
Interfascial planes as surgical landmarks for laparoscopic upper retroperitoneal surgery: a cadaveric and retrospectively clinical study
Highlight box
Key findings
• The interfascial plane (IFP) is a potential avascular space that can be used during laparoscopic surgery to improve the safety and efficacy of laparoscopic surgery for upper retroperitoneal lesions.
What is known and what is new?
• Retroperitoneal anatomy is complex and radiologists currently accept the concept of “IFP” to understand retroperitoneal anatomy, replacing Meyers’ tricompartmental theory.
• This study demonstrated that the IFP is an avascular space that can be dissected and expanded during laparoscopic surgery, and developed a novel technique of trans-interfascial plane procedures for laparoscopic upper retroperitoneal surgery (TIFP-LURS).
What is the implication, and what should change now?
• This study provides a deeper understanding of the retroperitoneal fascial anatomy and revealed its surgical applications with favorable safety and efficacy.
• Surgeons need deeper understanding of IFP in retroperitoneum to guide laparoscopic surgery process. TIFP-LURS can be considered as a feasible and preferable surgical approach for upper retroperitoneal lesions.
Introduction
Laparoscopic upper retroperitoneal surgery (LURS) is a minimally invasive surgical treatment for retroperitoneal lesions of the adrenal gland, kidney, ureter and associated blood vessels, nerves and lymph nodes (1). Interfascial planes (IFP) are important anatomical landmarks for LURS and an ideal workspace for bloodless operations (2,3).
Since Meyes proposed the classic tricompartmental theory based on radiology in 1972, and Japanese scholars proposed the subfascial plane theory, the anatomy of the retroperitoneal space has been developing (4-7). However, the retroperitoneal fascial anatomy related to LURS has not been fully elucidated (8). A deeper understanding of the retroperitoneal fascial anatomy and identification of a bloodless surgical plane will promote the development of urological surgery (3,9).
This study combined anatomical and histological observations to further understand the anatomical basis of fascial planes in relation to upper urinary tract surgery. We designed and optimized surgical methods for establishing operative IFP during laparoscopic surgery, namely trans-interfascial plane procedures for laparoscopic upper retroperitoneal surgery (TIFP-LURS). Furthermore, this study retrospectively compared the perioperative efficacy of TIFP-LURS with conventional laparoscopic upper urinary tract surgery (Con-LURS) performed by the same surgical team at our center, providing scientific evidence for further optimization and promotion of the laparoscopic surgery system in urology. We present this article in accordance with the TREND reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-632/rc).
Methods
Cadaveric study
The anatomical study was based on the dissection of one freezing male cadaver (70 years of age at death), one formalin-fixed male cadaver (61 years of age at death) and 30 fresh cadavers (18 males and 12 females) provided by the Laboratory of Anatomy of Zhongshan School of Medicine of Sun Yat-sen University, using one set of anatomic devices and one camera (PowerShot SX700 HS, Canon, Inc., Tokyo, Japan). All donors voluntarily declared before their deaths that their remains were donated for educational and scientific research purposes. The format of the informed consent form was in line with the guidelines of the China Organ Donation Administrative Center.
A frozen male cadaver (70 years of age at death) was used for sectional analysis. The specimens, including the thorax, abdomen and pelvis, was frozen at −80 ℃ and cut into 10-mm thick transverse sections to examine gross anatomy from the caudal aspect. One male cadaver (61 years of age at death) with no congenital and/or acquired abnormalities in the retroperitoneal structure was fixed by arterial perfusion with 10% formalin and preserved in 30% alcohol to prevent fungal growth and to maintain tissue softness. The formalin-fixed male cadaver was transversely cut at the intervertebral disc level between L1 and L2, and dissected from the anterior aspect. From the anterior aspect, the skin, muscles, and abdominal organs were removed, and the peritoneum and renal fascia (RF) were visualized to investigate the connective tissue continuity and vessel communication inside the RF in the retroperitoneal space. Thirty fresh cadavers (18 males and 12 females; mean age 65 years at death; range 45–78 years) were used for histological analysis. The cadavers were preserved at 4 ℃, and dissected from the anterior aspect, from which the fascial tissue was collected. Fascial tissue was fixed in 10% formalin, dehydrated and embedded in paraffin. After cutting into 5 µm thick sections, the histological sections were processed for hematoxylin and eosin (H&E) and Masson’s trichrome staining. All images were obtained using a microscope (CX-41; Olympus, Tokyo, Japan).
Clinical study population
Retrospectively, subjects eligible for the study were continuous patients with upper retroperitoneal disease (more details in Table 1) who underwent Con-LURS between January 2012 to November 2018, and who underwent TIFP-LURS between October 2018 to July 2021, as performed by one experienced surgical team including surgeons, nurses and anesthetists. Patients with incomplete clinical data, those who underwent emergency surgery due to urgent conditions or surgery on organs other than the urinary system at the same time, and those who underwent palliative tumor reduction surgery were excluded from the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Committee of The Sixth Affiliation Hospital of Sun Yat-sen University (No. 2022ZSLYEC-171) and individual consent for this retrospective analysis was waived.
Table 1
| Characteristics | TIFP-LURS (n=229) | Con-LURS (n=121) | t/χ2/Z | P value |
|---|---|---|---|---|
| Sex | χ2=0.086 | 0.77 | ||
| Male | 123 (53.7) | 63 (52.1) | ||
| Female | 106 (46.3) | 58 (47.9) | ||
| Age (years) | 49.22±14.94 | 52.41±14.17 | t=1.929 | 0.055 |
| Body mass index (kg/m2) | 22.86±1.76 | 22.78±1.87 | t=−0.391 | 0.70 |
| Location (side) | χ2=0.009 | 0.92 | ||
| Right | 111 (48.5) | 58 (47.9) | ||
| Left | 118 (51.5) | 63 (52.1) | ||
| ASA score | Ζ=−0.022 | 0.98 | ||
| 1 | 188 (82.1) | 99 (81.8) | ||
| 2 | 35 (15.3) | 20 (16.5) | ||
| 3 | 6 (2.6) | 2 (1.7) | ||
| Previous abdominal surgery | 23 (10.0) | 16 (13.2) | χ2=0.808 | 0.37 |
| Ipsilateral hydronephrosis | 79 (34.5) | 48 (39.7) | χ2=0.916 | 0.34 |
| Diagnosis | χ2=7.833 | 0.25 | ||
| Pyeloureteral stenosis | 51 (22.3) | 30 (24.8) | ||
| Adrenal disease | 62 (27.1) | 29 (24.0) | ||
| Renal cysts | 27 (11.8) | 24 (19.8) | ||
| Renal masses | 56 (24.5) | 22 (18.2) | ||
| UTUC | 14 (6.1) | 8 (6.6) | ||
| Nonfunctioning kidney | 15 (6.6) | 4 (3.3) | ||
| Other urinary disease | 4 (1.7) | 4 (3.3) | ||
| Disease condition | χ2=2.126 | 0.15 | ||
| Malignant | 80 (34.9) | 33 (27.3) | ||
| Benign | 149 (65.1) | 88 (72.7) | ||
| Surgery | χ2=9.045 | 0.17 | ||
| Pyeloureteroplasty | 29 (12.7) | 17 (14.0) | ||
| Adrenalectomy | 62 (27.1) | 29 (24.0) | ||
| Renal cyst decompression | 25 (10.9) | 24 (19.8) | ||
| Partial nephrectomy | 26 (11.4) | 9 (7.4) | ||
| Nephrectomy | 64 (27.9) | 27 (22.3) | ||
| Nephroureterectomy | 18 (7.9) | 9 (7.4) | ||
| Other reconstruction or dissection surgery | 5 (2.2) | 6 (5.0) |
Values are presented as mean ± standard deviation or number (%). t, using the Student’s t-test; χ2, using the chi-square test; Z, using the Mann-Whitney rank-sum test. TIFP-LURS, trans-interfascial planes procedures for laparoscopic upper retroperitoneal surgery; Con-LURS, conventional laparoscopic upper retroperitoneal surgery; ASA, American Society of Anesthesiologists; UTUC, upper tract urothelial carcinoma.
Surgery procedure
In the Con-LURS cohort, all patients underwent trans-retroperitoneal approach procedure.
In the TIFP-LURS cohort, patients underwent trans-abdominal approach procedure. Procedures for TIFP-LURS (left side):
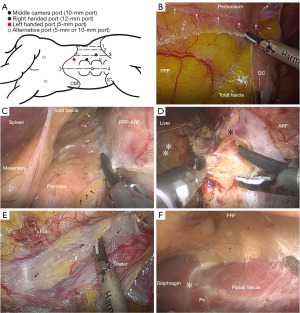
- Surgical position and trocar placement are presented in Figure 1.
- Mobilization of the colon. Incise along the white line of Toldt medially and access the IFP between Toldt fascia and primitive parietal peritoneum (PPP).
- Exposure of the retroperitoneum. Dissect along the IFP between Toldt fascia and PPP medially, and reflect the descending colon (DC) to expose the urinary organs. Stay outside the paler yellow anterior renal fascia (ARF) and avoid entering the brighter yellow posterior mesentery. Loose areolar tissues with minute vessels inside can be observed along the IFP between different fasciae, which can be dissected without hemorrhage.
- Renal hilum dissection. Dissect along the IFP among retropancreatic fascia, ARF and fasciae surrounding vessels, and expose the left renal vein (LV) and adrenal vein.
- Dissection along the ureter. Dissection along loose areolar tissues (black arrows) in the IFP between fasciae surrounding the left external iliac artery (LEIA) and Gerota’s fascia surrounding the ureter.
- Exposure of the psoas muscle. Enter the IFP between posterior renal fascia (PRF) and psoas fascia. Retract the kidney up but stay outside the PRF. The medial arcuate ligament of the diaphragm was the anatomical landmark continuous with the fasciae of the psoas muscle.
- Lesion removal or reconstruction.
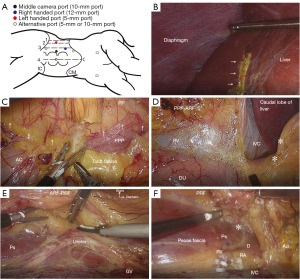
Procedures for TIFP-LURS in the right side is presented in Figure 2.
Outcomes
The primary endpoints were estimated blood loss, intraoperative complication rate (including severe hemorrhage, vascular injury, bowel injury, diaphragm injury, and other injuries) and postoperative complication rate and grading (according to the Dindo-Clavien classification) within 30 days (short-term). The secondary endpoints were duration of surgery, transfusion rate in surgery, conversion rate, length of hospital stay after surgery, and postoperative complication rate over 30 days (long-term) (including pyeloureteral stenosis, hydronephrosis, renal insufficiency, lumbar hernia, and others).
Statistical analysis
The results of the clinical study were analyzed using SPSS software (version 25.0, Chicago, USA). To compare the characteristics and differences between the two groups, the Student’s t-test or Mann-Whitney rank-sum test was used for continuous variables, and the Pearson Chi-square test or Fisher exact test was used for categorical variables, as appropriate. Univariate and multivariate logistic regression analyses were used to determine the potential risk factors for postoperative complications. P<0.05 was considered statistically significant. (The incidence of postoperative complications was assessed using univariate and multivariate logistic regression analyses with clinical factors. Multivariate analysis was performed using the forward elimination method. All results were considered statistically significant when the 2-sided P value was less than 0.05).
Results
Gross anatomy of the retroperitoneum
Transverse-sectional diagrams of the abdomen from a male cadaver (70 years of age at death) were observed from the caudal aspect (Figure 3A-3D). Multilaminar fasciae surrounding the kidney in the retroperitoneal space were observed in transverse sectional specimens, including the renal capsule (RC), perirenal fat (PeRF), RF, parietal peritoneum, and pararenal fat (PaRF). There was a membranous structure with a clear demarcation between the PeRF and PaRF.
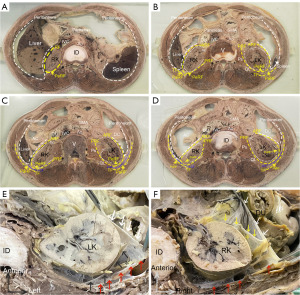
A transverse section of the retroperitoneum from a male cadaver (61 years of age at death) at the intervertebral disc level between L1 and L2 is presented in Figure 3E,3F. Fibrous connective tissue was observed between the PRF and muscular fascia. The vessel travelled and branched within the sub-peritoneal layer or internal space of the ARF independently, but no vessel anastomosis was observed between the two layers of fascia, which may be due to different embryonic origins.
Histological observation of the retroperitoneum
Regarding specimens taken from the anterior multilaminar fascia of the left kidney at the L1 vertebral body level (Figure 4A-4E), a fibrous connective tissue gap was observed between the peritoneum and ARF. As presented in Figure 4B-4E, the vessel ran in the internal space (sub-mesothelial tissue) of the peritoneum, and fascial tissue consisted of mesothelial and sub-mesothelial tissue, whereas the fusion layer of different fasciae consisted of multilaminar parallel strands of collagen fibers, which was pink in HE staining (Figure 4D) and blue in Masson staining (Figure 4E).
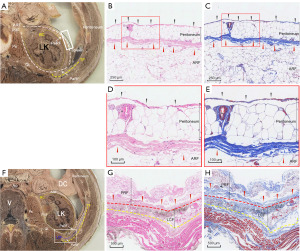
The specimens taken from the lateral multilaminar fascia of the left kidney at the L2 vertebral body level (Figure 4F-4H) indicated an observative fusion layer between the PRF and lateral conal fascia (LCF) and between the LCF and muscular fascia, which can be completely dissected in the LURS procedure.
Schematic diagram of retroperitoneal anatomy and TIFP procedures in surgery
Based on the result of anatomic and histological observation of the retroperitoneum above, and the concept of “IFP”, the transverse sectional diagram of the left part of the retroperitoneum was drawn (Figure 5). The IFP is a potential space within a multilaminar membranous structure, indicated by asterisks (*) with different shapes and colors. We considered ARF continuous with PRF, which was composed of both mesothelial layer and submesothelial loose connective tissue. We considered the combination of the PPP, cone-like fat, and ARF as the traditional anterior layer of Gerota’s fascia, and the combination of the PRF, LCF and fasciae of the psoas muscle and quadratus lumborum muscle as the posterior layer of Gerota’s fascia (also known as Zuckerkandl’s fascia). We considered the Toldt fascia sandwiched between the overlying mesothelial layer of the mesocolon and the underlying mesothelial layer of the PPP. The anterior layer of Gerota’s fascia extended transversely in two directions: medially to fuse with the fascia overlying the adventitia layer of the aorta and IVC, laterally to fuse with Toldt fascia and the posterior layer, and then tapered at the area below the reflection of the visceral and parietal peritoneum. The IFP sandwiched by different fasciae comprised areolar tissues with minute vessels inside. Therefore, surgical dissection plane is readily developed within IFP.

We designed the TIFP-LURS (pink dashed line) using transperitoneal approach, which included: (I) incise along the white line of Toldt and get the access to IFP between Toldt fascia and PPP, which could mobilize and reflect the colon; (II) dissect along the IFP and medially get the access to the IFP between PPP and ARF, which actually stay outside ARF; (III) identify cone-like fat as an anatomical landmark and laterally dissect along the IFP between RF (ARF and PRF) and LCF; (IV) dissect along the IFP between PRF and LCF and then get the access to the IFP between PRF and fasciae of the psoas muscle and quadratus lumborum muscle, which divide the lateral and dorsal attachments of urinary system; (V) communicate the IFP between RF (ARF and PRF) and fascia around blood vessels, which could control and dissect the renal hilum with great care.
Clinical results
A total of 121 patients were included in the Con-LURS cohort and 229 patients were included in the TIFP-LURS cohort. There were no significant differences in baseline characteristics between the two cohorts (Table 1).
The primary endpoints of TIFP-LURS were significantly lower than those of Con-LURS, including estimated blood loss (72.24±134.97 vs. 118.23±229.78 mL, P=0.044), intraoperative complication rate [2/229 (0.9%) vs. 6/121 (5.0%), P=0.040], and postoperative complication rate [6/229 (2.6%) vs. 10/121 (8.3%), P=0.004].
The following secondary endpoints were identified in favor of TIFP-LURS versus Con-LURS: shorter length of hospital stay after surgery (P=0.007) and lower long-term postoperative complication rate (P=0.022). No significant differences were noted in the duration of surgery, transfusion rate during surgery, or the rate of conversion to open surgery (P>0.05). The detailed data are presented in Table 2.
Table 2
| Characteristics | TIFP-LURS (n=229) | Con-LURS (n=121) | t/χ2/Ft | P value |
|---|---|---|---|---|
| Duration of surgery (min) | 262.47±112.97 | 243.37±126.19 | t=−1.444 | 0.15 |
| Estimated blood loss (mL) | 72.24±134.97 | 118.23±229.78 | t=2.025 | 0.044* |
| Transfusion in surgery | 5 (2.2) | 5 (4.1) | χ2=0.495 | 0.48 |
| Intraoperative complications | 2 (0.9) | 6 (5.0) | χ2=4.228 | 0.04* |
| Severe hemorrhage | 0 | 2 (1.7) | ||
| Vascular injury | 1 (0.4) | 1 (0.8) | ||
| Bowel injury | 1 (0.4) | 1 (0.8) | ||
| Diaphragm injury | 0 | 1 (0.8) | ||
| Other injury | 0 | 1 (0.8) | ||
| Conversions to open surgery | 4 (1.7) | 5 (4.1) | χ2=0.972 | 0.32 |
| Length of hospital stay after surgery (day) | 6.08±3.42 | 7.16±3.76 | t=2.699 | <0.01* |
| Postoperative complications (short-term) | 45 (19.7) | 37 (30.6) | χ2=5.270 | 0.02* |
| Grade I | 16 (7.0) | 9 (7.4) | χ2=0.024 | 0.88 |
| Fever | 5 (2.2) | 5 (4.1) | ||
| Noninfectious diarrhea | 2 (0.9) | 0 (0.0) | ||
| Hematuria | 4 (1.7) | 2 (1.7) | ||
| Wound infection | 5 (2.2) | 2 (1.7) | ||
| Grade II | 25 (10.9) | 27 (22.3) | χ2=8.129 | <0.01* |
| Hemorrhage treated by hemostatic agents | 6 (2.6) | 10 (8.3) | ||
| Lymphatic leakage treated by agents | 14 (6.1) | 8 (6.6) | ||
| Infection treated by antibiotics | 5 (2.2) | 9 (7.4) | ||
| Grade IIIa | 2 (0.9) | 1 (0.8) | Ft | >0.99 |
| Hemorrhage treated by endovascular embolization | 2 (0.9) | 1 (0.8) | ||
| Grade IIIb | 2 (0.9) | 0 | Ft | 0.55 |
| Hemorrhage treated by surgery | 1 (0.4) | 0 | ||
| Urinary leakage treated by surgery | 1 (0.4) | 0 | ||
| Grade IV | 0 | 0 | Ft | >0.99 |
| Postoperative complications (long-term) | 6 (2.6) | 10 (8.3) | χ2=5.782 | 0.02* |
| Pyeloureteral stenosis | 1 (0.4) | 2 (1.7) | ||
| Hydronephrosis | 0 | 3 (2.5) | ||
| Renal insufficiency | 5 (2.2) | 3 (2.5) | ||
| Lumbar hernia | 0 | 1 (0.8) | ||
| Others | 0 | 1 (0.8) |
Values are presented as mean ± standard deviation or number (%). *, P<0.05. Postoperative complications (short-term) according to Dindo-Clavien classification. t, using the Student’s t-test; χ2, using the chi-square test; Ft, using the Fisher exact test. TIFP-LURS, trans-Interfascial planes procedures for laparoscopic upper retroperitoneal surgery; Con-LURS, conventional laparoscopic upper retroperitoneal surgery.
On univariate analysis, patients age, disease condition (malignant or benign), surgery procedure (TIFP or conditional), duration of surgery and estimated blood loss predicted the risk of short-term postoperative complications. However, on multivariate analysis, disease condition [odds ratio (OR) =4.726, 95% CI: 2.562–8.717, P<0.001], surgery procedure (OR =0.469, 95% CI: 0.260–0.848, P=0.012) and estimated blood loss (OR =1.003, 95% CI: 1.001–1.005, P=0.009) were independent predictors of the risk of short-term postoperative complications (Table 3).
Table 3
| Variable | Total (n=350) | Complications (n=82) | Univariable | Multivariable | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||||
| Sex | 1.246 (0.757–1.002) | 0.39 | Not included in the model | NC | |||
| Male | 186 (53.1) | 47 (57.3) | |||||
| Female | 164 (46.9) | 35 (42.7) | |||||
| Age (years) | 1.019 (1.002–1.037) | 0.03* | Not included in the model | NC | |||
| BMI (kg/m2) | 1.005 (0.918–1.212) | 0.45 | Not included in the model | NC | |||
| Location | 1.325 (0.807–2.175) | 0.27 | Not included in the model | NC | |||
| Right | 169 (48.3) | 44 (53.7) | |||||
| Left | 181 (51.7) | 38 (46.3) | |||||
| ASA score | 1.092 (0.216–5.515) | 0.92 | Not included in the model | NC | |||
| ≥3 | 8 (2.3) | 2 (2.4) | |||||
| <3 | 342 (97.7) | 80 (97.6) | |||||
| Disease condition | 5.466 (3.221–9.277) | <0.001* | 4.726 (2.562–8.717) | <0.01* | |||
| Malignant | 113 (32.3) | 51 (62.2) | |||||
| Benign | 237 (67.7) | 31 (37.8) | |||||
| Previous abdominal history | 39 (11.1) | 9 (11.0) | 0.978 (0.444–2.154) | 0.96 | Not included in the model | NC | |
| Ipsilateral hydro-nephrosis | 127 (36.3) | 30 (36.6) | 1.017 (0.608–1.700) | 0.95 | Not included in the model | NC | |
| Surgery procedure | 0.555 (0.355–0.921) | 0.02* | 0.469 (0.260–0.848) | 0.01* | |||
| TIFP | 229 (65.4) | 45 (54.9) | |||||
| Con | 121 (34.6) | 37 (45.1) | |||||
| Duration of surgery | 1.005 (1.003–1.008) | <0.001* | 1.002 (0.999–1.001) | 0.26 | |||
| Estimated blood loss | 1.005 (1.003–1.007) | <0.001* | 1.003 (1.001–1.005) | <0.01* | |||
| Conversion | 9 (2.6) | 1 (1.2) | 0.401 (0.049–3.256) | 0.39 | Not included in the model | NC | |
Values are presented as number (%) unless otherwise specified. *, P<0.05. Postoperative complications (short-term) according to Dindo-Clavien classification. OR, odds ratio; CI, confidence interval; BMI, body mass index; TIFP, trans-interfascial planes procedures for laparoscopic upper retroperitoneal surgery; Con, conventional laparoscopic upper retroperitoneal surgery; NC, not calculated; ASA, American Society of Anesthesiologists.
Discussion
Surgical-related IFP refer to the multilaminar, avascular, loose connective tissue gap between the surface fascia of different organs in the abdominal and retroperitoneal spaces (2,3). Owing to the complexity of embryonic development of the main organs (urinary system organs) in the retroperitoneum, the IFP structure in the retroperitoneal space is the most complex. Radiologists currently accept the concept of “IFP” to understand retroperitoneal anatomy (10), replacing Meyers’ classic tricompartmental theory. Despite much research on retroperitoneal anatomy, its anatomical structure, embryonic origin and developmental process still need further exploration, especially the anatomical relationship between the LCF, Gerota’s fascia and Zuckerkandl’s fascia, and how to understand the IFP to guide the optimization of surgical pathways.
According to the results of anatomic and histological observation in this study, “fascia tissue” is a three-dimensional structure that contains blood vessels and lymphatic pathways. The retroperitoneal space forms a layered structure with various layers of fascia, providing the potential to establish and expand a safe surgical space between fascial planes. The core principle of TIFP-LURS is to dissect and expand the inherent potential spaces between the organs of different embryonic origins during laparoscopic surgery. This allows for the creation of surgical operating planes free of blood vessels or adipose tissue, and ultimately removes or reconstructs upper urinary tract lesions.
The laparoscopic technique originated with the use of cystoscope and has revolutionized modern surgery. In 1976, Cortesi first reported its use for diagnosing undescended testicles (11). With advancements in equipment, laparoscopy has been used to treat abdominal organ lesions. In 1990, Clayman successfully performed the first laparoscopic nephrectomy (12). In 1992, Higashihara and Gagner reported successful laparoscopic adrenalectomies and Gaur developed a technique for expanding the retroperitoneal space using a balloon (13-15). Since then, laparoscopy has been rapidly developed in urology and has become the “gold standard” for many diseases because of its advantages over open surgery. New technologies such as 4K and 3D video, laparo-endoscopic single-site surgery, and robot-assisted surgery have greatly promoted innovation in urological surgery.
Currently, the main surgical approaches for LURS are trans-abdominal and retroperitoneal approaches. Both are technically mature and there is no statistically significant difference in efficacy between them for skilled surgeons performing Con-LURS (16-18). Surgeons can select the appropriate approach based on preoperative imaging, patient status, and surgical experience. In this study, the Con-LURS group used the retroperitoneal approach to directly access the lesion area and reduce interference with abdominal organs. The transabdominal approach was used in the TIFP-LURS group to obtain a larger operating space and clearer anatomical structures.
We contended that discussions such as “transabdominal or retroperitoneal approach” represent practical summaries of technical details. Ultimately, their theoretical foundations must rest on the understanding of anatomical structures. In 2015, Mike described the technique of “fascial anatomy” in colorectal cancer surgery, which involves finding the layers that make up the “holy plane” (19) and Gong (20) advanced a surgical theory named “membrane anatomy” in gastrointestinal surgery. Furtherly, several gastroenterologists confirmed surgical landmark based on fascial anatomy researches (21,22). With these surgical concepts in gastrointestinal surgery, we advanced the “TIFP” surgical theory based on the comprehension of retroperitoneal anatomy.
The key to performing TIFP-LURS successfully is to identify the correct surgical plane, specifically an avascular IFP. The challenge lies in preserving the integrity of the multiple thin layers of the fascia while smoothly entering the IFP. TIFP-LURS is based on IFP anatomy. Its technical characteristics include the realization of re-embryonic and “paginization” operations during the surgical process. “Paginization” refers to the concept of separating an object into layers, like pages in a book, which means establishing IFP in bloodless areas between the fusion edges of two series of organ surface fasciae in TIFP surgery. Through paginization operation, the retroperitoneal fascia layers are freed and a bloodless operating space is established. Following the path of rotation and translation during organ embryonic development (morphogenesis), surgical operation is performed to restore the organ position during embryonic development and achieve lesion removal and upper urinary tract reconstruction or repair. This surgical idea can be summarized as re-embryonic operation.
In this study, the results showed no statistically significant differences in disease type, surgical type, or preoperative data between the Con-LURS and TIFP-LURS groups (P>0.05) (Table 1), indicating the comparability of perioperative data between the two groups. Compared to Con-LURS, TIFP-LURS offers a larger operating space and employs paganization and re-embryonic surgical techniques. These differences in surgical approaches may account for variations in perioperative outcomes, and were measured by indicators such as duration of surgery, estimated blood loss, length of hospital stay, and the incidence and severity of postoperative complications.
Previous research indicated that factors that may affect the occurrence of postoperative complications after LURS include patient, surgical, and perioperative management factors (23). Patient factors included age >60 years, male sex, tumor disease, ascites, American Society of Anesthesiologists (ASA) grade ≥3, preoperative anemia, and hypoalbuminemia (24-27). However, BMI and obesity are not independent risk factors for laparoscopic nephrectomy or partial nephrectomy (28). Surgical factors include the use of minimally invasive surgery in pediatric urology and the use of a retroperitoneal approach in laparoscopic partial nephrectomy (29). Perioperative management factors include an increased investment in patient care resources (23). Currently, there is no consensus regarding the factors that affect postoperative complications.
In the multivariable analysis in this study, TIFP procedure was an independent protective factor for decreasing the incidence of postoperative complications, while prolonged duration of surgery and increased blood loss in surgery were independent risk factors for increasing incidence of postoperative complications. These factors may lead to the incidence of postoperative complications after LURS through the following mechanisms: (I) prolonged surgery time could increase abdominal carbon dioxide absorption and increase the burden on cardiopulmonary function in elderly or high ASA grade patients. (II) Increased blood loss during surgery may increase the risk of hypothermia, and increased intraoperative bleeding (30). (III) Paginization operation in TIFP-LURS can reduce intraoperative bleeding by minimizing damage to the microvascular network in the fasciae. TIFP-LN can reduce damage to perirenal tissue by freeing the kidney and renal fascia through the fascial space. TIFP-LA can expose important blood vessels and prevent intraoperative injuries.
The novelty of this study is that we found anatomic basis for TIFP-LURS through cadaveric study, and furtherly developed a completely described technique of TIFP-LURS and indicated its advantages in perioperative outcomes. Specifically, on the one hand, according to observation results of cadaveric study, we provided new understanding about retroperitoneal fascia anatomy, and highlighted the IFP as potential avascular space that can be used during LURS. One the other hand, according to the new understanding of anatomy, we developed technique of TIFP-LURS, and demonstrated its advantage in perioperative outcomes, including less blood loss, lower operative complication rate, shorter hospital stay. Additionally, the concept of TIFP procedure theoretically is applicable to different surgical approaches or types. It is possible useful for trans-retroperitoneal approach, for the reason that the understanding of IFP in retroperitoneal fascia anatomy and the TIFP procedure help urologists to better dissect perirenal structure and complete LURS. But further clinical study is required.
This study had several limitations. The number of anatomical and histological specimens observed was relatively small, which may have affected the results owing to anatomical variations. Meanwhile, direct evidence from embryological observations is lacking. The clinical study was a single-center, non-randomized retrospective comparison of surgical outcomes, which may introduce various retrospective biases.
Conclusions
Fasciae is a three-dimensional structure containing a microvascular network, and the IFP are potential avascular spaces that can be dissected and expanded during laparoscopic surgery. TIFP-LURS is a novel and feasible surgical approach based on IFP anatomy, which can reduce intraoperative bleeding and postoperative complications, and improve the safety and efficacy of laparoscopic surgery for upper retroperitoneal lesions.
Acknowledgments
All authors thank the Laboratory of Anatomy of Zhongshan School of Medicine of Sun Yat-sen University for providing cadavers for this study.
Funding: This work was supported by a grant from
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-632/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-632/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-632/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-632/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Committee of The Sixth Affiliation Hospital of Sun Yat-sen University (No. 2022ZSLYEC-171) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosevear HM, Montgomery JS, Roberts WW, et al. Characterization and management of postoperative hemorrhage following upper retroperitoneal laparoscopic surgery. J Urol 2006;176:1458-62. [Crossref] [PubMed]
- Ochi A, Muro S, Adachi T, et al. Zoning inside the renal fascia: The anatomical relationship between the urinary system and perirenal fat. Int J Urol 2020;27:625-33. [Crossref] [PubMed]
- Ishikawa K, Nakao S, Nakamuro M, et al. The retroperitoneal interfascial planes: current overview and future perspectives. Acute Med Surg 2016;3:219-29. [Crossref] [PubMed]
- Meyers MA, Whalen JP, Peelle K, et al. Radiologic features of extraperitoneal effusions. An anatomic approach. Radiology 1972;104:249-57. [Crossref] [PubMed]
- Chesbrough RM, Burkhard TK, Martinez AJ, et al. Gerota versus Zuckerkandl: the renal fascia revisited. Radiology 1989;173:845-6. [Crossref] [PubMed]
- Liang JT, Huang J, Chen TC, et al. The Toldt fascia: A historic review and surgical implications in complete mesocolic excision for colon cancer. Asian J Surg 2019;42:1-5. [Crossref] [PubMed]
- Molmenti EP, Balfe DM, Kanterman RY, et al. Anatomy of the retroperitoneum: observations of the distribution of pathologic fluid collections. Radiology 1996;200:95-103. [Crossref] [PubMed]
- Takahashi R, Furubayashi N, Nakamura M, et al. Surgical considerations of the renal fascia and the retroperitoneal space around the kidney. J Bodyw Mov Ther 2012;16:392-6. [Crossref] [PubMed]
- Höckel M. Morphogenetic fields of embryonic development in locoregional cancer spread. Lancet Oncol 2015;16:e148-51. [Crossref] [PubMed]
- Lee SL, Ku YM, Rha SE. Comprehensive reviews of the interfascial plane of the retroperitoneum: normal anatomy and pathologic entities. Emerg Radiol 2010;17:3-11. [Crossref] [PubMed]
- Cortesi N, Ferrari P, Zambarda E, et al. Diagnosis of bilateral abdominal cryptorchidism by laparoscopy. Endoscopy 1976;8:33-4. [Crossref] [PubMed]
- Clayman RV, Kavoussi LR, Soper NJ, et al. Laparoscopic nephrectomy. N Engl J Med 1991;324:1370-1. [Crossref] [PubMed]
- Higashihara E, Tanaka Y, Horie S, et al. A case report of laparoscopic adrenalectomy. Nihon Hinyokika Gakkai Zasshi 1992;83:1130-3. [Crossref] [PubMed]
- Gagner M, Lacroix A, Bolté E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med 1992;327:1033. [Crossref] [PubMed]
- Gaur DD. Laparoscopic operative retroperitoneoscopy: use of a new device. J Urol 1992;148:1137-9. [Crossref] [PubMed]
- Ozcan L, Polat EC, Onen E, et al. Comparison between Retroperitoneal and Transperitoneal Approaches in the Laparoscopic Treatment of Bosniak Type I Renal Cysts: A Retrospective Study. Urol J 2015;12:2218-22. [PubMed]
- Gavriilidis P, Camenzuli C, Paspala A, et al. Posterior Retroperitoneoscopic Versus Laparoscopic Transperitoneal Adrenalectomy: A Systematic Review by an Updated Meta-Analysis. World J Surg 2021;45:168-79. [Crossref] [PubMed]
- Constantinides VA, Christakis I, Touska P, et al. Systematic review and meta-analysis of retroperitoneoscopic versus laparoscopic adrenalectomy. Br J Surg 2012;99:1639-48. [Crossref] [PubMed]
- Mike M, Kano N. Laparoscopic surgery for colon cancer: a review of the fascial composition of the abdominal cavity. Surg Today 2015;45:129-39. [Crossref] [PubMed]
- Gong JP. Rise and mix of membrane anatomy. Zhonghua Wei Chang Wai Ke Za Zhi 2019;22:401-5. [PubMed]
- Wu Q, Wei M, Zhang X, et al. Distinctive features of small vessels on the mesorectal and parietal pelvic fascia as important landmarks in guiding precise inter-fascial dissection for low rectal cancer. Surg Endosc 2022;36:1657-65. [Crossref] [PubMed]
- Wedel T, Heimke M, Fletcher J, et al. The retrocolic fascial system revisited for right hemicolectomy with complete mesocolic excision based on anatomical terminology: do we need the eponyms Toldt, Gerota, Fredet and Treitz? Colorectal Dis 2023;25:764-74. [Crossref] [PubMed]
- Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth 2016;117:601-9. [Crossref] [PubMed]
- Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg 2010;4:5. [Crossref] [PubMed]
- Longo WE, Virgo KS, Johnson FE, et al. Risk factors for morbidity and mortality after colectomy for colon cancer. Dis Colon Rectum 2000;43:83-91. [Crossref] [PubMed]
- Wang Y, Li H, Ye H, et al. Postoperative infectious complications in elderly patients after elective surgery in China: results of a 7-day cohort study from the International Surgical Outcomes Study. Psychogeriatrics 2021;21:158-65. [Crossref] [PubMed]
- Yoo PS, Mulkeen AL, Frattini JC, et al. Assessing risk factors for adverse outcomes in emergent colorectal surgery. Surg Oncol 2006;15:85-9. [Crossref] [PubMed]
- Sperling CD, Xia L, Berger IB, et al. Obesity and 30-Day Outcomes Following Minimally Invasive Nephrectomy. Urology 2018;121:104-11. [Crossref] [PubMed]
- Tejwani R, Young BJ, Wang HS, et al. Open versus minimally invasive surgical approaches in pediatric urology: Trends in utilization and complications. J Pediatr Urol 2017;13:283.e1-9. [Crossref] [PubMed]
- Weiskopf RB, Feiner J, Hopf H, et al. Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans. Anesthesiology 2006;104:911-20. [Crossref] [PubMed]


