
Rucaparib monotherapy in the heavily pre-treated metastatic castrate-resistant prostate cancer setting: practical considerations and alternate treatment approaches
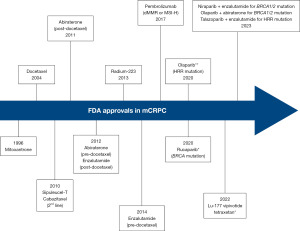
Over the past few years, poly-ADP ribose polymerase inhibitors (PARPi) have emerged as a guideline-approved, biomarker-selected treatment approach for the management of metastatic castrate-resistant prostate cancer (mCRPC). Following the publication of multiple trials in this disease space, numerous such agents, either alone or in combination with androgen receptor pathway inhibitors (ARPI) have gained Food and Drug Administration (FDA) approval across the mCRPC treatment paradigm (Figure 1): (I) rucaparib in May 2020 for mCRPC patients with deleterious BReast CAncer (BRCA) mutations who had been previously treated with an ARPI and taxane-based chemotherapy (1) [TRITON2 (2)]; (II) olaparib in May 2020 for patients with deleterious or suspected homologous recombination repair (HRR) gene-mutated mCRPC with progression following prior abiraterone or enzalutamide (3) [PROfound (4)]; (III) olaparib in combination with abiraterone and prednisone (or prednisolone) in May 2023 for mCRPC patients with deleterious or suspected deleterious BRCA mutations (5) [PROpel (6)]; (IV) talazoparib in combination with enzalutamide in June 2023 for mCRPC patients with HRR gene mutations (7) [TALAPRO-2 (8)]; and (V) niraparib in combination with abiraterone acetate plus prednisone in August 2023 for mCRPC patients with deleterious or suspected deleterious BRCA mutations (9) [MAGNITUDE (10)].
Updated, final results of TRITON2 were recently published by Abida et al. (11). To summarize, this is an international, open-label, phase II trial that evaluated the safety and efficacy of rucaparib 600 mg twice daily in mCRPC patients with DNA damage response (DDR) gene alterations who had progressed after one to two lines of an ARPI and one-taxane based chemotherapy. The efficacy cohort included 277 patients, of whom 172 (62.1%) had a deleterious germline or somatic BRCA alteration with 59 (21.3%), 15 (5.4%), seven (2.5%), 11 (4.0%), and 13 (4.7%) having ataxia-telangiectasia mutated gene (ATM), cyclin-dependent kinase 12 (CDK12), checkpoint kinase 2 (CHEK2), partner and localizer of BRCA2 (PALB2), and other DDR gene mutations, respectively. A confirmed objective response was observed in 46% of BRCA patients with measurable disease (complete response: 10%). A superior response was observed among BRCA2 patients (48% versus 30% for BRCA1), which is potentially secondary to an increased frequency of biallelic mutations among BRCA2 patients and a greater coexistence of TP53 mutations among BRCA1-mutated men (12). Objective response was consistent irrespective of whether the BRCA mutation was somatic or germline and whether other DDR mutations were present or absent. All four patients with PALB2 mutations and measurable disease had an objective partial response, with none of the ATM-, CDK12-, CHEK2-mutated patients experiencing an objective response. A confirmed prostate-specific antigen (PSA) response with ≥50% decrease from baseline (PSA50) was observed in 53% and 55% of BRCA and PALB2-mutated patients, compared to 3.4–14% among patients with other DDR gene mutations. The median overall survival was 17.2 months for BRCA patients, compared to 11.1–14.6 months among ATM, CDK12, and CHEK2-mutated patients (Table 1). Grade 3 or worse treatment-emergent adverse events were observed in 64% of patients, with the most common being anemia/decreased hemoglobin (29%) and fatigue (11%). Significantly, 33% of patients required at least one transfusion. While not reported in the TRITON2 overall safety population, known potential long-term sequalae of these agents, particularly if used in earlier settings, include myelodysplastic syndrome and bone marrow failure (13).
Table 1
| Outcome | BRCA1 (n=22) | BRCA2 (n=150) | ATM (n=59) | CDK12 (n=15) | CHEK2 (n=7) | PALB2 (n=11) | Other (n=13) |
|---|---|---|---|---|---|---|---|
| ORR (%) | 30 | 48 | 0 | 0 | 0 | 100 | 25 |
| DoR, median (months) | 15.5 | – | – | – | 10.1 | – | |
| rPFS, median (months) | 10.7 | 5.3 | – | – | 13.6 | 5.8 | |
| PSA50 (%) | 18 | 59 | 3.40 | 6.70 | 14 | 55 | 23 |
| PSA90 (%) | 20 | 0 | 0 | 14 | 9.10 | 23 | |
| Time to PSA progression (months) | 6.5 | 3.1 | – | – | 7.5 | – | |
| OS, median (months) | 17.2 | 14.6 | 13.9 | 11.1 | 17.7 | 10.5 | |
BRCA, BReast CAncer; ATM, ataxia-telangiectasia mutated gene; CDK12, cyclin-dependent kinase 12; CHEK2, checkpoint kinase 2; PALB2, partner and localizer of BRCA2; ORR, objective response rate; DoR, duration of response; rPFS, radiographic progression-free survival; PSA50, 50% decrease in PSA level; PSA90, 90% decrease in PSA level; PSA, prostate-specific antigen; OS, overall survival.
These results add to the existing evidentiary base for rucaparib in the mCRPC disease space, with the recently published TRITON3 phase III trial demonstrating that rucaparib 600 mg twice daily in mCRPC patients with a BRCA or ATM alteration and who had disease progression following treatment with an ARPI improved imaging-based progression-free survival from a median of 6.4 to 10.2 months [P<0.001; (14)]. These results ‘move up’ rucaparib along the mCRPC treatment paradigm from the 3rd to 2nd line setting. As with other drugs in this disease space, such as docetaxel and ARPIs, there is a concerted effort to move up these agents further up the prostate cancer treatment landscape. Ongoing phase III trials in the metastatic hormone sensitive prostate cancer (mHSPC) space include TALAPRO-3 (NCT04821622), evaluating the combination of talazoparib plus enzalutamide, and AMPLITUDE (NCT04497844), evaluating the combination of niraparib plus abiraterone, both in mHSPC patients with HRR gene alterations. The phase II ZZ-First (NCT04332744) trial is evaluating the addition of talazoparib to enzalutamide in unselected mHSPC patients. In the high-risk localized and locally advanced setting, the NADIR (NCT04037254) three-arm trial is comparing radiotherapy + androgen deprivation therapy (ADT) to each of niraparib alone and niraparib + radiotherapy/ADT. Results of these trials are expected to become available in the next few years and will determine where PARP inhibitors will ultimately fit in the prostate cancer treatment landscape.
The efficacy of rucaparib in TRITON2 highlights its potential role in the post-ARPI/docetaxel setting and adds to the multitude of treatment options for patients in the ‘3rd line’ treatment setting, which also includes cabazitaxel and lutetium-177 (177Lu)-PSMA-617. The optimal treatment approach for patients in this setting remains uncertain. While it is currently recommended that all patients with metastatic prostate cancer undergo testing for germline and somatic tumor mutations, with patients harboring actionable mutations such as BRCA1/2 ideally benefiting from a biomarker-selected treatment approach with PARPi, the reality is that the majority of patients in clinical practice do not undergo genetic testing (15,16). Another complicating factor is that the best method for detecting an HRR deficiency has yet to be determined. While obtaining genetic testing from a fresh metastatic tumor biopsy is considered optimal, it is often unavailable and can be technically challenging to obtain in select metastatic cases (e.g., bone biopsy samples). Use of circulating tumor DNA remains an option in this setting, along with an archival sample of metastatic or primary tumor tissue, although limitations in this scenario include longitudinal accumulation of additional somatic mutations that are not present in this older sample, multifocal/multiclonal disease not representative of other tumor sites, potential degradation of DNA after years in paraffin, and potential false positive results secondary to clonal hematopoiesis of indeterminate potential (CHIP). Reliance on blood or saliva samples for germline testing does not evaluate for somatic alterations and cannot discern monoallelic from biallelic alterations, missing approximately half of actionable BRCA1/2 alterations.
Given the challenges associated with routine genetic testing in clinical practice, this may consequentially lead to the ‘practical’ utilization of 177Lu-PSMA-617 and cabazitaxel in mCRPC patients progressing following prior APRI and docetaxel treatment (17,18). Both agents have demonstrated overall survival benefits in this setting and their use may be applicable to a broader population of mCRPC patients. In TheraP, eligible patients were required to have high expression on prostate-specific membrane antigen positron emission tomography/computed tomography (PSMA-PET/CT), with at least one site with a maximum standardized uptake value (SUVmax) ≥20) and no sites of fluorodeoxyglucose (FDG)-positive/PSMA-negative disease. Based on these criteria, 200/291 patients (68.7%) were eligible for study inclusion (18). Conversely in the VISION trial, patients were required to have PSMA-positive disease on the basis of a central review of 68Ga-PSMA-11 staging scans, with PSMA positivity defined as uptake greater in the metastatic lesions than in the liver. Further, they could have no PSMA-negative metastatic lesions. Of 1,003 scanned patients, 869 (86.6%) were deemed trial eligible (19). Given the increased availability and utilization of prostate-specific PSMA-PET/CT, along with most mCRPC patients expressing PSMA-positive disease in the post-ARPI/docetaxel setting, we may observe 177Lu-PSMA-617 being preferentially utilized in this setting. Additionally, as of current, no biomarker testing is required prior to cabazitaxel administration.
In conclusion, we note that the results of the TRITON2 trial are an important addition to the current mCRPC literature and solidify rucaparib as a treatment option for mCRPC patients with disease progression following ARPI and docetaxel treatment. With the increased availability of treatment options in this disease setting, a nuanced approach to the management of these patients will be required to enhance guideline-concordant exposure to the maximal possible number of lines of therapy with alternate mechanisms of action in order to maximize survival outcomes in this high-risk population.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Andrology and Urology. The article has undergone external peer review.
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-671/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-671/coif). Neil E. Fleshner reports grants and personal fees from Janssen, Astellas, Bayer, and Sanofi; grants from Nucleix, Progenix, and the Ontario Institute for Cancer Research; and personal fees from Amgen, Abbvie, Ferring, Verity Pharmaceuticals, and POINTBiopharma outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- FDA grants accelerated approval to rucaparib for BRCA-mutated metastatic castration-resistant prostate cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistant-prostate (accessed on October 9, 2023).
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol 2020;38:3763-72. [Crossref] [PubMed]
- FDA approves olaparib for HRR gene-mutated metastatic castration-resistant prostate cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-hrr-gene-mutated-metastatic-castration-resistant-prostate-cancer (accessed on October 9, 2023).
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020;382:2091-102. [Crossref] [PubMed]
- FDA D.I.S.C.O. Burst Edition: FDA approval of Lynparza (olaparib), with abiraterone and prednisone, for BRCA-mutated metastatic castration-resistant prostate cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-lynparza-olaparib-abiraterone-and-prednisone-brca-mutated#:~:text=On%20May%2031%2C%202023%2C%20the,FDA%2Dapproved%20companion%20diagnostic%20test (accessed on October 9, 2023).
- Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evid 2022;1:EVIDoa2200043.
- FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-enzalutamide-hrr-gene-mutated-metastatic-castration-resistant-prostate (accessed on October 9, 2023).
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet 2023;402:291-303. [Crossref] [PubMed]
- FDA approves niraparib and abiraterone acetate plus prednisone for BRCA-mutated metastatic castration-resistant prostate cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-niraparib-and-abiraterone-acetate-plus-prednisone-brca-mutated-metastatic-castration (accessed on December 21, 2023).
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol 2023;41:3339-51. [Crossref] [PubMed]
- Abida W, Campbell D, Patnaik A, et al. Rucaparib for the Treatment of Metastatic Castration-resistant Prostate Cancer Associated with a DNA Damage Repair Gene Alteration: Final Results from the Phase 2 TRITON2 Study. Eur Urol 2023;84:321-30. [Crossref] [PubMed]
- Taza F, Holler AE, Fu W, et al. Differential Activity of PARP Inhibitors in BRCA1- Versus BRCA2-Altered Metastatic Castration-Resistant Prostate Cancer. JCO Precis Oncol 2021;5:PO.21.00070.
- Morice PM, Leary A, Dolladille C, et al. Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: a safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol 2021;8:e122-34. [Crossref] [PubMed]
- Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or Physician's Choice in Metastatic Prostate Cancer. N Engl J Med 2023;388:719-32. [Crossref] [PubMed]
- Kurian AW, Abrahamse P, Furgal A, et al. Germline Genetic Testing After Cancer Diagnosis. JAMA 2023;330:43-51. [Crossref] [PubMed]
- Zhen JT, Syed J, Nguyen KA, et al. Genetic testing for hereditary prostate cancer: Current status and limitations. Cancer 2018;124:3105-17. [Crossref] [PubMed]
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med 2019;381:2506-18. [Crossref] [PubMed]
- Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 2021;397:797-804. [Crossref] [PubMed]
- Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2021;385:1091-103. [Crossref] [PubMed]


