
Risk factors for distant metastasis and prognosis of the penile cancer with distant metastasis
Highlight box
Key findings
• This study investigated risk factors of penile cancer (PC) with distant metastasis (DM). Also, nomograms for predicting DM, overall survival and cancer specific survival of PC patients were developed.
What is known and what is new?
• PC is a rare malignant tumor, whose DM is associated with the poorest outcomes. The risk factors associated with PC have been investigated.
• We expanded the array of risk factors from a larger cohort, and established N stage and tumor size as critical predictors of DM in the predicting nomogram.
What is the implication, and what should change now?
• The nomograms based on these factors exhibited a good performance in predicting PC prognosis and DM, thus benefiting disease management. External validation and complete data on metastatic sites and metastatic patterns should be needed for further improvement of the nomogram performance.
Introduction
Penile cancer (PC), primarily penile squamous cell carcinoma (PSCC), is a rare malignancy that can be classified based on human papillomavirus (HPV) infection and inflammation-related etiology (1,2). A substantial proportion of cases are associated with HPV infection. Certain studies have underscored the crucial role of inflammatory factors, particularly penile infection and chronic irritation, in tumorgenesis or progression (3,4). Additionally, obesity, circumcision, and smoking are also implicated in the etiology of PC (5,6). PC may present with penile ulceration or malign priapism (3).
Low prevalence of PC has led to the management of PC is undervalued. Despite its scarcity, the global burden of PC is not negligible, with an estimated 2050 new diagnoses and 470 deaths anticipated in 2023 cancer statistics (7). Additionally, the period from 2000 to 2018 marked a notable rise in PC mortality in the United States (U.S.) (8). PC exhibits a marked epidemiological variation, significantly more prevalent in developing nations, with an incidence of 10% surpassing those in developed regions like the U.S. From 1998 to 2003, the incidence among Hispanics in the U.S. was 72% higher than that in non-Hispanics, potentially reflecting the involvement of lifestyle-related factors (9,10).
PC can be diagnosed using non-invasive imaging techniques, such as ultrasound and magnetic resonance imaging. Therapeutic strategies aim at preserving sexual and urinary function as much as possible, involving conservative to radical surgeries, or adjuvant therapies (radiation therapy, chemotherapy, etc.) (11). However, early detection is often disrupted by misdiagnosis, leading to loss of optimal opportunities for treatment (8).
Previous studies on PC prognostic factors have been limited by small sample sizes, rendering their findings less convincing. The extent of nodal metastasis, reported in previous studies, is closely associated with survival outcomes (11). Moreover, distant metastasis (DM) is associated with the poorest outcomes, evidenced by a 5-year survival rate of merely 16% (8). Risk factors including positive surgical margins were found associated with higher recurrence rate (10). Yet, risk factors about DM in PC have been rarely analyzed. This gap underscores the need for a predictive model, such as a nomogram, to enhance disease management and prognosis, an area currently under-explored in PC research. To address this, our study investigated the risk factors, utilized them to construct a nomogram for predicting the DM and prognosis in PC. We present this article in accordance with the TRIPOD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-24-92/rc).
Methods
Data sources and collection
We utilized the Surveillance, Epidemiology, and End Results (SEER) database, a comprehensive source of U.S. cancer incidence and mortality data, to collect data of PC available since 1973. Specifically, we extracted data from dataset of Incidence-SEER Research Data (17 Registries, Nov 2022 Sub, 2000–2020)-Linked To County Attributes-Total U.S., meticulously selected all PC cases using International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) site recode during 2000–2020. Essential data, including demographic, clinical and histopathological characteristics, and treatment-related information, were collected. Strict inclusion criteria were set as follows to ensure robust data integrity and meaningful analysis.
Inclusion criteria: (I) confirmed diagnosis of PC, with histopathological validation; (II) first-onset; (III) general data available, including age at diagnosis, race, marital status, tumor details [site, size, grade, and Tumor Node Metastasis (TNM) staging], treatment information (surgery, radiation therapy, chemotherapy, etc.), and survival outcomes (survival time, tumor-specific death status, survival status); (IV) a minimum survival time of one month. Cases not meeting these conditions were excluded. Case screening flowchart is presented in Figure 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).

Statistical analysis
The Kaplan-Meier method was employed for survival analysis, and the Log-rank test for comparing survival curves of overall survivals (OS) times across patient subgroups. Univariate and multivariate Cox proportional hazard regression analyses were preformed to exhibit the correlations between PC prognosis of OS and cancer specific survivals (CSS) and clinical factors. Logistic regression analysis further investigated risk factors for PC metastasis, with statistical significance set at P<0.05. Initial data were extracted utilizing SEER*Stat (v8.3.5), while SPSS 26.0 (IBM, Armonk, New York, USA) and R3.6.0 (Statistical Computing, Vienna, Austria) supported subsequent detailed analyses and visual representation. The receiver operating characteristic (ROC) curve determined cutoff values for continuous variables, such as age at diagnosis and tumor size.
Construction and validation of the nomograms
Following the logistic regression and the cox regression analysis, predictive nomograms were constructed. Weighted estimators of each covariate were derived from Cox regression coefficients and variance estimates. The highest b coefficient of each variable was converted into a 0–100 scale. These nomograms were run to predict CSS and OS at 1, 3, and 5 years, as well as the DM rates of PC patients. The predictive accuracy of these nomograms was evaluated by ROC curves and internal calibration curves.
Results
Clinicopathological characteristics of PC patients
In total, 1,488 PC cases were identified, with 40.7% aged over 69.5 years and 91.3% as Whites/Other (Black patients, 8.7%). The proportions of married and unmarried patients were approximately equal. A large proportion (64.6%) of tumors originated from the glans penis. The cases were categorized into 58.4% with tumor sizes ≤34.5 mm and 41.6% with >34.5 mm. Additionally, tumors were classified into T1, T2, or T3 stages, with a majority (80.0%) with no lymph node extension. Surgery remained the primary treatment (98.9%), with radiation (8.8%) and chemotherapy (11.4%) less adopted. The clinicopathological characteristics are summarized in Table 1.
Table 1
| Characteristics | Value, n (%) |
|---|---|
| Age, years | |
| ≤69.5 | 883 (59.3) |
| >69.5 | 605 (40.7) |
| Race | |
| White | 1,276 (85.8) |
| Black | 129 (8.7) |
| Other | 83 (5.5) |
| Marital status | |
| Married | 909 (61.1) |
| Other | 579 (38.9) |
| Tumor site | |
| Prepuce | 270 (18.1) |
| Glans penis | 961 (64.6) |
| Body of penis | 125 (8.4) |
| Overlapping lesion of penis | 132 (8.9) |
| Tumor size, mm | |
| ≤34.5 | 869 (58.4) |
| >34.5 | 619 (41.6) |
| Grade | |
| I (well differentiated) | 418 (28.1) |
| II (moderately differentiated) | 718 (48.3) |
| III (poorly differentiated) | 341 (22.9) |
| IV (undifferentiated) | 11 (0.7) |
| T stage | |
| Ta | 3 (0.2) |
| T1 | 698 (46.9) |
| T2 | 468 (31.5) |
| T3 | 302 (20.3) |
| T4 | 17 (1.1) |
| N stage | |
| N0 | 1,191 (80.0) |
| N1 | 94 (6.3) |
| N2 | 111 (7.5) |
| N3 | 92 (6.2) |
| M stage | |
| M0 | 1,455 (97.8) |
| M1 | 33 (2.2) |
| Surgery | |
| No | 17 (1.1) |
| Yes | 1,471 (98.9) |
| Radiation | |
| Yes | 131 (8.8) |
| No | 1,357 (91.2) |
| Chemotherapy | |
| Yes | 170 (11.4) |
| No | 1,318 (88.6) |
Prognosis of PC with DM
DM was observed in a small fraction (2.2%) of the cases, with the lung being the most frequent metastatic site (30.3%). OS rates at 1, 3, and 5 years post-diagnosis were 35%, 17%, and 13%, respectively. Nevertheless, cases with survival time exceeding 1, 3, and 5 years account for 42.4%, 12.1%, and 9.1%, respectively. Tumor size served as a significant independent prognostic factor for PC with DM (Figure 2).

Nomogram for DM prediction
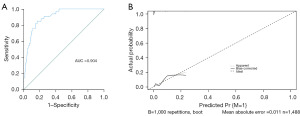
Univariate and multivariate analyses identified larger tumor size [odds ratio (OR) 3.088; 95% confidence interval (CI): 1.281–7.443], higher N stage (N1: OR 5.148; 95% CI: 1.222–21.683; N2: OR 10.298; 95% CI: 3.163–33.533; N3: OR 35.626; 95% CI: 12.425–102.147) as significant risk factors for DM in PC patients, with specific ORs indicating a strong association (Table 2). Based on the two predictors, the nomogram was established, achieving an AUC of 0.904 in predicting metastasis. The internal calibration curve further confirmed its accuracy (Figures 3,4).
Table 2
| Factors | No. of patients | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Age of diagnosis, years | ||||||||
| ≤69 | 883 | 1.0 | 1.0 | |||||
| >69 | 605 | 0.831 | 0.406–1.701 | 0.612 | 1.283 | 0.579–2.844 | 0.540 | |
| Race | ||||||||
| Other | 83 | 1.0 | 1.0 | |||||
| White | 1,276 | 0.598 | 0.178–2.010 | 0.406 | 0.442 | 0.115–1.699 | 0.235 | |
| Black | 129 | 0.420 | 0.069–2.568 | 0.348 | 0.355 | 0.052–2.447 | 0.293 | |
| Marital status | ||||||||
| Other | 579 | 1.0 | 1.0 | |||||
| Married | 909 | 0.760 | 0.380–1.519 | 0.437 | 0.838 | 0.390–1.799 | 0.650 | |
| Tumor site | ||||||||
| Prepuce | 270 | 1.0 | 1.0 | |||||
| Glans & body | 1,086 | 1.567 | 0.541–4.541 | 0.408 | 0.809 | 0.246–2.659 | 0.727 | |
| Other* | 132 | 2.078 | 0.512–8.443 | 0.306 | 0.663 | 0.136–3.240 | 0.612 | |
| Tumor size, mm | ||||||||
| ≤34.5 | 869 | 1.0 | 1.0 | |||||
| >34.5 | 619 | 4.530 | 2.029–10.111 | <0.001 | 3.088 | 1.281–7.443 | 0.012 | |
| Grade | ||||||||
| I & II | 1,136 | 1.0 | 1.0 | |||||
| III & IV | 352 | 2.765 | 1.378–5.545 | 0.004 | 1.449 | 0.667–3.147 | 0.348 | |
| T | ||||||||
| Ta & T1 & T2 | 1,169 | 1.0 | 1.0 | |||||
| T3 & T4 | 319 | 3.578 | 1.787–7.165 | <0.001 | 1.100 | 0.479–2.528 | 0.822 | |
| N | ||||||||
| N0 | 1,191 | 1.0 | 1.0 | |||||
| N1 | 94 | 6.511 | 1.602–26.462 | 0.009 | 5.148 | 1.222–21.683 | 0.026 | |
| N2 | 111 | 13.283 | 4.387–40.284 | <0.001 | 10.298 | 3.163–33.533 | <0.001 | |
| N3 | 92 | 44.767 | 17.149–116.860 | <0.001 | 35.626 | 12.425–102.147 | <0.001 | |
| Surgery | ||||||||
| No | 17 | 1.0 | 1.0 | |||||
| Yes | 1,471 | 0.356 | 0.046–2.765 | 0.323 | 0.293 | 0.031–2.784 | 0.285 | |
| Radiation | ||||||||
| No | 1,357 | 1.0 | 1.0 | |||||
| Yes | 131 | 3.465 | 1.530–7.847 | 0.003 | 0.929 | 0.372–2.318 | 0.874 | |
*, overlapping lesion of penis. HR, hazard ratio; CI, confidence interval.

Nomogram for OS and CSS prediction
Further multivariate analysis identified critical prognostic factors for OS and CSS in PC patients, including age (OR 2.412; 95% CI: 2.064–2.817), race (OR 1.689; 95% CI: 1.116–2.558), tumor size, TNM staging (T stage: OR 1.471; 95% CI: 1.220–1.772; N1: OR 1.602; 95% CI: 1.205–2.130; N2: OR 1.530; 95% CI: 1.152–2.033; N3: OR 1.915; 95% CI: 1.393–2.632; M stage: OR 2.419; 95% CI: 1.580–3.705), and chemotherapy (OR 1.411; 95% CI: 1.077–1.847) related to a worse OS. While age (OR 1.591; 95% CI: 1.267–1.999), tumor size (OR 1.508; 95% CI: 1.200–1.898), pathological grade (OR 1.384; 95% CI: 1.088–1.759), TNM staging (T stage: OR 1.502; 95% CI: 1.162–1.941; N1: OR 2.272; 95% CI: 1.561–3.307; N2: OR 2.341; 95% CI: 1.641–3.339; N3: OR 2.987; 95% CI: 2.026–4.405; M stage: OR 2.700; 95% CI: 1.684–4.328) and chemotherapy (OR 1.677; 95% CI: 1.215–2.315) were associated with a poor CSS (Tables 3,4). Consequently, we constructed nomograms predicting 1-, 3-, and 5-year OSs and CSSs, validated by ROC curves (AUC for OS: 0.72, 0.69, 0.69, respectively; AUC for CSS: 0.73, 0.70, 0.69, respectively) (Figures 5,6). The accuracy of these nomograms was confirmed by internal calibration curves.
Table 3
| Factors | No. of patients | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Age of diagnosis, years | ||||||||
| ≤69 | 883 | 1.0 | 1.0 | |||||
| >69 | 605 | 2.102 | 1.183–2.437 | <0.001 | 2.412 | 2.064–2.817 | <0.001 | |
| Race | ||||||||
| Other | 83 | 1.0 | 1.0 | |||||
| White | 1,276 | 1.139 | 0.934–1.864 | 0.116 | 1.187 | 0.836–1.683 | 0.338 | |
| Black | 129 | 1.585 | 1.055–2.382 | 0.027 | 1.689 | 1.116–2.558 | 0.013 | |
| Marital status | ||||||||
| Other | 579 | 1.0 | 1.0 | |||||
| Married | 909 | 0.781 | 0.673–0.907 | 0.001 | 0.869 | 0.746–1.014 | 0.074 | |
| Tumor site | ||||||||
| Prepuce | 270 | 1.0 | 1.0 | |||||
| Glans & body | 1,086 | 1.187 | 0.974–1.446 | 0.089 | 0.887 | 0.720–1.091 | 0.256 | |
| Other* | 132 | 1.405 | 1.038–1.903 | 0.028 | 0.950 | 0.690–1.307 | 0.751 | |
| Tumor size, mm | ||||||||
| ≤34.5 | 869 | 1.0 | 1.0 | |||||
| >34.5 | 619 | 1.585 | 1.367–1.837 | <0.001 | 1.351 | 1.156–1.580 | <0.001 | |
| Grade | ||||||||
| I & II | 1,136 | 1.0 | 1.0 | |||||
| III & IV | 352 | 1.505 | 1.278–1.772 | <0.001 | 1.081 | 0.907–1.288 | 0.383 | |
| T | ||||||||
| Ta & T1 & T2 | 1,169 | 1.0 | 1.0 | |||||
| T3 & T4 | 319 | 1.669 | 1.411–1.974 | <0.001 | 1.471 | 1.220–1.772 | <0.001 | |
| N | ||||||||
| N0 | 1,191 | 1.0 | 1.0 | |||||
| N1 | 94 | 1.764 | 1.342–2.319 | <0.001 | 1.602 | 1.205–2.130 | 0.001 | |
| N2 | 111 | 1.848 | 1.434–2.380 | <0.001 | 1.530 | 1.152–2.033 | 0.003 | |
| N3 | 92 | 2.830 | 2.175–3.682 | <0.001 | 1.915 | 1.393–2.632 | <0.001 | |
| M | ||||||||
| M0 | 1,455 | 1.0 | 1.0 | |||||
| M1 | 33 | 4.533 | 3.091–6.706 | <0.001 | 2.419 | 1.580–3.705 | <0.001 | |
| Surgery | ||||||||
| No | 17 | 1.0 | 1.0 | |||||
| Yes | 1,471 | 0.822 | 0.440–1.534 | 0.538 | 0.683 | 0.358–1.301 | 0.246 | |
| Radiation | ||||||||
| No | 1,357 | 1.0 | 1.0 | |||||
| Yes | 131 | 1.474 | 1.164–1.868 | 0.001 | 0.871 | 0.655–1.158 | 0.342 | |
| Chemotherapy | ||||||||
| No | 1,318 | 1.0 | 1.0 | |||||
| Yes | 170 | 1.895 | 1.538–2.334 | <0.001 | 1.411 | 1.077–1.847 | 0.012 | |
*, overlapping lesion of penis. OS, overall survival; HR, hazard ratio; CI, confidence interval.
Table 4
| Factors | No. of patients | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Age of diagnosis, years | ||||||||
| ≤69.5 | 883 | 1.0 | 1.0 | |||||
| >69.5 | 605 | 1.304 | 1.051–1.619 | 0.016 | 1.591 | 1.267–1.999 | <0.001 | |
| Race | ||||||||
| Other | 83 | 1.0 | 1.0 | |||||
| White | 1,276 | 1.254 | 0.758–2.074 | 0.379 | 1.215 | 0.729–2.022 | 0.455 | |
| Black | 129 | 1.391 | 0.763–2.536 | 0.281 | 1.573 | 0.854–2.896 | 0.146 | |
| Marital status | ||||||||
| Other | 579 | 1.0 | 1.0 | |||||
| Married | 909 | 0.884 | 0.710–1.100 | 0.268 | 1.039 | 0.829–1.301 | 0.742 | |
| Tumor site | ||||||||
| Prepuce | 270 | 1.0 | 1.0 | |||||
| Glans & body | 1,086 | 1.564 | 1.136–2.153 | 0.006 | 1.062 | 0.759–1.485 | 0.726 | |
| Other* | 132 | 2.012 | 1.290–3.136 | 0.002 | 1.098 | 0.685–1.759 | 0.698 | |
| Tumor size, mm | ||||||||
| ≤34.5 | 869 | 1.0 | 1.0 | |||||
| >34.5 | 619 | 1.942 | 1.566–2.408 | <0.001 | 1.508 | 1.200–1.898 | <0.001 | |
| Grade | ||||||||
| I & II | 1,136 | 1.0 | 1.0 | |||||
| III & IV | 352 | 2.069 | 1.654–2.588 | <0.001 | 1.384 | 1.088–1.759 | 0.008 | |
| T | ||||||||
| Ta & T1 & T2 | 1,169 | 1.0 | 1.0 | |||||
| T3 & T4 | 319 | 2.305 | 1.836–2.895 | <0.001 | 1.502 | 1.162–1.941 | 0.002 | |
| N | ||||||||
| N0 | 1,191 | 1.0 | 1.0 | |||||
| N1 | 94 | 2.885 | 2.021–4.118 | <0.001 | 2.272 | 1.561–3.307 | <0.001 | |
| N2 | 111 | 3.572 | 2.629–4.853 | <0.001 | 2.341 | 1.641–3.339 | <0.001 | |
| N3 | 92 | 5.595 | 4.111–7.614 | <0.001 | 2.987 | 2.026–4.405 | <0.001 | |
| M | ||||||||
| M0 | 1,455 | 1.0 | 1.0 | |||||
| M1 | 33 | 7.182 | 4.687–11.005 | <0.001 | 2.700 | 1.684–4.328 | <0.001 | |
| Surgery | ||||||||
| No | 17 | 1.0 | 1.0 | |||||
| Yes | 1,471 | 0.535 | 0.253–1.132 | 0.102 | 0.495 | 0.228–1.077 | 0.076 | |
| Radiation | ||||||||
| No | 1,357 | 1.0 | 1.0 | |||||
| Yes | 131 | 2.174 | 1.617–2.923 | <0.001 | 0.787 | 0.550–1.125 | 0.189 | |
| Chemotherapy | ||||||||
| No | 1,318 | 1.0 | 1.0 | |||||
| Yes | 170 | 3.445 | 2.695–4.403 | <0.001 | 1.677 | 1.215–2.315 | 0.002 | |
*, overlapping lesion of penis. CSS, cancer specific survival; HR, hazard ratio; CI, confidence interval.


Discussion
PC inflicts patients with physical dysfunction and psychological distress. Incidence of PC varies by age, race and many other factors (12,13). Despite its rarity, its aggressive behavior gives PC high propensity to DM, underscoring the necessity for enhanced management (14). The typical clinical presentations of primary PC include ulcerated lesion, while secondary penile tumors present with a persistent and painful erection. A case of primary PC with painful erection was reported and a poor prognosis was observed in it (3).
In the U.S., patients with PC DM face poor prospects, with a 5-year survival rate of merely 16% in 2022 (8). Previous research has identified age, tumor grade, size, and TNM staging as prognostic factors of PC (15,16). In contrast, here we expanded the array of risk factors from a larger cohort, thus augmenting their accuracy in nomograms in predicting patients’ survival. Uniquely, our research pioneers in establishing a nomogram in predicting PC DM with a high AUC of 0.904, signifying its potential as a prognostic model. Prior research has pointed out that advanced age as a significant prognostic factor for PC. This aligns with the findings of Cancer Research UK showing that 32% of new cases during 2016–2018 aged over 75 years, and the mortality of those aged over 90 years peaked during 2017–2019 (11). Our study reaffirms this, noting a significant portion of patients aged over 69.5 years, accounting for 40.7% of the total cohort. Moreover, tumor size, pivotal in primary surgical strategy determination, was defined as a predicator for PC prognosis and DM, as evidenced by prior studies clarifying the link between smaller tumors (56–78% patients with tumor ≤5 cm) and improved 5-year survival rates (17). Additionally, Pinkheaw et al. have found that only advanced primary tumor stages stand out as significant prognostic markers for disease-free survival (18). In their previous work, Yang et al. explored the prognostic implications of various factors in 906 PC cases during 2010–2015 (19). Our study involved a longer array of risk factors with a larger sample size, significantly enhancing the reliability of our prognostic model. The predictive strength of our nomograms was proven by AUC values and calibration curves of OS and CSS. Furthermore, the univariate and multivariate analyses confirmed advanced age as a key prognostic indicator for poor outcomes in PC patients. Recent studies have identified cardiovascular diseases as the primary non-cancerous cause of mortality in PC patients. As patients’ age grows, the occurrences of chronic conditions, such as diabetes, cardiac disorders, and hypertension, increase the risk of mortality (1,20). Notably, our findings identified tumor grade as a significant risk factor for CSS rather than OS. Particularly, patients with higher-grade tumors (Grade III & IV) demonstrated poorer CSS outcomes, compared to those with lower-grade tumors (Grade I & II), aligning with the previous results from studies on poorly differentiated and undifferentiated carcinomas. Despite gradual advancements in treatments, the paramountcy of surgery remains (19,21). The European Association of Urology-American Society of Clinical Oncology Collaborative Guideline advocates complete tumor removal, while preserving sexual and urinary functions as primary treatment goals (11). A previous clinical study in 2021 indicated that palliative care does not impact mortality rates (22). Contrastingly, our research did not corroborate treatment as an independent prognostic factor, potentially due to our limited sample size.
Our study reaffirms the adverse prognosis associated with DM in PC patients, emphasizing the value of early detection and tailored treatment (23). Notably, initial diagnosis shows a DM incidence of 1% to 10% in prior studies. Our nomogram may be expected to enhance the management of PC via precisely predicting DM. Consequently, DM was pathologically confirmed in 2.2% of our cases, with 1-, 3-, and 5-year survival rates of 35%, 17%, and 13%, respectively.
Notably, our analysis established N stage as a critical predictor of metastasis, signifying the role of lymph node involvement. The hazard ratio of N stage escalated with the degree of lymph node involvement. Furthermore, tumor size was proven to be a key predictor in our DM-predicting nomogram. In contrast, tumor location, though considered as a risk factor, showed minimal impact on prognosis or metastasis rate in PC (24,25). Our study acknowledges certain limitations, including the lack of external validation and incomplete data on metastatic sites and metastatic patterns. It requires further research to improve the performance of our nomogram.
Conclusions
This study highlighted the significant association of larger tumor sizes and higher N stages with an increased DM risk. Additionally, age, tumor size, TNM staging were identified as independent prognostic factors. The nomograms based on these factors exhibited a good performance in predicting PC prognosis and DM, thus benefiting disease management.
Acknowledgments
Funding: This study was supported in part by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-24-92/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-24-92/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-24-92/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhan X, Chen L, Jiang M, et al. Get insight into the cause of death distribution and epidemiology of penile squamous cell carcinoma: A population-based study. Cancer Med 2022;11:2308-19. [Crossref] [PubMed]
- Bourlon MT, Verduzco-Aguirre H, Molina E, et al. Patterns of Treatment and Outcomes in Older Men With Penile Cancer: A SEER Dataset Analysis. Front Oncol 2022;12:926692. [Crossref] [PubMed]
- Unal S, Alijla AS, Ocal BG, et al. Primary Penile Squamous Cell Cancer-Related Malignant Priapism in a Cystectomized Patient: A Case Report. Cureus 2022;14:e31875. [Crossref] [PubMed]
- Graafland NM, Verhoeven RH, Coebergh JW, et al. Incidence trends and survival of penile squamous cell carcinoma in the Netherlands. Int J Cancer 2011;128:426-32. [Crossref] [PubMed]
- Barnes KT, McDowell BD, Button A, et al. Obesity is associated with increased risk of invasive penile cancer. BMC Urol 2016;16:42. [Crossref] [PubMed]
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol 2009;27:141-50. [Crossref] [PubMed]
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Deng X, Liu Y, Zhan X, et al. Trends in Incidence, Mortality, and Survival of Penile Cancer in the United States: A Population-Based Study. Front Oncol 2022;12:891623. [Crossref] [PubMed]
- Ritch CR, Soodana-Prakash N, Pavan N, et al. Racial disparity and survival outcomes between African-American and Caucasian American men with penile cancer. BJU Int 2018;121:758-63. [Crossref] [PubMed]
- Bandini M, Spiess PE, Pederzoli F, et al. A risk calculator predicting recurrence in lymph node metastatic penile cancer. BJU Int 2020;126:577-85. [Crossref] [PubMed]
- Brouwer OR, Albersen M, Parnham A, et al. European Association of Urology-American Society of Clinical Oncology Collaborative Guideline on Penile Cancer: 2023 Update. Eur Urol 2023;83:548-60. [Crossref] [PubMed]
- Fu L, Tian T, Yao K, et al. Global Pattern and Trends in Penile Cancer Incidence: Population-Based Study. JMIR Public Health Surveill 2022;8:e34874. [Crossref] [PubMed]
- Hansen BT, Orumaa M, Lie AK, et al. Trends in incidence, mortality and survival of penile squamous cell carcinoma in Norway 1956-2015. Int J Cancer 2018;142:1586-93. [Crossref] [PubMed]
- Veeratterapillay R, Teo L, Asterling S, et al. Oncologic Outcomes of Penile Cancer Treatment at a UK Supraregional Center. Urology 2015;85:1097-103. [Crossref] [PubMed]
- Okutan M, Boran F, Ergün A, et al. Hydrophobic surface modification and characterization of melamine foam. Turk J Chem 2023;47:591-604. [Crossref] [PubMed]
- Yang J, Pan Z, He Y, et al. Competing-risks model for predicting the prognosis of penile cancer based on the SEER database. Cancer Med 2019;8:7881-9. [Crossref] [PubMed]
- Krishna S, Shanbhogue K, Schieda N, et al. Role of MRI in Staging of Penile Cancer. J Magn Reson Imaging 2020;51:1612-29. [Crossref] [PubMed]
- Pinkheaw N, Sathitruangsak C, Tanthanuch M, et al. Real world data of recurrent and survival rates of penile cancer patients in Songklanagarind hospital: Tumor stage as a predictor for disease-free survival. Int J Urol 2024;31:144-53. [Crossref] [PubMed]
- Yang S, Chang W, Zhang B, et al. Development and validation of a predictive model for penile cancer based on the surveillance, epidemiology, and end results database and multi-center cases. J Cancer Res Clin Oncol 2023;149:13665-76. [Crossref] [PubMed]
- Pierpont ME, Brueckner M, Chung WK, et al. Genetic Basis for Congenital Heart Disease: Revisited: A Scientific Statement From the American Heart Association. Circulation 2018;138:e653-711. [Crossref] [PubMed]
- Boehm WU, Piontek D, Latarius S, et al. The Clinical Complexity of Penile Cancer: Current Clinical-Epidemiological Data from the Database of the Free State of Saxony/Germany. Urol Int 2022;106:706-15. [Crossref] [PubMed]
- Davaro FM, Weinstein D, Siddiqui SA, et al. A Lack of Palliative Therapy Use in Patients With Advanced Penile Cancer. J Palliat Care 2021;36:98-104. [Crossref] [PubMed]
- Chahoud J, Kohli M, Spiess PE. Management of Advanced Penile Cancer. Mayo Clin Proc 2021;96:720-32. [Crossref] [PubMed]
- Reyes ME, Borges H, Adjao MS, et al. Novel Prognostic Models for Patients With Penile Carcinoma. Cancer Control 2020;27:1073274820924728. [Crossref] [PubMed]
- Li K, Le X, Wang J, et al. Tumor Location May Independently Predict Survival in Patients With M0 Squamous Cell Carcinoma of the Penis. Front Oncol 2022;12:927088. [Crossref] [PubMed]


