
Does age impact clinical outcomes of radical nephroureterectomy in the elderly?—results from a multicenter retrospective study
Highlight box
Key findings
• In carefully selected cases, radical nephroureterectomy can be a safe and effective option, even for elderly (75–84 years old) and very elderly (≥85 years old) upper tract urothelial carcinoma (UTUC) patients.
What is known and what is new?
• The appropriateness of nephroureterectomy in elderly UTUC patients has received little attention in the literature. Published studies have presented variable and sometimes contrasting clinical outcomes.
• There have been few reports on UTUC patients in very elderly patients due to its rarity.
• This study, using the largest database, investigates the clinical outcomes of Japanese UTUC patients aged ≥85 years old who had undergone radical nephroureterectomy.
What is the implication, and what should change now?
• The efficacy of surgical interventions for UTUC appears to be consistent across different age groups. This finding suggests that patients aged 85 years and older could also be considered for nephroureterectomy, assuming they are physically robust enough to endure the surgery and its associated demands.
Introduction
The world is witnessing a significant surge in the elderly population, resulting in an increased percentage of individuals aged ≥65 years in the global populace (1). This could lead to increased public health issues. For example, Japan has experienced an upswing in cancer incidence rates among patients aged ≥75 years old (2). When managing elderly cancer patients, there is a tendency to employ less-aggressive treatment modalities. This has triggered continual debate over the appropriateness and implications of such approaches, particularly in relation to surgical procedures (3).
Upper tract urothelial carcinoma (UTUC) remains a relatively rare diagnosis, representing about 10% of renal tumors and 5% of urothelial malignancies. Nevertheless, its incidence has been increasing in recent years (4,5). Radical nephroureterectomy coupled with bladder cuff excision is the accepted primary treatment for UTUC, offering prospects for durable disease remission. However, high-risk UTUC, typified by features such as high-grade pathology or marked invasiveness, is frequently associated with notable recurrence and progression risks (6).
The appropriateness of nephroureterectomy in elderly patients with UTUC has received little attention in the literature. Published studies have presented variable and sometimes contrasting clinical outcomes. As such, the potential benefits and prognosis of radical nephroureterectomy in elderly populations continue to be debated (7-11). In this context, we aimed to elucidate the association between age and clinical outcomes in patients with UTUC who underwent radical nephroureterectomy. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-24-37/rc).
Methods
Patient demographics
We analyzed patients subjected to nephroureterectomy from January 2012 to December 2021 at the Jikei University Hospital and its 16 associated facilities (JIKEI-YAYOI Collaborative Group). Patients with incomplete clinical records (n=104), ambiguous pathological results (n=2), or those diagnosed with non-urothelial carcinoma (n=23) were removed from the study, leaving 847 qualified patients for analysis (Figure 1). Genitourinary surgeons conducted the operations, and the decision between open and laparoscopic methodologies depended on the circumstances of each individual patient and the judgment of the surgeon. A standard radical nephroureterectomy with bladder cuff removal was uniformly performed across all participating centers. The scope and performance of lymph node dissection were made by the overseeing physician. Induction for neoadjuvant chemotherapy and adjuvant chemotherapy was primarily based on clinical and pathological confirmation of T3/4 and N+ disease, respectively. The final decision regarding the type of chemotherapy induction was made after a comprehensive discussion between the patient and the urologist or medical oncologist. Data procured for evaluation included baseline demographic and clinicopathological metrics, operation specifics, and subsequent postoperative and oncological outcomes. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research was approved by the Jikei University Institutional Review Board [protocol No. 33-260(10878)]. Informed consent was obtained from all subjects involved in the study.

Pathological evaluation
We collected data including baseline demographics, clinicopathological traits, operative specifics, and subsequent oncological outcomes. Patients who underwent nephroureterectomy were stratified into four age groups at the time of the procedure: young (≤64 years), intermediate (65–74 years), elderly (75–84 years), and very elderly (≥85 years). Tumor staging adhered to the American Joint Committee on Cancer guidelines (8th edition, 2017). Lymphovascular invasion (LVI) was determined based on the presence of tumor cells within the endothelial linings of the vascular or lymphatic channels. Tumor grading and variant histology classification followed the criteria delineated by the World Health Organization in 2016 (12).
Follow-up
Non-urothelial tract recurrence (NUTR) was characterized by the manifestation of disease outside the urinary tract and bladder. Postoperative surveillance involved routine evaluations including complete blood count, hepatic and renal function assays, and abdominal computed tomography (CT) scans every 3–6 months for the initial 2 postoperative years, transitioning to biannual evaluations after that. Perioperative complications were assessed using the Clavien-Dindo classification.
Statistical analysis
Associations between categorical and continuous variables were assess using chi-square and Kruskal-Wallis tests, respectively. A logistic regression model was adopted to identify determinants of postoperative complications. Survival analyses including non-urothelial tract recurrence-free survival (NUTRFS), cancer-specific survival (CSS), and overall survival (OS) were conducted using the Kaplan-Meier method with log-rank tests as needed, commencing from the nephroureterectomy date. In the analysis of NUTRFS, patients who had no documented recurrence or death by the time of the final follow-up were considered censored. For CSS, patients were censored at the final follow-up if there was no documented cancer-specific death or death from causes other than UTUC. In the assessment of OS, patients who had not been documented as deceased by the final follow-up were also censored. Variables impacting intravesical recurrence-free survival (RFS), NUTRFS, CSS, and OS were evaluated via Cox proportional hazards regression models. P value <0.05 was taken to indicate statistical significance. All statistical analyses were performed using Stata (version 13.1, TX, USA).
Results
Patient characteristics
Descriptive statistics for the patients are presented in Table 1. Of the 847 participants, 177 (20.9%) were in the young category, 300 (35.4%) were intermediate, 312 (36.8%) were elderly, and 58 (6.8%) were very elderly. The median age was 74 years, with a spread from 32 to 94 years. The median follow-up duration was 28 months. Lymph node dissection was performed in 318 patients (37.5%). Of those who underwent lymph node dissection, 70 (22.0%) exhibited positive lymph node involvement. Laparoscopic nephroureterectomy was the choice for 615 patients (72.6%), with no instances of open conversion. Neoadjuvant and adjuvant chemotherapy were prescribed to 70 (8.3%) and 120 (14.2%) patients, respectively.
Table 1
| Variables | Total (n=847) | Young (n=177) | Intermediate (n=300) | Elderly (n=312) | Very elderly (n=58) | P value |
|---|---|---|---|---|---|---|
| Age (years) | 74 [67–79] | 61 [55–63] | 71 [68–73] | 79 [77–81] | 87 [86–89] | <0.001 |
| Follow-up (months) | 28 [15–53] | 34 [15–62] | 30 [17–57] | 25 [12–44] | 25 [12–37] | <0.001 |
| Sex | 0.15 | |||||
| Male | 606 (71.5) | 135 (76.3) | 218 (72.7) | 217 (69.6) | 36 (62.1) | |
| Female | 241 (28.5) | 42 (23.7) | 82 (27.3) | 95 (30.4) | 22 (37.9) | |
| Body mass index (kg/m2) | 22.8 [13.5–50.2] | 23.4 [15.4–50.2] | 22.75 [14.0–32.0] | 22.39 [15.2–33.0] | 22.13 [13.5–31.2] | <0.001 |
| ECOG performance status | <0.001 | |||||
| 0 | 644 (76.0) | 155 (87.6) | 245 (81.7) | 217 (69.6) | 27 (46.6) | |
| 1 | 154 (18.2) | 16 (9.0) | 42 (14.0) | 74 (23.7) | 22 (37.9) | |
| ≥2 | 49 (5.8) | 6 (3.4) | 13 (4.3) | 21 (6.7) | 9 (15.5) | |
| Laterality | 0.39 | |||||
| Right | 410 (48.4) | 84 (47.5) | 136 (45.3) | 162 (51.9) | 28 (48.3) | |
| Left | 437 (51.6) | 93 (52.5) | 164 (54.7) | 150 (48.1) | 30 (51.7) | |
| Hydronephrosis | 0.19 | |||||
| Absent | 406 (47.9) | 87 (49.2) | 156 (52.0) | 140 (44.9) | 23 (39.7) | |
| Present | 441 (52.1) | 90 (50.8) | 144 (48.0) | 172 (55.1) | 35 (60.3) | |
| Operative method | 0.16 | |||||
| Open | 234 (27.6) | 59 (33.3) | 73 (24.3) | 88 (28.2) | 14 (24.1) | |
| Laparoscopic | 615 (72.6) | 118 (66.7) | 227 (75.7) | 224 (71.8) | 44 (75.9) | |
| Tumor location | 0.005 | |||||
| Renal pelvis | 412 (48.6) | 106 (56.9) | 145 (48.3) | 140 (44.9) | 21 (36.2) | |
| Ureter | 381 (45.0) | 59 (33.3) | 141 (47.0) | 151 (48.4) | 30 (51.7) | |
| Both | 54 (6.4) | 12 (6.8) | 14 (4.7) | 21 (6.7) | 7 (12.1) | |
| Clinical T stage | 0.40 | |||||
| cTis/Ta/T1 | 36 (4.3) | 3 (1.7) | 14 (4.7) | 15 (4.8) | 4 (6.9) | |
| cT2 | 385 (45.5) | 72 (40.7) | 147 (49.0) | 139 (44.6) | 27 (46.6) | |
| cT3 | 235 (27.7) | 58 (32.8) | 74 (24.7) | 89 (28.5) | 14 (24.1) | |
| cT4 | 170 (20.1) | 37 (20.9) | 57 (19.0) | 65 (20.8) | 11 (19.0) | |
| NR | 21 (2.5) | 7 (4.0) | 8 (2.7) | 4 (1.3) | 2 (3.4) | |
| Clinical N stage | 0.65 | |||||
| cN0 | 781 (92.2) | 160 (90.4) | 280 (93.3) | 283 (90.7) | 58 (100.0) | |
| cN1 | 66 (7.8) | 17 (9.6) | 20 (6.7) | 29 (9.3) | 0 (0.0) | |
| Neoadjuvant chemotherapy | 0.01 | |||||
| Absent | 777 (91.7) | 156 (88.1) | 270 (90.0) | 293 (93.9) | 58 (100.0) | |
| Present | 70 (8.3) | 21 (11.9) | 30 (10.0) | 19 (6.1) | 0 (0.0) | |
| Pathological findings | ||||||
| Histology | 0.009 | |||||
| Pure UC | 813 (96.0) | 172 (97.2) | 295 (98.3) | 293 (93.9) | 53 (91.4) | |
| UC with variant | 34 (4.0) | 5 (2.8) | 5 (1.7) | 19 (6.1) | 5 (8.6) | |
| Pathological T stage | 0.50 | |||||
| pTis/pTa/pT1 | 366 (43.2) | 89 (50.3) | 128 (42.7) | 128 (41.0) | 21 (36.2) | |
| pT2 | 102 (12.0) | 21 (11.9) | 35 (11.7) | 38 (12.2) | 8 (13.8) | |
| pT3 | 334 (39.4) | 58 (32.8) | 125 (41.7) | 125 (40.1) | 26 (44.8) | |
| pT4 | 45 (5.3) | 9 (5.1) | 12 (4.0) | 21 (6.7) | 3 (5.2) | |
| Pathological N stage | 0.043 | |||||
| pN0 | 248 (29.3) | 65 (36.7) | 76 (25.3) | 92 (29.5) | 15 (25.9) | |
| pN+ | 70 (8.3) | 19 (10.7) | 21 (7.0) | 29 (9.3) | 1 (1.7) | |
| pNx | 529 (62.4) | 93 (52.5) | 203 (67.7) | 191 (61.2) | 42 (72.4) | |
| Tumor grade | 0.004 | |||||
| High | 632 (74.6) | 115 (65.0) | 221 (73.7) | 246 (78.9) | 50 (86.2) | |
| Low | 145 (17.1) | 45 (25.4) | 56 (18.7) | 39 (12.5) | 5 (8.6) | |
| NR | 70 (8.3) | 17 (9.6) | 23 (7.7) | 27 (8.7) | 3 (5.2) | |
| Concomitant CIS | 0.43 | |||||
| Absent | 713 (84.2) | 155 (87.6) | 250 (83.3) | 262 (84.0) | 46 (79.3) | |
| Present | 134 (15.8) | 22 (12.4) | 50 (16.7) | 50 (16.0) | 12 (20.7) | |
| LVI | 0.02 | |||||
| Absent | 565 (66.7) | 129 (72.9) | 207 (69.0) | 189 (60.6) | 40 (69.0) | |
| Present | 282 (33.3) | 48 (27.1) | 93 (31.0) | 123 (39.4) | 18 (31.0) | |
| Adjuvant chemotherapy | 0.02 | |||||
| Absent | 727 (85.8) | 147 (83.1) | 252 (84.0) | 271 (86.9) | 57 (98.3) | |
| Present | 120 (14.2) | 30 (16.9) | 48 (16.0) | 41 (13.1) | 1 (1.7) |
Data are presented as median [IQR] or n (%). ECOG, Eastern Cooperative Oncology Group; NR, not reported; UC, urothelial carcinoma; CIS, carcinoma in situ; LVI, lymphovascular invasion; IQR, interquartile range.
Clinical outcomes
During the surveillance period, 225 (26.6%) patients experienced metastases. In total, 137 (16.2%) experienced cancer-specific death and 182 (21.5%) had any case of death. The 3-year NUTRFS, CSS, and OS rates were 71.7%, 82.3%, and 77.9%, respectively (refer to Table S1). Kaplan-Meier analyses did not indicate significant differences in RFS and CSS among the age groups (Figure 2). However, OS rates were notably lower for the intermediate, elderly, and very elderly groups compared to the young group. Table S1 lists the 3-year NUTRFS, CSS, and OS rates stratified by age.
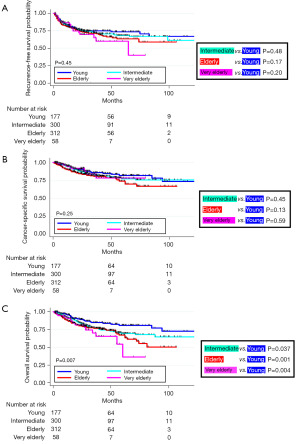
Multivariate analysis
Employing the Cox regression model, pathological staging ≥ T3, residual lymph node involvement, and LVI were identified as independent determinants for RFS. The same factors significantly influenced CSS and OS. While NUTRFS and CSS did not significantly differ by age, OS rates were again notably lower for the intermediate, elderly, and very elderly groups than for the young group (Table 2).
Table 2
| Covariant | Non-urothelial tract RFS | CSS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |||
| Age | |||||||||||
| Young | Ref. | Ref. | Ref. | ||||||||
| Intermediate | 1.03 | 0.66–1.60 | 0.91 | 1.14 | 0.63–2.05 | 0.67 | 1.83 | 1.03–3.27 | 0.03 | ||
| Elderly | 0.94 | 0.60–1.47 | 0.77 | 1.34 | 0.75–2.40 | 0.33 | 2.06 | 1.16–3.67 | 0.01 | ||
| Very elderly | 0.97 | 0.50–1.86 | 0.92 | 0.94 | 0.37–2.38 | 0.89 | 2.43 | 1.14–5.18 | 0.02 | ||
| Sex | |||||||||||
| Male | Ref. | Ref. | Ref. | ||||||||
| Female | 1.13 | 0.83–1.53 | 0.43 | 0.87 | 0.58–1.31 | 0.51 | 0.72 | 0.50–1.04 | 0.08 | ||
| ECOG performance status | |||||||||||
| 0–1 | Ref. | Ref. | Ref. | ||||||||
| ≥2 | 1.26 | 0.63–2.51 | 0.51 | 1.15 | 0.50–2.64 | 0.75 | 1.22 | 0.61–2.42 | 0.57 | ||
| Tumor location | |||||||||||
| Renal pelvis | Ref. | Ref. | Ref. | ||||||||
| Ureter | 1.18 | 0.83–1.66 | 0.36 | 1.12 | 0.71–1.76 | 0.62 | 1.19 | 0.82–1.75 | 0.36 | ||
| Both | 1.12 | 0.64–1.96 | 0.69 | 0.56 | 0.23–1.37 | 0.21 | 0.74 | 0.34–1.58 | 0.44 | ||
| Hydronephrosis | |||||||||||
| Absent | Ref. | Ref. | Ref. | ||||||||
| Present | 1.08 | 0.77–1.51 | 0.65 | 1.25 | 0.80–1.96 | 0.33 | 1.13 | 0.78–1.65 | 0.52 | ||
| Pathological T stage | |||||||||||
| ≤ pT2 | Ref. | Ref. | Ref. | ||||||||
| ≥ pT3 | 2.51 | 1.72–3.65 | <0.001 | 2.78 | 1.67–4.64 | <0.001 | 2.05 | 1.36–3.08 | <0.001 | ||
| Histology | |||||||||||
| Pure UC | Ref. | Ref. | Ref. | ||||||||
| UC with variant histology | 1.43 | 0.80–2.58 | 0.23 | 1.52 | 0.72–3.20 | 0.27 | 1.77 | 0.95–3.27 | 0.07 | ||
| Lymph node status | |||||||||||
| pN0 | Ref. | Ref. | Ref. | ||||||||
| pN+ | 2.01 | 1.26–3.20 | 0.003 | 2.2 | 1.24–3.88 | 0.007 | 2.02 | 1.21–3.39 | 0.007 | ||
| pNx | 1.04 | 0.74–1.46 | 0.83 | 1.05 | 0.68–1.62 | 0.83 | 0.98 | 0.68–1.41 | 0.91 | ||
| LVI | |||||||||||
| Absent | Ref. | Ref. | Ref. | ||||||||
| Present | 3.36 | 2.39–4.72 | <0.001 | 3.17 | 2.06–4.87 | <0.001 | 2.64 | 1.84–3.80 | <0.001 | ||
| Tumor grade | |||||||||||
| Low | Ref. | Ref. | Ref. | ||||||||
| High | 1.88 | 0.99–3.58 | 0.053 | 4.27 | 1.31–13.90 | 0.01 | 1.50 | 0.82–2.76 | 0.19 | ||
| NR | 1.21 | 0.54–2.69 | 0.64 | 2.63 | 0.70–9.84 | 0.15 | 0.75 | 0.32–1.75 | 0.50 | ||
| Adjuvant chemotherapy | |||||||||||
| Absent | Ref. | Ref. | Ref. | ||||||||
| Present | 1.01 | 0.70–1.45 | 0.96 | 1.18 | 0.77–1.83 | 0.45 | 1.11 | 0.74–1.66 | 0.62 | ||
Young: ≤64 years; intermediate: 65–74 years; elderly: 75–84 years; very elderly: ≥85 years. RFS, recurrence-free survival; CSS, cancer-specific survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; UC, urothelial carcinoma; LVI, lymphovascular invasion; NR, not reported; Ref., reference.
Postoperative complications
Table S2 provides a breakdown. Grade III and above complications encompassed wound infections (n=3), ileus (n=2), subcutaneous hematoma (n=1), postoperative bleeding (n=1), cardiovascular events (n=1), and pancreatic fistulas (n=1). Postoperative complications did not lead to any fatalities. Multivariable logistic regression revealed age wasn’t a significant predictor for postoperative complications. Notably, an Eastern Cooperative Oncology Group (ECOG) performance status ≥2 was the sole significant risk contributor (odds ratio =2.73; 95% confidence interval: 1.01 to 7.42; P value =0.048, Table 3).
Table 3
| Covariant | Complication | ||
|---|---|---|---|
| OR | 95% CI | P value | |
| ECOG performance status | |||
| 0–1 | Ref. | ||
| ≥2 | 2.73 | 1.01–7.42 | 0.048 |
| Body mass index | 0.97 | 0.89–1.06 | 0.55 |
| Sex | |||
| Male | Ref. | ||
| Female | 1.09 | 0.56–2.12 | 0.79 |
| Lymph node dissection | |||
| Absent | Ref. | ||
| Present | 1.34 | 0.68–2.62 | 0.39 |
| Age | |||
| Young | Ref. | ||
| Intermediate | 1.31 | 0.46–3.75 | 0.62 |
| Elderly | 1.88 | 0.68–5.18 | 0.22 |
| Very elderly | 2.37 | 0.63–8.94 | 0.20 |
Young: ≤64 years; intermediate: 65–74 years; elderly: 75–84 years; very elderly: ≥85 years. ECOG, Eastern Cooperative Oncology Group; OR, odds ratio; CI, confidence interval; Ref., reference.
Discussion
We investigated the clinical ramifications of age post-nephroureterectomy in patients diagnosed with localized UTUC. Postoperative complication rates after surgery did not significantly differ among the age groups. Similarly, there were no pronounced statistical differences in NUTRFS or CSS by age. Consequently, our results indicate that radical nephroureterectomy might be safely and effectively adopted in judiciously chosen cases, including elderly and very elderly patients. Therefore, age alone should not dictate the viability of this surgical technique for such patients. To the best of our understanding, this research represents the most expansive study to have probed the oncological outcomes in older patients undergoing radical nephroureterectomy within an Asian context (7,8). Notably, this was also the first study to explore the safety of this procedure in very elderly patients (age ≥85 years). We collated an extensive dataset from diverse centers, which may have potentially countered biases due to individual clinician preferences and varying patient demographics across institutions.
Several studies have considered the clinical consequences of advancing age on the oncological outcomes of UTUC patients (7-10). Shariat et al. studied 1,453 patients that had undergone radical nephroureterectomy across 13 establishments. They reported that older age at the time of surgery correlated with diminished survival rates. In multivariate analysis with age treated as a categorical variable, they found that the HR was significantly elevated for CSS and OS for those aged above 80 years (9). Moreover, Ferro et al. evaluated 1,387 patients who underwent the same procedure and reported that those who were 70 years or older had inferior CSS and OS versus younger counterparts (10). Two Japanese studies presented different outcomes (7,8). Yamada et al. assessed 451 UTUC patients after radical nephroureterectomy and found that while OS was lower in patients aged ≥80 years old, CSS remained comparable across age groups (7). Koterazawa et al. studied 283 patients and reported negligible differences in 5-year CSS rates between elderly (≥80 years old) and those <80 years old. Their 5-year OS rates were 43% for the elderly and 63% for the others, revealing discernible, albeit statistically nonsignificant, variation (8). In addition, Chromecki et al. found that older age at the time of nephroureterectomy was associated with worse clinical results after surgery. However, in multivariable analyses that incorporated ECOG performance status, age was solely correlated with diminished OS not impacting CSS (11).
Our findings align with these latter three studies (7,8,11). There are two potential explanations for the variation in results across different studies. First, differences in life expectancy across racial demographics could be in play. Japan ranks fourth in global life expectancy, averaging approximately 85.0 years, outpacing both the United States and many European nations (13). This might correlate with resilience to surgical stress, particularly in older cohorts. Second, as Chromecki et al. (11) underscored, ECOG performance status is pivotal in forecasting oncological outcomes. When patients present with optimal performance status, age might not be the primary determinant, encouraging surgeons to advocate for radical nephroureterectomy. Recently, the Geriatric 8 (G8) assessment tool has demonstrated utility in selecting older candidates suitable for uro-oncologic procedures (14). Although we used the ECOG performance metric, G8 data were not captured. As the G8 has been reported to provide a more precise assessment of surgical tolerance (15), we plan to focus on its impact in future research.
The aggregate rates of postoperative complications and grade III or higher complications were 6.61% and 1.06%, respectively. Ileus was the most prevalent postoperative complication, with 21 out of the 56 patients experiencing it after surgery (1.1%, 2.0%, 3.5%, and 3.4% across the age categories from youngest to oldest). In multivariable analysis, there was no significant correlation between age groups and complication rates. Thus, our data suggest that even the very elderly can safely undergo UTUC treatment. Generally, aging is concomitant with a waning physiological reserve, even in those without manifest comorbidities (16). This can compromise an older individual’s stress tolerance, potentially heightening postoperative complication risks and in-hospital mortality rates (17). It is pertinent to highlight that most patients nowadays opt for laparoscopic or robot-assisted laparoscopic radical nephroureterectomy, both of which are associated with lower complication rates than open surgery (8). In our cohort, approximately 80% of the elderly underwent laparoscopic radical nephroureterectomy. Our results are in line with Koterazawa et al., indicating analogous postoperative complication rates among elderly and younger patients following laparoscopic radical nephroureterectomy (8). In terms of postoperative complication risk factors after such surgery, ECOG performance status ≥2 was the sole significant predictor. This is in line with several previous studies that have reported that suboptimal performance status is linked to heightened postoperative risks for complications such as myocardial infarction and wound infections (18-20). Hence, when UTUC patients show a commendable performance status, their age should not deter clinicians from recommending nephroureterectomy.
Our study had some inherent limitations to acknowledge. Although multi-centric, its retrospective design and relatively short follow-up period are notable constraints. Further, our follow-up protocols lacked robust standardization, evident in the variation in examination types and imaging intervals. In addition, lymph node dissection decisions were made by individual physicians, potentially impacting oncological outcomes. Finally, given that pathology data were sourced from individual centers without a centralized review, diagnostic inconsistencies might have arisen.
Conclusions
In conclusion, we demonstrate there were no statistically significant differences in NUTRFS or CSS across the four assessed age groups. This suggests that even for patients aged 85 years old and older, surgical treatment might be a viable therapeutic choice, assuming they can withstand the inherent surgical stress and procedure invasiveness.
Acknowledgments
The authors thank Textcheck Inc. for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-24-37/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-24-37/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-24-37/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-24-37/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research was approved by the Jikei University Institutional Review Board [protocol No. 33-260(10878)]. Informed consent was obtained from all subjects involved in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- De Luca d'Alessandro E, Bonacci S, Giraldi G. Aging populations: the health and quality of life of the elderly. Clin Ter 2011;162:e13-8. [PubMed]
- Hotta T. Cancer Statistics in Japan [Internet]. Tokyo: The editorial borad of the Cancer Statisctics in Japan. 2021 March [cited 2023 Oct 24]. Available online: https://ganjoho.jp/public/qa_links/report/statistics/pdf/cancer_statistics_2021.pdf
- Audisio RA, Bozzetti F, Gennari R, et al. The surgical management of elderly cancer patients; recommendations of the SIOG surgical task force. Eur J Cancer 2004;40:926-38. [Crossref] [PubMed]
- Redrow GP, Matin SF. Upper tract urothelial carcinoma: epidemiology, high risk populations and detection. Minerva Urol Nefrol 2016;68:350-8. [PubMed]
- Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol 2000;164:1523-5. [Crossref] [PubMed]
- Lughezzani G, Burger M, Margulis V, et al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol 2012;62:100-14. [Crossref] [PubMed]
- Yamada Y, Ikeda M, Hirayama T, et al. Noninferior oncological outcomes in adults aged 80 years or older compared with younger patients who underwent radical nephroureterectomy for upper tract urothelial carcinoma. Asia Pac J Clin Oncol 2023;19:305-11. [Crossref] [PubMed]
- Koterazawa S, Kanno T, Kobori G, et al. Clinical outcomes following laparoscopic radical nephroureterectomy in octogenarians. Int J Clin Oncol 2023;28:155-62. [Crossref] [PubMed]
- Shariat SF, Godoy G, Lotan Y, et al. Advanced patient age is associated with inferior cancer-specific survival after radical nephroureterectomy. BJU Int 2010;105:1672-7. [Crossref] [PubMed]
- Ferro M, Chiujdea S, Vartolomei MD, et al. Advanced Age Impacts Survival After Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma. Clin Genitourin Cancer 2024;22:27-37. [Crossref] [PubMed]
- Chromecki TF, Ehdaie B, Novara G, et al. Chronological age is not an independent predictor of clinical outcomes after radical nephroureterectomy. World J Urol 2011;29:473-80. [Crossref] [PubMed]
- Humphrey PA, Moch H, Cubilla AL, et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol 2016;70:106-19. [Crossref] [PubMed]
- Tsugane S. Why has Japan become the world's most long-lived country: insights from a food and nutrition perspective. Eur J Clin Nutr 2021;75:921-8. [Crossref] [PubMed]
- Yamada Y, Taguchi S, Kume H. Surgical Tolerability and Frailty in Elderly Patients Undergoing Robot-Assisted Radical Prostatectomy: A Narrative Review. Cancers (Basel) 2022;14:5061. [Crossref] [PubMed]
- Park DH, Yoo S, Do MT, et al. Geriatric assessment using the G8 to predict postoperative complications in patients undergoing major uro-oncologic surgery: Comparison with the Charlson Comorbidity Index. J Geriatr Oncol 2022;13:426-31. [Crossref] [PubMed]
- Evers BM, Townsend CM Jr, Thompson JC. Organ physiology of aging. Surg Clin North Am 1994;74:23-39. [Crossref] [PubMed]
- Polanczyk CA, Marcantonio E, Goldman L, et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med 2001;134:637-43. [Crossref] [PubMed]
- Kocher NJ, Canes D, Bensalah K, et al. Incidence and preoperative predictors for major complications following radical nephroureterectomy. Transl Androl Urol 2020;9:1786-93. [Crossref] [PubMed]
- Martinez-Salamanca JI, Shariat SF, Rodriguez JC, et al. Prognostic role of ECOG performance status in patients with urothelial carcinoma of the upper urinary tract: an international study. BJU Int 2012;109:1155-61. [Crossref] [PubMed]
- Raman JD, Lin YK, Shariat SF, et al. Preoperative nomogram to predict the likelihood of complications after radical nephroureterectomy. BJU Int 2017;119:268-75. [Crossref] [PubMed]


