
Refined step-by-step narrative review of robotic radical nephroureterectomy in the management of upper tract urothelial carcinoma
Introduction
Background
Urothelial carcinoma (UC) is the fourth most common solid organ malignancy (1). About 5–10% occur in the upper urinary tract including the renal calyces, renal pelvis, or ureter, known collectively as upper tract UC (UTUC). While UC of the bladder is well-studied, UTUC studies are less common. Compared to lower urinary tract UC, UTUC has a higher rate of local invasion at diagnosis (2). The management of UTUC varies according to tumor grade, stage, location, and focality. Solitary, low-grade tumors and those in highly comorbid patients can be managed with endoscopic fulguration however for many upper tract lesions, particularly large, multifocal, high-grade, or renal pelvis tumors, the standard management is nephroureterectomy. Adoption of robot-assisted laparoscopic surgery for management of both UTUC and muscle-invasive bladder cancer (MIBC) has been increasing internationally over time. Two recent manuscripts from Germany document such advancements in surgical management from mainly open to minimally invasive over the study time periods 2006–2019 (3,4).
The first open nephroureterectomy was described in 1898 though in the initial reports, the distal ureter was left intact (5). Subsequently, it was noted that a significant portion of these patients had tumor recurrences in the distal ureter (6). As such, the operation was modified to include the entire kidney, extra- and intra-mural ureter by excising a bladder cuff. As minimally invasive urological techniques were developed, Clayman et al. successfully applied laparoscopy to UTUC by performing the first laparoscopic nephroureterectomy (7). With the advent of the da Vinci® robotic surgical system, surgeons had the advantage of improved visualization and dexterity. The first robot-assisted nephroureterectomy was performed in 2006 taking advantage of the unparalleled advantages of the robotic system (8,9). However, due to limitations in robotic arm placement with the initial robotic platforms, surgeons were forced to re-dock the robot for excision of the distal ureter and bladder cuff in most cases, increasing operative times (10). Innovative surgical approaches were developed in an effort to improve the technique including using a dual-port/hybrid-port technique to address the limitation of needing to adjust port size, location, and utility when transitioning from the nephrectomy aspect of the case to the ureterectomy portion (11). With the da Vinci® S robotic surgical system, efforts were additionally made to prevent re-docking and re-positioning of the patient with a “baseball diamond” port placement approach—which was first utilized in a porcine model followed by humans (12). Advanced visibility, motion, and the seven degrees of freedom associated with the fourth generation da Vinci® Xi robotic system affords precise excision and closure of a true bladder cuff in a more simplified and straightforward fashion. Robotic arms can be placed relatively close together, the boom to swing according to surgeon needs, and the camera can “hop”, or be placed through any robotic 8 mm port, allowing for robotic nephroureterectomy to be performed without re-docking. The surgical technique has been improved over time with advancements in the da Vinci® surgical system including the ability to use targeting to optimize patient clearance and the location of the robotic arms and synchronized table motion with the patient bed synchronized with the surgical robot. These improvements have obviated the need for dedicated separate incisions to address the distal ureter and bladder cuff, which has been shown to reduce operating room (OR) time and cost (13-17). There has recently been consideration of nuancing the decision to perform a bladder cuff excision in patients with low-risk UTUC (18). There are certain situations where a bladder cuff excision has been deferred, such as those with competing risk of continued anesthesia due to severe co-morbidities, however in general it continues to be performed at our institution for all patients otherwise (19).
Rationale and knowledge gap
Robot-assisted technology has been widely adapted among urologists throughout the world. As practicing urologists, trainees, and those interested in learning robotic surgery advance their application of various techniques in nephroureterectomy, we felt it would be worthwhile to share the approach that has been fine-tuned at our high-volume center over the last decade.
Objective
We seek to provide a logical approach to performing radical nephroureterectomy with bladder cuff excision (RNU/BCE) and regional lymphadenectomy that may be translatable for urologists interested in either adapting this refined robotic technique as part of UTUC management and/or optimizing their existing surgical approach in radical nephroureterectomy. We present this article in accordance with the Narrative Review reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-209/rc).
Methods
A review of local experience in RNU/BCE was undertaken and summarized. A literature review was performed regarding iterations of the robot-assisted technique and its adaptation over time from open surgery to laparoscopic surgery prior to the introduction of the da Vinci® surgical systems. Table 1 summarizes the search strategy implemented.
Table 1
| Items | Specification |
|---|---|
| Date of search | 08/22/2022 |
| Databases and other sources searched | PubMed |
| Search terms used | Robot-assisted nephroureterectomy, bladder cuff excision, laparoscopic nephroureterectomy |
| Timeframe | Up to August 22, 2022 |
| Inclusion criteria | English articles |
| Selection process | All authors reviewed articles included in final manuscript to ensure relevance |
| Any additional considerations, if applicable | Several articles were selected for inclusion based on their established role as seminal manuscripts beyond those identified in formal literature review |
Description of surgical technique
The approach for RNU/BCE and regional lymphadenectomy is broken down to a step-by-step approach systematically. The rationale behind certain technical nuances is provided for perspective, which may be of highest interest to those seeking to optimize their technique based on the experience of the authors.
Pre-operative evaluation
Pre-operatively, patients are evaluated in accordance with guideline recommendations including history and physical examination, comprehensive metabolic panel, complete blood count, urinalysis, urine culture, urine cytology, and cystourethroscopy. Most patients are referred with cross-sectional imaging; computed tomography (CT) including delayed images “urogram protocol” should be completed for all patients, except, in general, for those with estimated glomerular filtration rate (eGFR) <40 mL/min/1.73 m2. Those with impaired renal function are strongly considered for magnetic resonance imaging with “urogram protocol” as the risk of nephrogenic systemic fibrosis (NSF) is significantly low to almost non-existent with novel group II gadolinium-based contrast agents (GBCAs). Specifically, the 2023 American College of Radiology (ACR) “Manual on Contrast Media” states that the “risk of NSF among patients exposed to standard or lower than standard doses of group II GBCAs is sufficiently low or possibly nonexistent such that assessment of renal function with a questionnaire or laboratory testing is optional prior to intravenous administration” (20). If office cystoscopy reveals a concomitant bladder tumor, this is addressed by transurethral resection of bladder tumor with instillation of intravesical chemotherapy, if indicated, on a separate surgical date prior to proceeding with radical nephroureterectomy.
Endoscopic resection may be considered in lieu of nephroureterectomy for those with non-bulky low-grade UTUC. Well-selected patients with high-grade disease may be candidates for nephron-sparing surgery, such as those with isolated mid- and distal-ureteral UTUC, with appropriate reconstruction as indicated (e.g., ileal ureter, primary ureteroplasty with or without graft or flap interposition, and ureteral reimplantation). Patients not fitting these categories, deemed medically appropriate, are candidates to robot-assisted nephroureterectomy. Of note, when feasible, neoadjuvant chemotherapy is encouraged for the appropriate patient (21). The impact of neoadjuvant chemotherapy in UTUC continues to be explored in randomized controlled trials however appears to be promising in retrospective studies (22). All patients are planned for template-based regional lymphadenectomy at the time of surgery.
Patients are instructed to start drinking clear liquids only following breakfast the morning before surgery, which may be continued until 2 hours before arrival to the hospital for surgery, in accordance with local Anesthesiology protocol. We instruct patients to take two bisacodyl (Dulcolax®) 5 mg tablets in the afternoon the day before surgery and to self-administer a saline (Fleet®) or Glycerine enema (if renal function is compromised) the evening before and morning of surgery. If pre-operative urine culture is positive, the patient receives appropriate antibiotics before surgery. Anticoagulation and antiplatelet agents (except for aspirin 81 mg) are temporarily held in coordination with the appropriate prescriber weighing the relative risks and benefits of doing so.
Positioning
Upon entering the OR, the patient is placed in the supine position for the induction of anesthesia. A Foley catheter is placed in the standard aseptic technique. Once appropriately anesthetized, the patient is placed in a full 75°–90° flank positioning with all pressure points appropriately padded. The lower leg is flexed approximately 30° and the upper leg is straight with two pillows used between the legs as a bolster to avoid pressure injury. An axillary roll is placed under the axilla on the underside of the patient and this contralateral arm is placed on and secured to an arm board. The ipsilateral “up” arm is typically secured to the patient’s side as it lies. An Allen arm rest is occasionally required in large patients if the robot will not be able to be docked to the ports without colliding with the ipsilateral arm at the side. The back is supported by two back rests in the form of Stulberg hip positioners. The bed is flexed approximately 30°–40° to maximize space between the costal margin and the iliac crest. Caution should be taken that over-flexion may cause the space provided by abdominal insufflation to decrease such that manipulation of a large kidney may become technically more challenging without enough anterior space within the abdomen. The patient is secured to the OR table with thick silk tape wrapped over foam padding at least three times around the table at the level of the chest, pubic symphysis, and lower extremities. The bed may be tilted to the left or right as necessary and additional Trendelenburg may be incorporated to optimize positioning.
The abdomen is prepped and draped in the usual sterile manner with two 2% chlorhexidine gluconate/70% isopropyl alcohol applicators (ChloraPrep™) and 3M™ Ioban™ cut into strips, which are used to secure sterile towels to the prepped field.
Abdominal insufflation and port placement
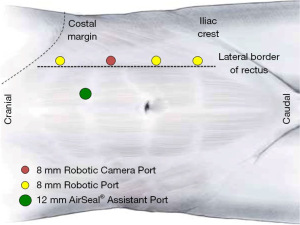
Abdominal access is performed with a Veress needle that is placed just lateral to the anticipated location of the ports at the lateral border of the ipsilateral rectus sheath. The abdomen is insufflated to 15 mmHg for initial port placement. We typically use bariatric trocars to standardize the approach to all patients. An appropriate location for the camera port is selected and an 8 mm incision is sharply made lateral to the anticipated location of the rectus sheath approximately 8 cm inferior to the costal margin. A blunt tip trocar is placed in the standard fashion at this site followed by insertion of the 30° up, down, or lateral camera angle to facilitate placement of all under ports under direct vision. Adjustments to initial abdominal access location may be necessary if the patient has had prior abdominal surgery and adhesions are anticipated. The remainder of ports are placed in a straight line just lateral to the rectus muscle approximately 6–7 cm from each other. The most inferior port may occasionally be placed just medial to this line towards midline as this port is important while performing distal ureteral dissection, bladder cuff excision, and cystorrhaphy in our popularized and most widely-adopted technique of port placement without redocking and repositioning (13,23,24). A 12 mm CONMED AirSeal® port is utilized as an assistant port and is placed in the midline or horizontally through the rectus muscle between and medial to the most superior and second-most inferior 8 mm robotic trocars. Prior to placement of the 12 mm AirSeal® port, a 0 polyglactin 910 tie is passed with a Carter-Thomason suture passer to the superior and inferior aspects of the horizontal incision at the anticipated location of the trocar intraperitoneally under live laparoscopic visualization to facilitate perfect port closure at the conclusion of the case. Passage of the suture prior to trocar passage improves efficiency and ensures a reliable fascial closure for the 12 mm port. A summary of port placement for transperitoneal da Vinci® Xi RNU/BCE (left-sided) may be seen in Figure 1. Port placement for a contralateral (right) approach would be essentially a mirror image of this.
The robot is docked from behind the patient’s back in the standard technique. Targeting is infrequently necessary and this step is often skipped. After docking, insufflation is decreased to 14 mmHg and in elderly patients and/or those with extensive co-morbidities with sensitivity in hemodynamics related to intraabdominal pneumoperitoneum, the pressure can be dropped to 10 mmHg where it remains for the majority of the operation. Close communication with anesthesiology is critical to ensure no significant hemodynamic or physiologic effects of insufflation are encountered. If required due to hypercapnia, the insufflation can be adjusted to as low as 5 mmHg for the safety of the patient without converting to open surgery. For left-sided operations, a Fenestrated Bipolar is placed through the most superior port, and from superior to inferior are placed the robotic 30° camera, Monopolar Curved Scissors, and ProGrasp™. For right-sided operations, the Fenestrated Bipolar and Monopolar Curved Scissors are swapped to start the operation. Energy settings vary from surgeon-to-surgeon however we typically start the case with all set at “3” and adjust accordingly throughout the case. The robotic assistant works facing the patient, as does the surgical technologist. We only use one assistant port and through this, a laparoscopic suction irrigator is utilized by the bedside assistant for general gentle tissue retracting and smoke evacuation. We additionally set the CONMED Insufflation Management System on “high” for smoke evacuation to optimize visualization.
Instillation of intravesical chemotherapy
Once the robot has been docked and the procedure is set to commence, intravesical chemotherapy is instilled by the circulating registered nurse and the urethral catheter is capped. This dwells for 1 hour within the bladder during the nephrectomy aspect of the operation. Earlier, we have used mitomycin C but presently, 2,000 mg gemcitabine is instilled intravesically as part of institutional GEMINI protocol (25). The circulating nurse drains this during the operation at 1 hour, prior to the distal ureterectomy and bladder cuff excision. Early instillation ensures maximum dwell time prior to performing this aspect of the case. However, if not instilled at that time, gemcitabine is instilled within 24 hours of surgery in the perioperative period.
Mobilization of the colon
The camera is placed in the 30° down position. The colon is mobilized medially by incising along the white line of Toldt with appropriate retraction medially. The bedside assistant and ProGrasp™ can be utilized to augment medial retraction as needed. This incision is carried inferiorly to the level of the pelvic inlet and cranially to permit access to the upper pole of the kidney. The plane between Gerota’s fascia and the colonic mesocolon is developed. There are multiple ways to approach proceeding with a nephrectomy; this depends on surgeon experience and preference. In some instances, the lower pole of the kidney is identified and lifted anteriorly with the fourth arm and the ureter and gonadal vein are identified. The ureter is retracted anteriorly and the Psoas muscle is identified. The plane along the Psoas muscle and lower pole is carried cephalad until the renal hilum is identified and dissected appropriately. Alternatively, the renal hilum may be approached directly without first mobilizing the lower pole, gonadal vein, and ureter. This is the standard approach in our experience in an effort to maximize efficiency unless anatomic or patient-specific factors dictate proceeding first with a lower pole and ureteral dissection. We prefer controlling the renal artery as soon as possible by applying one Weck® Hem-o-lok® extra-large clip, which will de-vascularize the kidney. Thereafter, we place another clip below the level of the tumor on the ureter to prevent downward seeding during manipulation.
Dissection and control of the renal hilum
The dissection of the renal hilum is carried out in the standard fashion, meticulously, with care to avoid injury to the main renal artery, vein, adrenal vessels, as well as accessory and lumbar veins. The dissection is carried out with appropriate retraction with the ProGrasp™ and with additional retraction as needed by the bedside assistant. We routinely utilize an absorbent white strip for gentle blunt dissection. After freeing, appropriately separating, and skeletonizing the renal vessels, extra-large Weck® Hem-o-lok® clips are typically applied to the proximal aspect of each the renal artery and vein followed by application of a vascular load laparoscopic surgical stapler passed by the bedside assistant distally to transect the vessels individually, starting with the renal artery and all significant branches or accessory arteries prior to taking the vein. There have been situations where passage of a stapler is not feasible and in an effort to optimize resource allocation/operative cost, clips alone have been used to control the renal hilum. In instances where the vessels are unable to be safely separated or efficiency is of the highest priority due to patient-related factors, the vessels are occasionally taken en bloc with a surgical stapler with vessel load. We do prefer and recommend applying one extra-large Weck® Hem-o-lok® clip over the stay side of the renal artery as the incidence of atherosclerosis is very high in elderly population which can affect the success of surgical stapling alone. If pre-operative CT demonstrates significant calcification, then we would strongly consider dividing the renal artery using extra-large Weck® Hem-o-lok® clips alone.
Freeing the kidney
Once the renal hilum has been controlled and transected and hemostasis is confirmed, the upper and lower pole are dissected along with the ureter. The proximal ureter is clipped immediately upon identification to minimize seeding of any renal pelvis lesions distally during the manipulation of the kidney and ureter that ensues. The upper and lower pole of the kidney are dissected and attachments transected, followed by the lateral attachments. This can be accomplished with the standard robotic instruments however occasionally a Vessel Sealer can be utilized throughout the case as the fourth arm to retract without opening a ProGrasp™, and then utilized in this scenario to efficiently complete the nephrectomy aspect of the case. Alternatively, tissue loads can be used via the laparoscopic surgical stapler that was already opened to apply vessel loads to the renal vessels to efficiently complete this step. Once the kidney has been freed circumferentially and hemostasis is confirmed, we proceed to performance of the distal ureterectomy and bladder cuff excision, followed by regional template lymphadenectomy. Typically, an adrenal gland-sparing approach is implemented unless there is an indication to take en masse (i.e., pre-operative imaging is suspicious for invasion or intraoperative findings suggest local tumor invasion).
Proximal ureteral dissection
As dissection carries from the proximal ureter to the mid and distal ureter, the camera port is changed to the trocar just inferior to its original location (now second-most inferior along the abdominal wall). In this way, the most inferior trocar becomes the right hand and the previous camera port becomes the left on a left-sided case and vice-versa for a right-sided procedure. The most superior port then becomes the “fourth arm” used for retraction. There is no need to completely undock the robot at this point; port hopping optimizes positioning to complete the distal ureterectomy and bladder cuff excision. With the patient Trumpf Medical™ TruSystem® 7000dV Surgical Table paired to the Xi™ robot, Integrated Table Motion can be employed to steepen the Trendelenburg to optimize patient positioning for the pelvic aspect of the operation if needed. In our experience, this is rarely necessary and adds additional time to OR utilization.
Distal ureterectomy, bladder cuff excision, and bladder repair
The distal ureter is next meticulously dissected with care to avoid injury and spillage to the ureter itself. Periodic Hem-o-lok® clips are placed along the ureter to minimize the risk of spillage with the first clip placed as soon as possible one the renal artery is controlled. If there is a known ureteral tumor, the clips are placed both proximal and distal, as feasible. Intraoperative ultrasound can be utilized to assess for ureteral patency as needed to localize the lesion(s) along the ureter. Gentle traction on the ureter is very helpful in completing this step. Once the distal ureter and intramural tunnel are identified, further traction is applied to tent up the bladder cuff to be excised. A 3-0 barbed suture is placed as a stay suture along the bladder cuff with care to avoid injury to the contralateral ureteral orifice. At this point, external suction is applied to the Foley catheter to minimize spillage of intravesical contents to the peritoneal cavity upon cystotomy. If there is difficulty in identifying the bladder cuff prior to cystotomy, indocyanine green admixed with sterile water can be directly instilled into the bladder via the urethral catheter and FireFly™ infrared immunofluorescence can be utilized at the robotic console to optimize localization. The cystotomy is performed in a controlled manner and the distal ureter with bladder cuff are excised en bloc with the remaining previously dissected proximal ureter and kidney. The specimen is immediately placed in an Endo Catch™ II bag once the cystotomy is closed. We typically perform a two-layer bladder closure with 3-0 barbed suture. The most inferior robotic port is extended to 15 mm and trocar removed to accommodate passage of the large Endo Catch™ II bag. This step can be easily undertaken as pneumoperitoneum is maintained via the assistant AirSeal® port.
Regional lymphadenectomy
Once the primary specimen has been appropriately bagged, attention is turned to regional lymphadenectomy based on a standard template, which has been previously described (17). With primary lesions in the renal pelvis on the right: hilar, para-caval, retrocaval, and interaortocaval nodal dissection is performed; on the left, hilar and para-aortic nodal dissection is performed. Primary lesions in the mid-ureter are addressed with, on the right, para-caval, retro-caval, interaortocaval, and common iliac lymph node dissection; on the left, para-aortic, common iliac, and internal iliac lymphadenectomy is performed. For lesions in the distal ureter, on the right, para-caval and pelvic lymph node dissection is performed whereas on the left para-aortic and pelvic nodal dissection is performed. Nodes are extracted in real-time by the bedside assistant using the laparoscopic grasper via the 12 mm AirSeal® port. The AirSeal® port is valveless and thus the specimens do not routinely get caught on the edges and are extracted intact. Each lymph node packet is sent separately to optimize histopathologic review and for localization of positive nodal disease, if present. If bulky nodal packets are taken, an Endo Catch™ I bag can be used and extracted at the time of the main specimen retrieval.
Specimen extraction and closure
Primary extraction of the en bloc kidney, ureter, and bladder cuff is performed within the Endo Catch™ II bag via an extension of the most inferior port site extended laterally, and occasionally, medially. Thus, a Gibson incision is created. Care must be taken to avoid injury to the inferior epigastric artery if the incision is carried medially. A muscle-splitting technique is utilized to optimize the strength of the closure and minimize post-operative pain. We extend the incision just as much as necessary to enable specimen extraction. The surgical incision is re-approximated in multiple layers, starting with the peritoneum with 0-polyglactin 910 suture in a running fashion. The internal oblique is re-approximated with care to avoid strangulation of the muscle fibers with a series of 0-polyglactin 910 interrupted horizontal mattress sutures. The external oblique fascia is re-approximated with #1 polydioxanone in a running fashion. Deep dermal 3-0 polyglactin 910 sutures are placed in an interrupted fashion followed by skin closure to all port sites with 4-0 poliglecaprone 25 in a subcuticular fashion followed by topical skin adhesive. Prior to skin closure of the 12 mm AirSeal® port, the previously placed fascial suture is tied down to close the fascial defect, which is confirmed with digital inspection post-closure. Drain placement is often deferred if the bladder closure is deemed water-tight and appropriate.
Post-operative management
Post-operatively, patients are typically admitted for overnight observation with bowel regimen and peri-operative antibiotics alone. An institutional enhanced recovery after surgery (ERAS) protocol is implemented to streamline peri-operative management. Diet is advanced if clear liquids are tolerated without nausea nor vomiting and ambulation is encouraged. The catheter is typically removed on post-operative days 5–7 and a cystogram is not routinely performed prior to catheter removal unless there is reason for concern regarding the bladder closure. The first surveillance cystoscopy is performed 3–4 months following surgery and surveillance continues in accordance with National Comprehensive Cancer Network® (NCCN) guidelines.
Discussion
Technical nuances and alternative approaches
Table 2 demonstrates an overview of advancing surgical technology and standard operative approaches in RNU/BCE over time with improvements in robotic technology and surgeon-specific refinements.
Table 2
| Author | Year | N | Patient positioning | Approach to kidney | Approach to ureter | Approach to lymphadenectomy | Approach to bladder cuff |
|---|---|---|---|---|---|---|---|
| Rose et al. (26) | 2006 | 2 | Flank with anterior tilt | Robot-assisted retroperitoneal | Proximal: robot-assisted | N/A | Open |
| Distal: open | |||||||
| Nanigian et al. (8) | 2006 | 10 | Modified flank (kidney) | Laparoscopic transperitoneal | Robot-assisted | N/A | Robot-assisted |
| Lithotomy (distal ureter and bladder) | Two-layer closure | ||||||
| Polyglycolic acid | |||||||
| Hu et al. (27) | 2008 | 9 | 5 cases: flank to lithotomy | Laparoscopic; 1 case retroperitoneal, 8 cases transperitoneal | Robot-assisted | N/A | Robot-assisted |
| 4 cases: flank only | Two-layer closure | ||||||
| Polyglactin 910 | |||||||
| Park et al. (11) | 2009 | 11 | 6 cases: flank to lithotomy | Robot-assisted transperitoneal | Robot-assisted (re-docking/re-positioning) | N/A | Robot-assisted |
| 5 cases: flank only | Two-layer closure | ||||||
| Polyglactin 910 | |||||||
| Hemal et al. (13) | 2011 | 15 | Modified flank, table flexion, 15° Trendelenburg tilt | Robot-assisted transperitoneal | Robot-assisted (no re-docking/re-positioning) | Robot-assisted | Robot-assisted |
| Two-layer closure | |||||||
| Polyglactin 910 | |||||||
| Zargar et al. (16) | 2014 | 31 | Modified 60° flank, no table flexion, 15° Trendelenburg tilt | Robot-assisted transperitoneal | Robot-assisted | Robot-assisted | Robot-assisted |
| One-layer closure | |||||||
| Polyglactin 910 | |||||||
| Aboumohamed et al. (28) | 2015 | 60 | Modified flank, table flexion, 15° Trendelenburg tilt | Robot-assisted transperitoneal | Robot-assisted (no re-docking/re-positioning) | Robot-assisted | Robot-assisted |
| Two-layer closure | |||||||
| Polyglactin 910 | |||||||
| Patel et al. (15) | 2018 | 87 | Modified flank, table flexion, 15° Trendelenburg tilt | Robot-assisted transperitoneal | Robot-assisted (no re-docking/re-positioning) | Robot-assisted | Robot-assisted |
| Two-layer closure | |||||||
| Polyglactin 910 | |||||||
| Pathak et al. (29) | 2022 | 105 | Modified flank, table flexion, 15° Trendelenburg tilt | Robot-assisted transperitoneal | Robot-assisted (no re-docking/re-positioning) | Robot-assisted | Robot-assisted |
| Two-layer closure | |||||||
| V-Lok™ | |||||||
| Veccia et al. (30) | 2022 | 148 | 60° flank, flat or 15° Trendelenburg tilt | Robot-assisted transperitoneal | Robot-assisted (no re-docking/re-positioning) | Robot-assisted | Robot-assisted |
| V-Lok™ |
RNU/BCE, radical nephroureterectomy with bladder cuff excision; N/A, not available.
Patient-related factors occasionally require adjustments to the standard technique. One of these such adjustments include consideration of a retroperitoneal approach for radical nephroureterectomy in patients with frozen abdomens with no window for safe intraperitoneal access. Recently, Sparwasser et al. described an entirely retroperitoneal robotic approach for RNU/BCE completed in five consecutive patients with associated step-by-step technique. Their approach required one trocar placement followed by single re-docking after completing the nephrectomy aspect of the case without needing to entirely reposition the robot. No positive margins were reported with a mean tumor size of approximately 3 cm and retroperitoneal lymphadenectomy was performed in three patients (31).
Hand-assisted laparoscopic radical nephroureterectomy is also a technically feasible approach and has been well-described in the literature. Compared to open and straight laparoscopic radical nephroureterectomy, this approach has been shown to reduce operative time. Various techniques have been implemented to manage the distal ureter, intramural tunnel, and bladder cuff including second incision for open dissection, cystoscopic en bloc distal ureteral disarticulation, and division of the distal ureter using a surgical stapler (32). The management of the distal ureter is a significant limitation of this approach, as the precise and meticulous dissection of the distal ureter and en bloc resection with a bladder cuff and formal cystotomy closure is significantly optimized via the robot-assisted technique.
Single port (SP) RNU/BCE has also been described. In one recent series, the authors described placing the multichannel SP trocar at the level of the umbilicus +1 12 mm assistant port contralateral and inferolateral to the umbilicus. Following control of the renal hilum, the distal ureter is dissected and excised along with the bladder cuff en bloc before returning to the kidney to complete the nephrectomy aspect of the case. The SP robot is well-suited for working in small spaces, particularly in the pelvis for excision of the bladder cuff and bladder repair (33).
Conclusions
Robot-assisted RNU/BCE, regional lymphadenectomy, and intravesical chemotherapy is a refined surgical approach for appropriate candidates with UTUC. The described surgical technique has been advanced through multiple iterations of the da Vinci® robot and surgeon experience over the last two decades.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Andrology and Urology for the series “Upper Tract Urothelial Cancer”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-209/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-209/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-209/coif). The series “Upper Tract Urothelial Cancer” was commissioned by the editorial office without any funding or sponsorship. A.K.H. served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Andrology and Urology from November 2023 to October 2025. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Raman JD, Messer J, Sielatycki JA, et al. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int 2011;107:1059-64. [Crossref] [PubMed]
- Herout R, Baunacke M, Flegar L, et al. Upper tract urothelial carcinoma in Germany: epidemiological data and surgical treatment trends in a total population analysis from 2006 to 2019. World J Urol 2023;41:127-33. [Crossref] [PubMed]
- Flegar L, Kraywinkel K, Zacharis A, et al. Treatment trends for muscle-invasive bladder cancer in Germany from 2006 to 2019. World J Urol 2022;40:1715-21. [Crossref] [PubMed]
- Srirangam SJ, van Cleynenbreugel B, van Poppel H. Laparoscopic nephroureterectomy: the distal ureteral dilemma. Adv Urol 2009;2009:316807. [Crossref] [PubMed]
- Kimball FN, Ferris HW. Papillomatous tumor of the renal pelvis associated with similar tumors of the ureter and bladder. Review of literature and report of two cases. J Urol 1934;31:257-304. [Crossref]
- Clayman RV, Kavoussi LR, Figenshau RS, et al. Laparoscopic nephroureterectomy: initial clinical case report. J Laparoendosc Surg 1991;1:343-9. [Crossref] [PubMed]
- Nanigian DK, Smith W, Ellison LM. Robot-assisted laparoscopic nephroureterectomy. J Endourol 2006;20:463-5; discussion 465-6. [Crossref] [PubMed]
- Yates DR, Vaessen C, Roupret M. From Leonardo to da Vinci: the history of robot-assisted surgery in urology. BJU Int 2011;108:1708-13; discussion 1714. [Crossref] [PubMed]
- Lee Z, Cadillo-Chavez R, Lee DI, et al. The technique of single stage pure robotic nephroureterectomy. J Endourol 2013;27:189-95. [Crossref] [PubMed]
- Park SY, Jeong W, Ham WS, et al. Initial experience of robotic nephroureterectomy: a hybrid-port technique. BJU Int 2009;104:1718-21. [Crossref] [PubMed]
- Eun D, Bhandari A, Boris R, et al. Concurrent upper and lower urinary tract robotic surgery: strategies for success. BJU Int 2007;100:1121-5. [Crossref] [PubMed]
- Hemal AK, Stansel I, Babbar P, et al. Robotic-assisted nephroureterectomy and bladder cuff excision without intraoperative repositioning. Urology 2011;78:357-64. [Crossref] [PubMed]
- Patel MN, Aboumohamed A, Hemal A. Does transition from the da Vinci Si to Xi robotic platform impact single-docking technique for robot-assisted laparoscopic nephroureterectomy? BJU Int 2015;116:990-4. [Crossref] [PubMed]
- Patel MN, Hemal AK. Does Advancing Technology Improve Outcomes? Comparison of the Da Vinci Standard/S/Si to the Xi Robotic Platforms During Robotic Nephroureterectomy. J Endourol 2018;32:133-8. [Crossref] [PubMed]
- Zargar H, Krishnan J, Autorino R, et al. Robotic nephroureterectomy: a simplified approach requiring no patient repositioning or robot redocking. Eur Urol 2014;66:769-77. [Crossref] [PubMed]
- Pathak RA, Dutta R, Williams J, et al. Robotic radical nephro-ureterectomy for high-risk upper tract urothelial carcinoma: Step-by-step illustrative video of surgical technique. Urology Video Journal 2020;8:100068. [Crossref]
- Abrate A, Vella M, Mogorovich A, et al. Time to safely omit bladder cuff removal for low-risk upper tract urothelial carcinoma. Minerva Urol Nephrol 2021;73:417-20. [Crossref] [PubMed]
- Pathak RA, Hemal AK. Fate of residual ureteral stump in patients undergoing robot-assisted radical nephroureterectomy for high-risk upper tract urothelial carcinoma. Transl Androl Urol 2020;9:856-62. [Crossref] [PubMed]
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. 2023. Available online: https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf
- Kim DK, Lee JY, Kim JW, et al. Effect of neoadjuvant chemotherapy on locally advanced upper tract urothelial carcinoma: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2019;135:59-65. [Crossref] [PubMed]
- Wu Z, Li M, Wang L, et al. Neoadjuvant systemic therapy in patients undergoing nephroureterectomy for urothelial cancer: a multidisciplinary systematic review and critical analysis. Minerva Urol Nephrol 2022;74:518-27. [Crossref] [PubMed]
- Hemal AK, Eun D, Tewari A, et al. Nuances in the optimum placement of ports in pelvic and upper urinary tract surgery using the da Vinci robot. Urol Clin North Am 2004;31:683-92. viii. [Crossref] [PubMed]
- Pathak RA, Patel M, Hemal AK. Comprehensive Approach to Port Placement Templates for Robot-Assisted Laparoscopic Urologic Surgeries. J Endourol 2017;31:1269-76. [Crossref] [PubMed]
- Packiam VT, Leibovich BC, Thompson RH, et al. GEMINI: An open-label, single-arm, phase II trial of intraoperative gemcitabine intravesical instillation in patients undergoing radical nephroureterectomy for upper tract urothelial carcinoma. J Clin Oncol 2020;38:TPS594. [Crossref]
- Rose K, Khan S, Godbole H, et al. Robotic assisted retroperitoneoscopic nephroureterectomy -- first experience and the hybrid port technique. Int J Clin Pract 2006;60:12-4. [Crossref] [PubMed]
- Hu JC, Silletti JP, Williams SB. Initial experience with robot-assisted minimally-invasive nephroureterectomy. J Endourol 2008;22:699-704. [Crossref] [PubMed]
- Aboumohamed AA, Krane LS, Hemal AK. Oncologic Outcomes Following Robot-Assisted Laparoscopic Nephroureterectomy with Bladder Cuff Excision for Upper Tract Urothelial Carcinoma. J Urol 2015;194:1561-6. [Crossref] [PubMed]
- Pathak RA, Crain NA, Hemal AK. Radical robotic nephroureterectomy with bladder cuff excision: Overview of surgical technique. Urology Video Journal 2022;13:100119. [Crossref]
- Veccia A, Carbonara U, Derweesh I, et al. Single-stage Xi® robotic radical nephroureterectomy for upper tract urothelial carcinoma: surgical technique and outcomes. Minerva Urol Nephrol 2022;74:233-41. [Crossref] [PubMed]
- Sparwasser P, Epple S, Thomas A, et al. First completely robot-assisted retroperitoneal nephroureterectomy with bladder cuff: a step-by-step technique. World J Urol 2022;40:1019-26. [Crossref] [PubMed]
- Brown JA, Strup SE, Chenven E, et al. Hand-assisted laparoscopic nephroureterectomy: analysis of distal ureterectomy technique, margin status, and surgical outcomes. Urology 2005;66:1192-6. [Crossref] [PubMed]
- Kim KH, Ahn HK, Kim M, et al. Technique and perioperative outcomes of single-port robotic surgery using the da Vinci SP platform in urology. Asian J Surg 2023;46:472-7. [Crossref] [PubMed]


