S. epidermidis on silicone catheters (65). Many of these AMPs are formulated as ointments to treat existing infections rather than prevent biofilm formation. However, the orthopedic community has experimented with coatings infused with AMPs. Kazemzadeh-Narbat et al. examined a microporous calcium phosphate coating with the HHC-36 AMP (66). This coating only demonstrated a short duration as the inhibition of P. aeruginosa decreased from 92% to 77% from 4 to 24 hours. Unfortunately, AMPs currently lack long-term efficacy which limits their utility for penile prostheses, but their unique effects on the biomass component are promising for future therapies or techniques.
Bacteriophages
The use of bacteriophages is another potential bactericidal treatment. These phages are selective for bacteria and are able to kill persister cells (67). One such use of bacteriophages is the phage lysin PlySs2 which was recently demonstrated to have greater antibiotic effect and biofilm reduction against S. aureus than vancomycin on the surface of murine knee implants (68). Notably, there was the greatest effect when PlySs2 and vancomycin were combined, suggesting the possibility of combining bacteriophages and antibiotics for greater synergistic effects. Compared to antibiotics, bacteriophages are less likely to cause resistance in the bacterial population. Despite this advantage, the prospect of virus implantation in humans remains untested and its practicality uncertain.
Extracorporeal methods to reduce and control biofilms
Ultrasounds present a promising intervention for biofilm reduction. Ultrasounds can be used as pure sonication therapy or used in conjunction with microbubbles, which are polymer, protein, or lipid shells encasing a gas core (69). Ultrasound waves can cause the microbubbles to expand and contract until they implode. Ultrasound and ultrasound-targeted microbubble destruction (UTMD) can cause cavitations in the EPS which may lead to loss of structural integrity, increased antibiotic penetrance, direct cellular damage, and increased metabolic activity of cells by decreasing bacterial aggregation and quorum sensing (70). Ultrasound therapy is currently limited by biofilm thickness and concerns for local tissue damage or displacement of bacteria with the potential to seed in new locations (11). While sonication alone has not demonstrated antibiofilm activity in vivo, Zhao et al. recently demonstrated that UTMD in conjunction with an antibiotic, amikacin, could reduce the bacterial load in septic arthritis in porcine models (69). Ultrasound therapy, while promising, has yet to be widely tested in vivo but could provide an external mechanism to prevent biofilms.
“Smart” technology for biofilm detection
A “smart” orthopedic implant studied by Erlich et al. involves a biosensor that can detect bacterial quorum sensing and trigger antibiotic release (71). The biosensor is built into the implant and senses levels of ribonucleic acid (RNA)III-activating protein (RAP), which usually binds to a bacterial cell surface receptor as part of quorum sensing and ultimately upregulates virulence gene expression. When the biosensor detects high levels of RAP, high concentrations of inhibitory compounds to prevent biofilm formation and antibiotics are released. Additionally, the implant would have the ability to report all bacterial detections and treatments to both the patient and physician. This implant still requires prototype testing and clinical trial testing.
Refillable antibiotic coatings
Researchers have also examined refillable polymers that can be placed during implantation and refilled later with antibiotics if needed. Cyphert et al. developed a polymerized cyclodextrin with “affinity pockets” that utilize the affinity binding interaction between a drug and cyclodextrin to fill the pockets and allow for prolonged drug release (72). This polymer could be used as an implant coating and filled with antibiotics after implantation. The ability to refill antibiotics could address the short duration limitation of many antibiotic coatings. This technology could potentially enable device rescue instead of removal for infections.
The future for biofilm testing
The wide variety of antibiofilm therapies and strategies demonstrates that the current research landscape is not lacking. NGS is another addition to the research toolkit. NGS was able to detect multiple species of microbes when compared to a standard culture which was often monomicrobial (23). In a study looking at 83 implants, NGS detected microorganisms on 56% of devices compared to only 29% by standard culture (23). These advancements in microbial identification may aid surgeons in selecting the most efficacious antibiotics for each revision.
Limitations
New biofilm treatments are promising; however, they are still in the experimental phase. These would require stringent clinical trials before application in routine patient care. Many of the methods outlined here remain hypothetical in nature and may not demonstrate efficacy in vivo.
Clinical utility
Understanding the dynamics of biofilm formation and subsequent infection after penile prosthesis implantation presents promising improvements to clinical practice. Clinicians may adopt a more targeted approach to antibiotic treatments by considering the different bacterial profiles observed in infected versus non-infected IPPs. Additionally, the implementation of next-generation sequencing may enhance diagnostic accuracy and guide tailored antibiotic therapies. Although the majority of prostheses harbor biofilms, a significant proportion of them remain asymptomatic. Thus, there is need for proactive measures, such as preventing disruption of the biofilm or performing rigorous antibiotic washouts, during revision surgeries even in clinically non-infected cases. Finally, the resistance to conventional antibiotics demonstrated by biofilms underscores the importance of exploring novel therapeutic avenues. While many of these methods are experimental, future investigations and clinical trials present opportunities for clinicians to investigate novel treatments.
Conclusions
Biofilms present a difficult infectious challenge for penile prostheses due to their resistance to conventional antibiotics. However, as our understanding of biofilms continues to evolve, new treatment approaches are being investigated to help prosthetic urologists tackle these microbial communities. These treatments include methods to reduce bacterial adhesion and biofilm formation through surface modifications, biosurfactants, and disruption of fibrin binding. Novel antibiotic strategies under investigation also include AMPs, bacteriophages, and refillable antibiotic coatings. The treatments discussed in this paper offer a promising outlook for the battle against biofilms and it is likely that a combination of these strategies will be integral to further reducing the infection rate after penile prosthesis implantation.
Acknowledgments
Figures were created with BioRender.com.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Paul Chung, Aaron Lentz, and Gerard Henry) for the series “Genitourinary Prosthesis Infection” published in Translational Andrology and Urology. The article has undergone external peer review.
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-550/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-550/coif). The series “Genitourinary Prosthesis Infection” was commissioned by the editorial office without any funding or sponsorship. C.W. has been compensated for CME content creation for Oakstone Publishing and Medscape. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rojanasarot S, Williams AO, Edwards N, et al. Quantifying the number of US men with erectile dysfunction who are potential candidates for penile prosthesis implantation. Sex Med 2023;11:qfad010. [Crossref] [PubMed]
- Baas W, O'Connor B, Welliver C, et al. Worldwide trends in penile implantation surgery: data from over 63,000 implants. Transl Androl Urol 2020;9:31-7. [Crossref] [PubMed]
- Serefoglu EC, Mandava SH, Gokce A, et al. Long-term revision rate due to infection in hydrophilic-coated inflatable penile prostheses: 11-year follow-up. J Sex Med 2012;9:2182-6. [Crossref] [PubMed]
- Carson CC 3rd, Mulcahy JJ, Harsch MR. Long-term infection outcomes after original antibiotic impregnated inflatable penile prosthesis implants: up to 7.7 years of followup. J Urol 2011;185:614-8. [Crossref] [PubMed]
- Bernal RM, Henry GD. Contemporary patient satisfaction rates for three-piece inflatable penile prostheses. Adv Urol 2012;2012:707321. [Crossref] [PubMed]
- Hebert KJ, Kohler TS. Penile Prosthesis Infection: Myths and Realities. World J Mens Health 2019;37:276-87. [Crossref] [PubMed]
- Arciola CR, Campoccia D, Speziale P, et al. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012;33:5967-82. [Crossref] [PubMed]
- Welliver RC Jr, Hanerhoff BL, Henry GD, et al. Significance of biofilm for the prosthetic surgeon. Curr Urol Rep 2014;15:411. [Crossref] [PubMed]
- Werneburg GT, Lundy SD, Hettel D, et al. Microbe-metabolite interaction networks, antibiotic resistance, and in vitro reconstitution of the penile prosthesis biofilm support a paradigm shift from infection to colonization. Sci Rep 2023;13:11522. [Crossref] [PubMed]
- Herati AS, Lo EM. Penile prosthesis biofilm formation and emerging therapies against them. Transl Androl Urol 2018;7:960-7. [Crossref] [PubMed]
- Faller M, Kohler T. The Status of Biofilms in Penile Implants. Microorganisms 2017;5:19. [Crossref] [PubMed]
- O'Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol 2000;54:49-79. [Crossref] [PubMed]
- Muhammad MH, Idris AL, Fan X, et al. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front Microbiol 2020;11:928. [Crossref] [PubMed]
- Berne C, Ellison CK, Ducret A, et al. Bacterial adhesion at the single-cell level. Nat Rev Microbiol 2018;16:616-27. [Crossref] [PubMed]
- Yarwood JM, Bartels DJ, Volper EM, et al. Quorum sensing in Staphylococcus aureus biofilms. J Bacteriol 2004;186:1838-50. [Crossref] [PubMed]
- Songtanin B, Peterson CJ, Molehin AJ, et al. Biofilms and Benign Colonic Diseases. Int J Mol Sci 2022;23:14259. [Crossref] [PubMed]
- Licht MR, Montague DK, Angermeier KW, et al. Cultures from genitourinary prostheses at reoperation: questioning the role of Staphylococcus epidermidis in periprosthetic infection. J Urol 1995;154:387-90. [Crossref] [PubMed]
- Henry GD, Wilson SK, Delk JR 2nd, et al. Penile prosthesis cultures during revision surgery: a multicenter study. J Urol 2004;172:153-6. [Crossref] [PubMed]
- Silverstein AD, Henry GD, Evans B, et al. Biofilm formation on clinically noninfected penile prostheses. J Urol 2006;176:1008-11. [Crossref] [PubMed]
- Abouassaly R, Angermeier KW, Montague DK. Risk of infection with an antibiotic coated penile prosthesis at device replacement for mechanical failure. J Urol 2006;176:2471-3. [Crossref] [PubMed]
- Henry GD, Carson CC, Wilson SK, et al. Revision washout decreases implant capsule tissue culture positivity: a multicenter study. J Urol 2008;179:186-90; discussion 190. [Crossref] [PubMed]
- Gross MS, Phillips EA, Carrasquillo RJ, et al. Multicenter Investigation of the Micro-Organisms Involved in Penile Prosthesis Infection: An Analysis of the Efficacy of the AUA and EAU Guidelines for Penile Prosthesis Prophylaxis. J Sex Med 2017;14:455-63. [Crossref] [PubMed]
- Chung PH, Leong JY, Phillips CD, et al. Microorganism Profiles of Penile Prosthesis Removed for Infection, Erosion, and Mechanical Malfunction Based on Next-Generation Sequencing. J Sex Med 2022;19:356-63. [Crossref] [PubMed]
- Chandrapal J, Harper S, Davis LG, et al. Comparison of Penile Prosthesis Cultures Within Individual Patients After Removal/Replacement and Subsequent Salvage. Sex Med 2020;8:783-7. [Crossref] [PubMed]
- Leong JY, Capella CE, D'Amico MJ, et al. A scoping review of penile implant biofilms-what do we know and what remains unknown? Transl Androl Urol 2022;11:1210-21. [Crossref] [PubMed]
- Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2003;2:114-22. [Crossref] [PubMed]
- Hall CW, Mah TF. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev 2017;41:276-301. [Crossref] [PubMed]
- Henry GD, Wilson SK, Delk JR 2nd, et al. Revision washout decreases penile prosthesis infection in revision surgery: a multicenter study. J Urol 2005;173:89-92. [Crossref] [PubMed]
- Hoffman LR, D'Argenio DA, MacCoss MJ, et al. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005;436:1171-5. [Crossref] [PubMed]
- Driffield K, Miller K, Bostock JM, et al. Increased mutability of Pseudomonas aeruginosa in biofilms. J Antimicrob Chemother 2008;61:1053-6. [Crossref] [PubMed]
- Alav I, Sutton JM, Rahman KM. Role of bacterial efflux pumps in biofilm formation. J Antimicrob Chemother 2018;73:2003-20. [Crossref] [PubMed]
- Mulcahy JJ. Penile prosthesis infection: progress in prevention and treatment. Curr Urol Rep 2010;11:400-4. [Crossref] [PubMed]
- Wilson SK, Gross MS. Biofilm and penile prosthesis infections in the era of coated implants: 2021 update. Int J Impot Res 2022;34:411-5. [Crossref] [PubMed]
- Wilson SK, Costerton JW. Biofilm and penile prosthesis infections in the era of coated implants: a review. J Sex Med 2012;9:44-53. [Crossref] [PubMed]
- Wilson SK, Wen L, Rossello M, et al. Initial safety outcomes for the Rigicon Infla10® inflatable penile prosthesis. BJU Int 2023;131:729-33. [Crossref] [PubMed]
- Wilson SK, Salem EA, Costerton W. Anti-infection dip suggestions for the Coloplast Titan Inflatable Penile Prosthesis in the era of the infection retardant coated implant. J Sex Med 2011;8:2647-54. [Crossref] [PubMed]
- Wilson SK, Zumbe J, Henry GD, et al. Infection reduction using antibiotic-coated inflatable penile prosthesis. Urology 2007;70:337-40. [Crossref] [PubMed]
- Swords K, Martinez DR, Lockhart JL, et al. A preliminary report on the usage of an intracorporal antibiotic cast with synthetic high purity CaSO4 for the treatment of infected penile implant. J Sex Med 2013;10:1162-9. [Crossref] [PubMed]
- Isguven S, Chung PH, Machado P, et al. Minimizing Penile Prosthesis Implant Infection: What Can We Learn From Orthopedic Surgery? Urology 2020;146:6-14. [Crossref] [PubMed]
- Krzastek SC, Smith R. An update on the best approaches to prevent complications in penile prosthesis recipients. Ther Adv Urol 2019;11:1756287218818076. [Crossref] [PubMed]
- Swanton AR, Munarriz RM, Gross MS. Updates in penile prosthesis infections. Asian J Androl 2020;22:28-33. [Crossref] [PubMed]
- Kalra L, Camacho F, Whitener CJ, et al. Risk of methicillin-resistant Staphylococcus aureus surgical site infection in patients with nasal MRSA colonization. Am J Infect Control 2013;41:1253-7. [Crossref] [PubMed]
- Tanner J, Melen K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev 2021;8:CD004122. [PubMed]
- Eid JF, Wilson SK, Cleves M, et al. Coated implants and "no touch" surgical technique decreases risk of infection in inflatable penile prosthesis implantation to 0.46%. Urology 2012;79:1310-5. [Crossref] [PubMed]
- Campoccia D, Montanaro L, Arciola CR. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013;34:8533-54. [Crossref] [PubMed]
- Ahmed W, Zhai Z, Gao C. Adaptive antibacterial biomaterial surfaces and their applications. Mater Today Bio 2019;2:100017. [Crossref] [PubMed]
- Ruggieri MR, Hanno PM, Levin RM. Reduction of bacterial adherence to catheter surface with heparin. J Urol 1987;138:423-6. [Crossref] [PubMed]
- Awonusi BO, Li J, Li H, et al. In vivo study on heparin/poly-l-lysine-copper coating for surface functionalization of ureteral stent. Regen Biomater 2022;9:rbac083. [Crossref] [PubMed]
- Pike CM, Theriot CM. Mechanisms of Colonization Resistance Against Clostridioides difficile. J Infect Dis 2021;223:S194-200. [Crossref] [PubMed]
- Velraeds MM, van de Belt-Gritter B, van der Mei HC, et al. Interference in initial adhesion of uropathogenic bacteria and yeasts to silicone rubber by a Lactobacillus acidophilus biosurfactant. J Med Microbiol 1998;47:1081-5. [Crossref] [PubMed]
- Rodrigues LR. Inhibition of bacterial adhesion on medical devices. Adv Exp Med Biol 2011;715:351-67. [Crossref] [PubMed]
- Kwiecinski J, Na M, Jarneborn A, et al. Tissue Plasminogen Activator Coating on Implant Surfaces Reduces Staphylococcus aureus Biofilm Formation. Appl Environ Microbiol 2015;82:394-401. [Crossref] [PubMed]
- Hogan S, Kasotakis E, Maher S, et al. A novel medical device coating prevents Staphylococcus aureus biofilm formation on medical device surfaces. FEMS Microbiol Lett 2019;366:fnz107. [Crossref] [PubMed]
- Darling KE, Evans TJ. Effects of nitric oxide on Pseudomonas aeruginosa infection of epithelial cells from a human respiratory cell line derived from a patient with cystic fibrosis. Infect Immun 2003;71:2341-9. [Crossref] [PubMed]
- Charville GW, Hetrick EM, Geer CB, et al. Reduced bacterial adhesion to fibrinogen-coated substrates via nitric oxide release. Biomaterials 2008;29:4039-44. [Crossref] [PubMed]
- Holt J, Hertzberg B, Weinhold P, et al. Decreasing bacterial colonization of external fixation pins through nitric oxide release coatings. J Orthop Trauma 2011;25:432-7. [Crossref] [PubMed]
- Samuni Y, Goldstein S, Dean OM, et al. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta 2013;1830:4117-29. [Crossref] [PubMed]
- Zhao T, Liu Y. N-acetylcysteine inhibit biofilms produced by Pseudomonas aeruginosa. BMC Microbiol 2010;10:140. [Crossref] [PubMed]
- Costa F, Sousa DM, Parreira P, et al. N-acetylcysteine-functionalized coating avoids bacterial adhesion and biofilm formation. Sci Rep 2017;7:17374. [Crossref] [PubMed]
- Tetz GV, Artemenko NK, Tetz VV. Effect of DNase and antibiotics on biofilm characteristics. Antimicrob Agents Chemother 2009;53:1204-9. [Crossref] [PubMed]
- Sharma K, Pagedar Singh A. Antibiofilm Effect of DNase against Single and Mixed Species Biofilm. Foods 2018;7:42. [Crossref] [PubMed]
- Batoni G, Maisetta G, Esin S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim Biophys Acta 2016;1858:1044-60. [Crossref] [PubMed]
- Li X, Sun L, Zhang P, et al. Novel Approaches to Combat Medical Device-Associated BioFilms. Coatings 2021;11:294. [Crossref]
- de Breij A, Riool M, Cordfunke RA, et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci Transl Med 2018;10:eaan4044. [Crossref] [PubMed]
- Maisetta G, Grassi L, Di Luca M, et al. Anti-biofilm properties of the antimicrobial peptide temporin 1Tb and its ability, in combination with EDTA, to eradicate Staphylococcus epidermidis biofilms on silicone catheters. Biofouling 2016;32:787-800. [Crossref] [PubMed]
- Kazemzadeh-Narbat M, Kindrachuk J, Duan K, et al. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials 2010;31:9519-26. [Crossref] [PubMed]
- Tkhilaishvili T, Lombardi L, Klatt AB, et al. Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. Int J Antimicrob Agents 2018;52:842-53. [Crossref] [PubMed]
- Sosa BR, Niu Y, Turajane K, et al. 2020 John Charnley Award: The antimicrobial potential of bacteriophage-derived lysin in a murine debridement, antibiotics, and implant retention model of prosthetic joint infection. Bone Joint J 2020;102-B:3-10. [Crossref] [PubMed]
- Zhao N, Curry D, Evans RE, et al. Microbubble cavitation restores Staphylococcus aureus antibiotic susceptibility in vitro and in a septic arthritis model. Commun Biol 2023;6:425. [Crossref] [PubMed]
- He N, Hu J, Liu H, et al. Enhancement of vancomycin activity against biofilms by using ultrasound-targeted microbubble destruction. Antimicrob Agents Chemother 2011;55:5331-7. [Crossref] [PubMed]
- Ehrlich GD, Stoodley P, Kathju S, et al. Engineering approaches for the detection and control of orthopaedic biofilm infections. Clin Orthop Relat Res 2005;59-66. [Crossref] [PubMed]
- Cyphert EL, Zuckerman ST, Korley JN, et al. Affinity interactions drive post-implantation drug filling, even in the presence of bacterial biofilm. Acta Biomater 2017;57:95-102. [Crossref] [PubMed]


