
Efficacy and safety of platelet-rich plasma (PRP) in erectile dysfunction (ED): a systematic review and meta-analysis
Highlight box
Key findings
• Platelet-rich plasma (PRP) appears to be an effective and safe measure for treating erectile dysfunction (ED). Our research presents a novel therapeutic option for patients with ED.
What is known and what is new?
• The efficacy and safety of PRP as a novel treatment method in patients with ED are yet to be well understood.
• The results of meta-analysis showed that the use of PRP had some clinical effectiveness and a low incidence of side effects.
What is the implication, and what should change now?
• PRP is a safe and promising method for improving ED. Although PRP therapy is not considered a first-line treatment for ED, we recommend that patients with inadequate responses to other treatments consider trying PRP therapy.
Introduction
Erectile dysfunction (ED) is a common male disease that often occurs in men over 40 years old (1). The development of ED is closely related to factors such as diabetes, hypertension, aging, obesity, and lower urinary tract symptoms (1). Commonly used medications for ED, such as type 5 phosphodiesterase (PDE5) inhibitors, can relax the cavernous vessel through the nitric oxide pathway, allowing more blood to flow into the cavernous body (2,3). Additionally, alternative therapies including vacuum erection devices, shockwave therapy, and penile implants can also improve ED symptoms (3). Although the above-mentioned treatments can improve ED to some extent by enhancing hemodynamics, they cannot truly reverse the pathological changes of ED. Therefore, increasing attention has been focused on the field of regenerative medicine, aiming to utilize the body’s own cells for the treatment and reversal of pathological changes in ED (1).
Platelet-rich plasma (PRP), a plasma component derived from human blood, exhibits a platelet concentration of three to seven times higher than that of whole blood (4). PRP is notably enriched with a range of growth factors, including but not limited to platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), and vascular endothelial growth factor (VEGF) (5). When injury occurs, these growth factors are released at the site of the injury, promoting wound healing, vascular and neural remodeling (6), and having a significant role in the body’s recovery. The application of PRP in fields such as thoracic surgery, dermatology, and orthopedics has demonstrated positive effects (7-9). These characteristics have led to an increasing number of researchers exploring the role of PRP in ED. In recent years, several studies have indicated that PRP helps improve ED with a low incidence of adverse events (10,11).
Therefore, our objective was to integrate all available evidence to better assess the effectiveness and safety of PRP in the treatment of ED. This may provide an optional novel treatment modality for ED patients in the future. We present this article in accordance with the PRISMA reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-24-30/rc).
Methods
This systematic review and meta-analysis rigorously was registered with PROSPERO (CRD42023484693).
Search strategy
In our research, we meticulously searched for relevant studies using databases including PubMed, EMBASE, Web of Science, and the Cochrane Controlled Trials Register, covering the time frame from their respective starts to November 2023. The search strategy was based on the PICOS tool as follows: (P) population: patients with ED; (I) intervention: PRP; (C) control: placebo; (O) outcomes: effectiveness and safety; (S) study design: case-control, cohort studies, or randomized controlled trials (RCTs). The search strategy used different combinations of the following entry terms: “Erectile Dysfunction [MeSH]”, “Dysfunction, Erectile”, “Male Impotence”, “Impotence, Male”, “Male Sexual Impotence”, “Impotence, Male Sexual”, “Sexual Impotence, Male”, “Impotence”, “Platelet-Rich Plasma [MeSH]”, “Plasma, Platelet-Rich”, and “Platelet Rich Plasma”. For detailed search strategies, please refer to Appendix 1, Table S1. In additional, a manual search was performed using reference lists and cited articles in the identified studies, looking for further relevant publications.
Inclusion and exclusion criteria
The selection criteria for the articles were as follows: (I) the study covered male patients over 18 years old; (II) the study covered patients diagnosed with ED; (III) the study evaluated PRP; and (IV) the study reported on one or more of the following outcomes: International Index of Erectile Function-Erectile Function (IIEF-EF), minimal clinically important difference (MCID), peak systolic velocity (PSV), erectile hardness score (EHS), and visual analog scale (VAS).
The exclusion criteria were: (I) studies were unrelated to the topic; (II) animal experiments, commentaries, reviews, conference abstracts, secondary studies, and editorials; and (III) studies for which analysis data could not be obtained.
Study selection
We used Endnote literature management software for literature management to remove duplicate records. Two researchers read the titles and abstracts for assessment and classification to determine which articles should be included. During this process, the two researchers independently screened the literature, and if there were any disagreements, they were discussed and resolved by a third researcher. When there were missing of any required information, we reached out to the corresponding writers to ask for the missing data. These examinations were omitted if no figures were given or no reply was provided by the time of meta-analysis when the studies did not have all the necessary information.
Data extraction
Data were extracted by two independent researchers and comprised: (I) the initial author’s identity, publication year, and research country; (II) patient demographic information including the count of subjects, ages, body mass index (BMI), duration of ED, follow-up length, and initial outcome measures; and (III) outcome metrics such as the IIEF-EF scores measured at 1, 3, and 6 months subsequent to PRP treatment, the percentage of patients achieving the MCID during these time frames, post-PRP treatment evaluations of PSV, EHS, and VAS scores.
Quality assessment
The methodological quality of the included RCTs was evaluated using the modified Jadad scale. Studies with scores ranging from 1 to 3 were deemed to be of low quality, whereas those scoring between 4 and 7 were classified as high quality. For non-randomized studies, both single-arm and double-arm, the Methodological Index for Non-Randomized Studies (MINORS) scale was employed, assigning a maximum score of 16 for single-arm studies and 24 for double-arm studies (12).
Outcome measurements
Primary outcome involved evaluating patient scores on the IIEF-EF following PRP treatment. Widely recognized globally, the IIEF-EF scale serves as a standard tool for determining the severity of ED in patients. Additionally, the MCID stood as another crucial metric, employed to gauge PRP’s therapeutic effectiveness.
Secondary outcomes were composed of PSV, EHS, and the VAS. EHS is a metric that gauges the hardness of the penis. The safety of PRP was frequently assessed using the VAS, a standard tool for pain evaluation.
Data analysis
Using Review Manager 5.4 and StataMP 15.0, we conducted statistical evaluations of the results. We employed risk ratios (RRs) accompanied by 95% confidence intervals (CIs) to quantify the outcomes for binary variables, whereas mean differences (MDs) with 95% CI were calculated for continuous variables. The degree of variability across the studies, or heterogeneity, was assessed through the I2 (13). We utilized a random-effects model when I2 surpassed 50%, whereas for I2 values under 50%, a fixed-effects model was chosen. To verify the reliability and precision of our findings, we implemented the leave-one-out approach for sensitivity analysis. Additionally, funnel plots and Egger’s regression test were used to identify any potential publication bias, considering P values below 0.05 as significant (14). Sensitivity analysis and publication bias assessment were omitted if fewer than three studies were involved.
Results
Study selection and characteristics of included studies
We retrieved 367 articles from the database. Subsequently, we excluded 174 duplicated articles and 166 articles that were irrelevant to the topic, conference abstracts, secondary analyses, or animal studies. Finally, after reading the full text of the remaining 27 articles, we found that 17 articles did not meet the inclusion criteria and were excluded (reasons for exclusion included: mismatched study population, non-PRP treatment, lack of relevant outcome data). Ultimately, we included 10 literature references in this study (15-24) (Figure 1). Among them, 7 articles were prospective non-randomized studies (6 single-arm studies and 1 double-arm study) (15,18,19,21-24), and 3 articles were RCTs (16,17,20). A total of 559 ED patients were analyzed. This includes 446 patients receiving PRP treatment and 113 patients receiving placebo treatment. Research was carried out in various countries such as the United States, China, Italy, Turkey, France, Egypt, and Greece. The duration of follow-up in these studies varied, spanned from 1 to 6 months. A summary of the initial characteristics of the populations studied is provided in Table 1. Tables 2,3 also include baseline information for parameters like IIEF-EF, MCID, PSV, EHS, and VAS, along with post-treatment data specific to each region. In addition, we summarized the injection dose and injection site of PRP in ED patients (Appendix 2, Table S2).

Table 1
| Study | Country | Type of study | Follow-up time (months) | Intervention | No. of patients | Age (years) | BMI (kg/m2) | Duration of ED | Hypertension | Smoking | Diabetes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Francomano et al., 2023 (15) | Italy | Open-label, single-arm, multicenter | 1 | PRP | 150 | 51 (16.7) | 25 [20–30] | NA | NA | NA | NA |
| Taş et al., 2021 (21) | Turkey | Prospective single-arm study | 6 | PRP | 31 | 54.41 (8.74) | 30.82 (5.42) | 5.0 [3.0–8.0] years | 15 | NA | 15 |
| Schirmann et al., 2022 (19) | France | Prospective single-arm study | 6 | PRP | 15 | 55 [49–64] | NA | NA | 7 | 7 | 10 |
| Zaghloul et al., 2021 (24) | Egypt | Prospective single-arm study | 1 | PRR | 34 | 50.18 (8.64) | NA | 26.5 (23.83) months | 2 | 12 | 13 |
| Wong et al., 2021 (22) | China | Prospective single-arm study | 6 | PRP | 30 | 54.93 (8.31) | 25.74 (2.78) | NA | 12 | 9 | 5 |
| Raaia et al., 2019 (18) | Egypt | Prospective single-arm study | 3 | PRP | 30 | 55 [42–69] | NA | 4.7 [1–15] years | NA | NA | NA |
| Zaghloul et al., 2022 (23) | Egypt | Prospective study | 3 | PRP | 24 | 50.16 (6.89) | NA | NA | NA | NA | 24 |
| 24 | 52.33 (5.17) | NA | NA | NA | NA | 0 | |||||
| Poulios et al., 2021 (17) | Greece | RCT | 6 | PRP | 30 | 58 [51.5–62] | 29.4 [26.6–32.1] | 78 [48–120] months | 10 | 16 | 11 |
| Placebo | 30 | 59 [53.5–61] | 28.5 [26–30.4] | 60 [39–117] months | 8 | 19 | 4 | ||||
| Shaher et al., 2023 (20) | Egypt | RCT | 6 | PRP | 50 | 56 [46–65] | 25 [23–28.7] | 44.5 [33-53.25] months | 18 | 27 | 15 |
| Placebo | 50 | 54 [45–64] | 24.9 [22.75–27] | 41.5 [35–53.5] months | 14 | 28 | 17 | ||||
| Masterson et al., 2023 (16) | USA | RCT | 6 | PRP | 28 | 49 [38.5–55] | 27.9 [24.5–30.3] | NA | 8 | 2 | 3 |
| Placebo | 33 | 46 [42–56] | 28.5 [25.7–31.1] | NA | 10 | 0 | 3 |
Statistics are presented as mean (SD), median [IQR], or number. BMI, body mass index; ED, erectile dysfunction; PRP, platelet-rich plasma; NA, not applicable; RCT, randomized controlled trial; SD, standard deviation; IQR, interquartile range.
Table 2
| Outcomes | Francomano et al., 2023 (15) | Taş et al., 2021 (21) | Schirmann et al., 2022 (19) | Zaghloul et al., 2021 (24) | Wong et al., 2021 (22) | Raaia et al., 2019 (18) | Poulios et al., 2021 (17) | Shaher et al., 2023 (20) | Masterson et al., 2023 (16) | Zaghloul et al., 2022 (23) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diabetic ED group | Non-diabetic ED group | ||||||||||
| IIEF-EF | |||||||||||
| Pre-treatment | 12 (2.6) | 18 (10.74) | 11.8 (5.51) | 7.7059 (2.73617) | 12.034 (5.10) | 6.5 (5.1852) | 20.4 (2.9) | 18 (3.7037) | 17.4 (4.1263) | 8.04 (2.7) | 10.2 (0.9) |
| Post-treatment (months) | |||||||||||
| 1 | 19 (3.0) | 20 (11.11) | 16.8 (4.97) | 13.2059 (6.7724) | 13.93 (5.97) | 7.7 (9.6296) | 23.897 (4.2623) | 22 (3.7037) | 21 (7.2223) | NA | NA |
| 3 | NA | 20 (11.11) | 16.23 (5.1) | NA | 15.138 (5.06) | 10.2 (14.074) | 23.724 (4.3581) | 22 (3.7037) | 19.2 (7.6966) | 12.1 (5.6) | 14.8 (4.8) |
| 6 | NA | 20 (11.11) | 15.15 (6.44) | NA | 16.59 (5.5) | NA | 23.897 (4.1691) | 22 (3.3333) | 22.2 (6.9442) | NA | NA |
| PSV (cm/s) | |||||||||||
| Pre-treatment | 32 (5.5) | NA | NA | 49.3519 (16.2972) | NA | NA | NA | 19 (5.1852) | 47.2 (13.2814) | 33.335 (4.2504) | 28.97 (7.299) |
| Post-treatment | 42 (7.6) | NA | NA | 50.4669 (17.0809) | NA | NA | NA | 33 (3.8519) | 48.8 (15.7485) | 45.29 (1.223) | 39.08 (4.691) |
| EHS | |||||||||||
| Pre-treatment | NA | NA | 2.2507 (1.511) | NA | 2.31 (0.76) | NA | NA | NA | NA | NA | NA |
| Post-treatment | NA | NA | 2.3575 (1.063) | NA | 3.03 (0.82) | NA | NA | NA | NA | NA | NA |
Statistics are presented as mean (SD). ED, erectile dysfunction; IIEF-EF, International Index of Erectile Function-Erectile Function; NA, not applicable; PSV, peak systolic velocity; EHS, erectile hardness score; SD, standard deviation.
Table 3
| Outcomes | Poulios et al., 2021 (17) | Shaher et al., 2023 (20) | Masterson et al., 2023 (16) | |||||
|---|---|---|---|---|---|---|---|---|
| PRP | Placebo | PRP | Placebo | PRP | Placebo | |||
| IIEF-EF post-treatment (months) | ||||||||
| 1 | 23.897 (4.2623) | 20.39 (4.0764) | 22 (3.7037) | 19 (3.7037) | 21 (7.2223) | 21.6 (6.44729) | ||
| 3 | 23.724 (4.3581) | 20.22 (5.0332) | 22 (3.7037) | 19 (3.7037) | 19.2 (7.6966) | 20.6 (6.17763) | ||
| 6 | 23.897 (4.1691) | 19.42 (4.3004) | 22 (3.3333) | 19 (3.7037) | 22.2 (6.9442) | 20.5 (4.85479) | ||
| MCID (months) | ||||||||
| 1 | 22/29 | 7/28 | 38/50 | 9/50 | 14/24 | 15/28 | ||
| 3 | 20/29 | 10/26 | 36/50 | 8/50 | 10/24 | 13/25 | ||
| 6 | 20/29 | 7/26 | 35/50 | 8/50 | 12/20 | 10/24 | ||
| PSV (cm/s) | NA | NA | 33 (3.8519) | 20 (5.333) | 48.8 (15.7485) | 43.1 (14.296) | ||
| VAS | 2.5665 (0.7217) | 2.1667 (0.7628) | 1.52 (1.2) | 1.54 (1.3) | NA | NA | ||
Statistics are presented as mean (SD) or n/N. PRP, platelet-rich plasma; IIEF-EF, International Index of Erectile Function-Erectile Function; MCID, minimal clinically important difference; PSV, peak systolic velocity; VAS, visual analog scale; NA, not applicable; SD, standard deviation.
Quality assessment
After evaluating with the MINORS scale, among the included 7 non-randomized studies, the scores of 6 single-arm studies ranged from 13 to 15 (15,18,19,21,22,24), and the score of 1 two-arm study was 22 (23), which was acceptable for our meta-analysis (Appendix 3, Table S3). Additionally, the 3 included RCT studies were assessed using the modified Jadad scale (16,17,20), with scores ranging from 6 to 7, indicating that they were all high-quality studies (Appendix 3, Table S4).
Primary outcomes
Ten studies reported the outcome of IIEF-EF (15-24). The results of the single-arm meta-analysis (Figure 2) showed a significant improvement in IIEF-EF scores at 1 month (MD =4.05; 95% CI: 2.42, 5.68; P<0.001), 3 months (MD =3.73; 95% CI: 2.93, 4.53; P<0.001), and 6 months (MD =3.92; 95% CI: 3.00, 4.85; P<0.001) compared to baseline after PRP treatment. Similarly, the results of the double-arm meta-analysis (Figure 3) showed that the IIEF-EF scores in the PRP group were higher than the placebo group at 1 month (MD =2.47; 95% CI: 0.64, 4.31; P=0.008), 3 months (MD =2.14; 95% CI: −0.15, 4.44; P=0.07), and 6 months (MD =3.22; 95% CI: 2.13, 4.30; P<0.001). Sensitivity analysis indicated that the results did not significantly change when excluding any individual study (Appendix 4, Figures S1,S2).


Three studies reported the outcome of MCID (16,17,20). The results of the meta-analysis (Figure 4) showed that the PRP group had a higher occurrence of MCID than the placebo group at 1 month (RR =2.36; 95% CI: 0.95, 5.87; P=0.06), 3 months (RR =1.85; 95% CI: 0.71, 4.80; P=0.21), and 6 months (RR =2.50; 95% CI: 1.28, 4.87; P=0.007) after treatment (no statistically significant difference between the 1- and 3-month groups). Sensitivity analysis indicated that the results did not significantly change when excluding any individual study (Appendix 4, Figure S3).

Secondary outcomes
Four studies reported the outcomes of PSV (15,16,20,24). The results of the single-arm meta-analysis demonstrated (Figure 5A) a significant increase in PSV after PRP treatment compared to the baseline data (MD =8.53; 95% CI: 4.28, 12.79; P<0.001). The results of the two-arm meta-analysis showed (Figure 5B) that the PSV in the PRP group was higher than the placebo group (MD =10.32; 95% CI: 3.43, 17.22; P=0.003). Sensitivity analysis indicated that the results did not significantly change when excluding any individual study (Appendix 4, Figure S4).
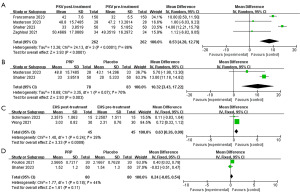
Two studies reported the outcomes of EHS (19,22). The meta-analysis results revealed (Figure 5C) no significant difference in EHS scores after PRP treatment compared to the baseline (MD =0.63; 95% CI: 0.26, 0.99; P<0.001).
Two studies reported the outcomes of VAS (17,20). The meta-analysis results showed (Figure 5D) no significant difference between the PRP group and the placebo group in terms of VAS (MD =0.24; 95% CI: −0.05, 0.54; P=0.11).
Publication bias
The funnel plot of IIEF-EF at 1-month follow-up after PRP treatment in single-arm studies showed significant asymmetry, and Egger’s regression test (P=0.006) indicated publication bias. Additionally, no publication bias was detected in the funnel plot and Egger’s regression test for other outcome measurements (Appendix 5, Figures S5-S8, Tables S5,S6).
Discussion
ED is a common male disorder in older men influenced by various factors. Reduction in tunica albuginea elastic fibers and smooth muscles of the corpus cavernosum, decline in gonadal function leading to decreased libido, and endothelial dysfunction are all reasons for the development of erectile impotence and reduced medication compliance (25). Additionally, increased oxidative stress caused by aging could result in endothelial dysfunction and corporal smooth muscle cell (CSMC) apoptosis, leading to relaxation impairment of smooth muscles (26). Several factors could potentially reduce the efficacy of PDE5 inhibitors. Intriguingly, while 60–65% of men with ED initially respond to PDE5 inhibitors, treatment failure is observed in 30–40% of these patients. It was found that primary reasons for this treatment failure include escalating endothelial dysfunction, advancing atherosclerosis, and the development of rapid tolerance. Consequently, PRP has been explored as a novel treatment approach for various diseases (27,28). PRP treatment for ED might be linked to cellular structural remodeling. Current research revealed that PRP is rich in growth factors such as fibroblast growth factor (FGF), PDGF, TGF-β, epidermal growth factor (EGF), VEGF, and insulin-like growth factor (IGF) (29). The plasma, abundant in nutrients, vitamins, hormones, electrolytes, and proteins, plays a pivotal role in cell survival, thereby being instrumental for cell differentiation and proliferation (30). Moreover, a couple of studies have reported that intracavernous injection of PRP in the corpus cavernosum post-cavernous nerve injury can alleviate ED by reducing markers of cell apoptosis and fibrosis (29,31). Hence, our study focused on examining the effectiveness and safety of PRP treatment for ED. The research findings suggest that PRP treatment is a safe and effective therapeutic strategy.
Efficacy
The erectile function (EF) domain of the IIEF serves as a primary measurement tool and is frequently utilized as the main endpoint in clinical trials targeting ED (32). In the study, there was a significant improvement in the IIEF-EF scores compared to baseline at 1, 3, and 6 months after PRP therapy. Specifically, the scores increased by 4.05 points at the first month, although there was a slight decrease in scores at the third month, it still increased by 3.73 points, and by the sixth month, the scores started to rise again, with an increase of 3.92 points. Additionally, compared to the placebo group, the score increments after PRP treatment at 1, 3, and 6 months were 2.47, 2.14, and 3.22 points, respectively. The data indicated that the enhancement in EF following PRP treatment seemed to become more notable as time progresses. Nonetheless, as mentioned by Rosen et al., there must be a minimum 4-point rise on the IIEF-EF questionnaire in order to establish a correlation between clinical improvement and the significance of the test. Hence, it is advised for readers to be cautious when interpreting our findings.
When evaluating the clinical effectiveness of therapies aimed at improving subjective outcomes, we needed to focus on the subjectivity of the IIEF-EF score scale. Therefore, we emphasized determining the degree of improvement that was important for patients, which was involved in the evaluation of the MCID. MCID was a patient-centered concept that reflects not only the magnitude of improvement but also the importance patients attach to the degree of change (33). In the studies we included, we found that specific changes in IIEF-EF scores may not be very obvious for judging efficacy. Therefore, we believed that adopting MCID may be more appropriate as a more accurate way to evaluate the outcome indicators of PRP treatment for ED. This helped ensure that we focused on changes that patients truly feel were important, rather than just statistical significance.
Meta-analysis results showed that at 1, 3, and 6 months after PRP therapy, the occurrence rates were 2.34, 1.85, and 2.5 times higher than those in the placebo group, indicating a certain effectiveness of PRP treatment. However, we found significant heterogeneity in the summary results of our 1-, 3-, and 6-month follow-ups. Upon analysis, it was found that this heterogeneity was primarily due to the study by Masterson et al. (16). In their study, both the PRP treatment group and the placebo treatment group were allowed to continue medication during the treatment period, which may have influenced the assessment of PRP efficacy. However, when excluding this study, no significant changes were observed in the summary results. Observations concurrently revealed that, as the duration of treatment extends, the cohort achieving the minimum clinical outcome benchmarks exhibited a gradual augmentation. This suggested an amelioration in the efficacy of PRP therapy for ED with an elongation of the treatment period. Nevertheless, despite thorough exploration of databases, studies focusing on an extended duration of PRP treatment remained elusive. Additionally, our study only included three articles, thus our conclusions need further validation and support from more high-quality studies in the future. Therefore, readers should exercise caution when interpreting our results.
The meta-analysis results of EHS showed no significant changes in the EHS scores after PRP treatment compared to baseline. This suggests that PRP appears to have poor effects on improving penile hardness in patients, which may also be attributed to the relatively short duration of PRP treatment. Further research is needed to validate these findings.
PSV is used to assess the blood flow in the corpus cavernosum arteries during penile erection. A single-arm meta-analysis showed that after PRP treatment, PSV increased by 8.53 cm/s compared to baseline. Animal studies by Wu et al. suggested that “optimized” PRP mixed with high-level growth factors exhibited a significant increase in intracavernosal pressure, nitric oxide synthase (NOS) levels, and mean arterial blood flow (31). However, the specific mechanisms of this phenomenon in the human body are not yet clear. There was significant heterogeneity in our results, which may be primarily attributed to the fact that subjects in two of the studies had received prior medication before PSV measurements, which could have influenced the results (16,20). However, after sensitivity analysis using the leave-one-out method, the summary results did not show significant changes. Therefore, readers should interpret our results with caution. Additionally, a two-arm meta-analysis showed that the PSV in the PRP group was 10.32 cm/s higher than that in the placebo group, indirectly indicating that PRP treatment helps improve blood flow in the penile corpus cavernosum, although there was high heterogeneity in these two studies (16,20).
Although the above discussions have demonstrated the efficacy of PRP to a certain extent, there is still no consensus on the optimal dosage, injection method, and frequency of PRP administration, which is a limitation of our study. Meanwhile, this is also a major source of heterogeneity between studies. For example, in the studies we included, the single dose of PRP ranged from 0.5 to 9 mL, or there were differences in the preparation method of PRP, which may have had some impact on the research results. Therefore, more high-quality studies are needed in the future to further validate the optimal treatment protocol for PRP therapy.
Furthermore, the heterogeneity between articles may stem from differences in the included populations. The criteria for including individuals are primarily assessed based on the IIEF-EF, categorizing the population into mild, moderate, or moderate ED. Due to the subjectivity in defining IIEF-EF scores for participant selection across different studies, there is a certain degree of variability in the chosen populations. However, following sensitivity analysis, the research results exhibit a certain robustness, indicating that the selection of the population did not impact the study outcomes. Nevertheless, future higher-quality studies are still needed to ensure greater applicability to the ED population undergoing PRP.
Safety
The VAS is often used to assess the degree of pain in patients, or to compare the severity between patients with similar conditions. In PRP therapy, VAS is used to evaluate patients’ pain perception during the injection process, in order to assess their tolerance.
The meta-analysis results of VAS showed no significant difference in VAS scores between the PRP group and the placebo group, and the VAS pain scores were relatively low after PRP injection. Poulios et al. expressed the view (17) that the increase in VAS scores after saline injection (placebo group) compared to PRP may be clinically irrelevant. However, only two studies reported VAS results, so our conclusions need further validation from more future studies. Additionally, the measurement of VAS is highly subjective, so caution is needed when interpreting the results.
In the qualitative analysis of the research, Shaher, Poulios, Zaghloul, Schirmann, and Raaia found that throughout the follow-up period (17-20,23), patients did not experience severe complications such as hematuria, local petechial bleeding, hematoma, bruises, ecchymosis, fibrous plaques, penile deformities, and abnormal penile erection. In Masterson’s report (16), one patient in the PRP group developed plaques, and in the placebo group, one patient had hematoma, but the rest of the patients did not show significant discomfort. Francomano’s study also did not report any serious adverse reactions (15), but 16 patients experienced pain during the injection process, and two patients had mild subcutaneous hematomas at the injection site. However, these two conditions disappeared within 3 days, indicating that they were within an acceptable range. Taş’s study also indicated that no patients felt pain during the injection process (21), but some patients had mild subcutaneous bruising, possibly due to repeated injections at the same site, with no other adverse reactions reported. Despite two studies not reporting adverse reactions, the adverse reaction results reported in the above analysis of studies all suggest that PRP therapy is a relatively safe treatment option.
Strengths and limitations
By conducting this meta-analysis, our study provided the initial assessment of the safety and effectiveness of PRP treatment for ED. Noteworthy aspects of our research encompassed examining the disparities between post-PRP treatment outcomes and the initial state, as well as comparing results between the PRP and placebo groups.
However, our study also had some limitations. These mainly included: (I) the limited number of studies involved in some outcomes, which may affect the credibility of the results; (II) the inclusion criteria for the study population varied, making it impossible to conduct subgroup analyses to explore their impact on PRP treatment; (III) despite using a random-effects model, there was heterogeneity in some of the study results; (IV) the relatively short follow-up time included in the study may introduce some bias into the treatment effectiveness of PRP; and (V) the definition criteria for the dosage and frequency of PRP were not consistent across the studies, which may have influenced the results. These issues need to be addressed by more high-quality studies in the future.
Conclusions
The evaluation and meta-analysis of this study show that PRP has a certain effectiveness in treating ED. Our research provides a certain reference for the treatment of ED patients. However, most of the studies we included were single-arm designs, so more future RCTs are needed for further evaluation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-24-30/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-24-30/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-24-30/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shamloul R, Ghanem H. Erectile dysfunction. Lancet 2013;381:153-65. [Crossref] [PubMed]
- Le TV, Tsambarlis P, Hellstrom WJG. Pharmacodynamics of the agents used for the treatment of erectile dysfunction. Expert Opin Drug Metab Toxicol 2019;15:121-31. [Crossref] [PubMed]
- Burnett AL, Nehra A, Breau RH, et al. Erectile Dysfunction: AUA Guideline. J Urol 2018;200:633-41. [Crossref] [PubMed]
- Hesseler MJ, Shyam N. Platelet-rich plasma and its utility in medical dermatology: A systematic review. J Am Acad Dermatol 2019;81:834-46. [Crossref] [PubMed]
- Hall MP, Band PA, Meislin RJ, et al. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg 2009;17:602-8. [Crossref] [PubMed]
- El-Sharkawy H, Kantarci A, Deady J, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol 2007;78:661-9. [Crossref] [PubMed]
- Patel AN, Selzman CH, Kumpati GS, et al. Evaluation of autologous platelet rich plasma for cardiac surgery: outcome analysis of 2000 patients. J Cardiothorac Surg 2016;11:62. [Crossref] [PubMed]
- Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev 2016;2016:CD006899. [Crossref] [PubMed]
- Moraes VY, Lenza M, Tamaoki MJ, et al. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev 2013;CD010071. [PubMed]
- Alkhayal S. Platelet rich plasma as a treatment for erectile dysfunction. J Sex Med 2022;19:S189. [Crossref]
- Banno JJ, Kinnick TR, Roy L, et al. 146 The efficacy of platelet-rich plasma (PRP) as a supplemental therapy for the treatment of erectile dysfunction (ED): initial outcomes. J Sex Med 2017;14:e59-60. [Crossref]
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003;73:712-6. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Francomano D, Iuliano S, Dehò F, et al. Regenerative treatment with platelet-rich plasma in patients with refractory erectile dysfunction: short-term outcomes and predictive value of mean platelet volume. Minerva Endocrinol (Torino) 2023. [Epub ahead of print]. doi:
10.23736/S2724-6507.23.04060-5 .10.23736/S2724-6507.23.04060-5 - Masterson TA, Molina M, Ledesma B, et al. Platelet-rich Plasma for the Treatment of Erectile Dysfunction: A Prospective, Randomized, Double-blind, Placebo-controlled Clinical Trial. J Urol 2023;210:154-61. [Crossref] [PubMed]
- Poulios E, Mykoniatis I, Pyrgidis N, et al. Platelet-Rich Plasma (PRP) Improves Erectile Function: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial. J Sex Med 2021;18:926-35. [Crossref] [PubMed]
- Raaia MF, Elkhiat Y, El Guindi AM, et al. Efficacy and Safety of Intracavernosal Injection of Autologous Platelet Rich Plasma for Treatment of Erectile Dysfunction. Indian Journal of Public Health Research & Development 2019;10:1460-4. [Crossref]
- Schirmann A, Boutin E, Faix A, et al. Pilot study of intra-cavernous injections of platelet-rich plasma (P-shot®) in the treatment of vascular erectile dysfunction. Prog Urol 2022;32:1440-5. [Crossref] [PubMed]
- Shaher H, Fathi A, Elbashir S, et al. Is Platelet Rich Plasma Safe and Effective in Treatment of Erectile Dysfunction? Randomized Controlled Study. Urology 2023;175:114-9. [Crossref] [PubMed]
- Taş T, Çakıroğlu B, Arda E, et al. Early Clinical Results of the Tolerability, Safety, and Efficacy of Autologous Platelet-Rich Plasma Administration in Erectile Dysfunction. Sex Med 2021;9:100313. [Crossref] [PubMed]
- Wong SM, Chiang BJ, Chen HC, et al. A short term follow up for intracavernosal injection of platelet rich plasma for the treatment of erectile dysfunction. Urological Science 2021;32:171-6. [Crossref]
- Zaghloul AS, El-Nashaar AM, Said SZ, et al. Assessment of the intracavernosal injection platelet-rich plasma in addition to daily oral tadalafil intake in diabetic patients with erectile dysfunction non-responding to on-demand oral PDE5 inhibitors. Andrologia 2022;54:e14421. [Crossref] [PubMed]
- Zaghloul AS, Mahmoud ElNashar AER. Smoking status and the baseline international index of erectile function score can predict satisfactory response to platelet-rich plasma in patients with erectile dysfunction: A prospective pilot study. Andrologia 2021;53:e14162. [Crossref] [PubMed]
- Seftel AD. Erectile dysfunction in the elderly: epidemiology, etiology and approaches to treatment. J Urol 2003;169:1999-2007. [Crossref] [PubMed]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 1999;5:1403-9. [Crossref] [PubMed]
- Di Martino A, Boffa A, Andriolo L, et al. Leukocyte-Rich versus Leukocyte-Poor Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Double-Blind Randomized Trial. Am J Sports Med 2022;50:609-17. [Crossref] [PubMed]
- Keene DJ, Alsousou J, Harrison P, et al. Platelet rich plasma injection for acute Achilles tendon rupture: PATH-2 randomised, placebo controlled, superiority trial. BMJ 2019;367:l6132. [Crossref] [PubMed]
- Huang YC, Wu CT, Chen MF, et al. Intracavernous Injection of Autologous Platelet-Rich Plasma Ameliorates Hyperlipidemia-Associated Erectile Dysfunction in a Rat Model. Sex Med 2021;9:100317. [Crossref] [PubMed]
- Sharara FI, Lelea LL, Rahman S, et al. A narrative review of platelet-rich plasma (PRP) in reproductive medicine. J Assist Reprod Genet 2021;38:1003-12. [Crossref] [PubMed]
- Wu YN, Wu CC, Sheu MT, et al. Optimization of platelet-rich plasma and its effects on the recovery of erectile function after bilateral cavernous nerve injury in a rat model. J Tissue Eng Regen Med 2016;10:E294-304. [Crossref] [PubMed]
- Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822-30. [Crossref] [PubMed]
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407-15. [Crossref] [PubMed]


