
Diagnostic value of oxidation-reduction potential for male infertility: a systematic review and meta-analysis
Highlight box
Key findings
• This study showed that oxidation-reduction potential (ORP) levels were higher in infertile men than in fertile men and that ORP has a high diagnostic value in the diagnosis of male infertility.
What is known and what is new?
• The MiOXSYS system provides a static ORP that represents the actual redox equilibrium in a given sample, the higher the redox potential, the higher the oxidative stress.
• The validity of ORP for diagnosing male infertility has been evaluated in the current literature, but with varying results.
• In our evidence-based study on the diagnosis of male infertility by ORP measurement, we found that ORP has high sensitivity and specificity for diagnosing male infertility.
What is the implication, and what should change now?
• There is substantial evidence that ORP can be a valid and accurate diagnostic marker for male infertility patients. A new marker for male infertility screening can be used for evaluation, adding more information to the traditional semen analysis.
Introduction
An increasing number of couples worldwide are affected by infertility. Male factors affect 20% to 70% of couples with infertility (1). There are many causes of male infertility, and semen oxidative stress (OS) significantly contributes to the etiology of male infertility. Reactive oxygen species (ROS) are highly reactive oxidative radical reagents, including superoxide anion radicals (O2·−), hydrogen peroxide (H2O2), nitric oxide (NO·), and hydroxyl radicals (·OH), which in spermatozoa originate mainly from activated leukocytes in seminal plasma and mitochondria in spermatozoa. Leukocytospermia is one of the major causes for increased seminal OS. OS occurs when there is an overproduction of ROS or a deficiency of antioxidants, resulting in a disruption of the balance of oxidants and reducing agents (2). In most cases, sperm DNA damage is considered to be caused by oxidative and lipid peroxidation, and is associated with reduced fertilisation rates and increased abortion rates (3). During regular physiological fertilization, the redox potential is balanced. ROS levels are associated with physiological functions such as highly active sperm motility, energy acquisition, and acrosome response (2). However, excess ROS produces OS, and due to the sensitivity of spermatozoa to OS, oxidative and inflammatory processes lead to reduced sperm viability and sperm DNA integrity, which can severely affect male fertility (4,5). OS alters semen parameters (6,7). therefore, OS measurements indicate semen quality (8,9). Due to the multiple factors contributing to male infertility, the solitary semen parameter is not available as a valid biomarker (10,11). Numerous direct and indirect methods have been introduced to evaluate semen OS. However, measurement of only a single marker of oxidant or reducing agent can lead to a lack of standardization of results (12). The MiOXSYS system is a new technology that is based on the measurement of electronic potentials. By measuring electron transfer, a thorough measurement of oxidants and antioxidants is achieved, avoiding many drawbacks of conventional measurement methods (13,14). MiOXSYS is a constant current meter-based technology consisting of an analyser and disposable sensor. It provides a static oxidation-reduction potential (ORP) that represents the actual redox equilibrium in a given sample; the higher the redox potential, the higher the OS. Therefore, ORP reflects the oxidised state of a chemical system, including cellular systems. Biological fluids, including semen, also have an inherent ORP, which may be of clinical value as it relates to the state of biological and/or pathological processes. Thus, ORP can provide information about the health status of a patient. A high ORP level indicates OS, which is negatively associated with sperm parameters and can differentiate between the infertile and fertile males (15-17). ORP substitutes the need to measure each component (oxidants and antioxidants) separately, delivering a rapid and useful indicator that can be a critical addition to semen analysis for the determination of semen quality as well as fertility status (18). The validity of ORP for diagnosing male infertility has been evaluated in the current literature, but with varying results. In this article, we used a meta-analysis to quantitatively and comprehensively evaluate the current studies related to ORP diagnosis to investigate further the clinical value of ORP in diagnosing male infertility. We present this article in accordance with the PRISMA reporting checklist (19) (available at https://tau.amegroups.com/article/view/10.21037/tau-24-32/rc).
Methods
Literature search strategy
Systematic reviews and meta-analyses are expected to be registered in order to avoid publication bias (20). Hence, we registered the study with PROSPERO (No. CRD42022358030). A comprehensive systematic search of relevant publications up to April 2023 was performed in PubMed, Web of Science, Embase, and the Cochrane Library. The search consisted of the following terminologies: (“sterility, male” or “male infertility” or “subfertility, male” or “male reproduction” or “male fertility”) and (“redox potential”). There were no language restrictions when searching for documents. The necessary reference tracking of relevant literature was performed to avoid missing literature.
Inclusion and exclusion criteria
The literature was screened according to the Cochrane Collaboration Network’s inclusion criteria for diagnostic trials. The inclusion criteria were: (I) studies were prospective or retrospective literature related to male infertility; (II) patients were ≥18 years old; (III) the ORP extraction values for the diagnosis of male infertility include true positive (TP), false positive (FP), true negative (TN), and false negative (FN). These extraction values can be obtained directly from the original literature or indirectly calculated from the literature. Exclusion criteria: (I) repeated use of literature data; (II) literature data with incomplete extraction; (III) animal experiments, literature reviews, case reports, and conference proceedings.
Data extraction and quality evaluation
The two researchers extracted the information separately, and the extraction process was kept independently collected and organized. When disagreement occurred, one additional researcher was added. If there was still disagreement, it was fed back to the evidence-based medicine research discussion group to discuss and negotiate a consensus opinion. Information extracted from the study included first author, publication time, gold standard, number of infections, threshold, and specific values of TP, FP, TN, and FN were calculated directly or indirectly and summarized to produce a table. The quality of the included literature was evaluated using quality assessment of diagnostic accuracy studies (QUADAS) criteria, and the questions of the criteria were given a “yes”, “no”, or “unclear” rating. In case of disagreement, the resolution method was as described above.
Statistical analysis
Heterogeneity, pooled sensitivity, pooled specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), odds ratio (OR), diagnostic odds ratio (DOR) were analyzed using the Metadisc 1.4 medical software package, and all with their 95% confidence intervals (CI) as effect indicators. The subject receiver operating characteristic (SROC) curve were drawn, and the area under the curve (AUC) and Q* index were used for determining the diagnostic value. Threshold effects were assessed by the Spearman correlation coefficient between the logarithm of sensitivity and the logarithm of (1 − specificity), and non-threshold effects were examined by calculating the Cochrane-Q value of DOR. Deeks plots could be made for publication bias detection with the Stata 12.0 software, and a difference of P<0.05 was seen as statistically significant.
Results
Search strategy and cohort characteristics
A total of 359 papers were obtained after a scientifically standardized search of various databases and the necessary reference tracking of relevant literature all of which were imported into the NoteExpress Literature Management Software for initial screening, and 311 papers were obtained by excluding duplicates. Then 285 irrelevant articles were excluded by reading the titles and abstracts, 19 articles were excluded after full-text reading of the remaining 26 eligible articles, and 7 papers were finally included in the meta-analysis (16,17,21-25) (Figure 1, Table 1).
Table 1
| Study | Infertility criterion | Country | Publish time | Study year | TP, n | FP, n | TN, n | FN, n | Total sample, n | Cut-off (mV/106 sperm/mL) | AUC | Sensitivity | Specificity | Subject | Index | Predesign |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agarwal (16) | Proven infertility | USA | 2017 | Aug 2015 to Aug 2016 | 360 | 26 | 75 | 234 | 695 | 1.42 | 0.703 | 0.61 | 0.74 | Yes | Yes | No |
| Majzoub (21) | Abnormal sperm morphology | Qatar | 2018 | Jun 2016 to Jun 2017 | 841 | 24 | 76 | 328 | 1,269 | 1.73 | 0.8 | 0.72 | 0.76 | Yes | Yes | Yes |
| Arafa (22) | Abnormal semen quality | Qatar | 2018 | NA | 209 | 11 | 39 | 156 | 415 | 1.41 | 0.68 | 0.57 | 0.78 | No | Yes | Yes |
| Agarwal (23) | Abnormal semen parameters | USA | 2019 | NA | 1,857 | 118 | 81 | 36 | 2,092 | 1.34 | 0.765 | 0.98 | 0.41 | No | Yes | No |
| Karabulut (17) | Abnormal semen parameters | Turkey | 2021 | Apr 2018 to Aug 2019 | 29 | 27 | 55 | 10 | 121 | 0.415 | 0.688 | 0.74 | 0.67 | Yes | No | Yes |
| Majzoub (25) | Abnormal motile sperm count | Qatar | 2020 | Jan 2015 to Jan 2016 | 1,051 | 27 | 83 | 117 | 1,278 | 2.34 | 0.9 | 0.90 | 0.75 | Yes | Yes | No |
| Joao (24) | Abnormal semen parameters | Canada | 2022 | Oct 2017 to Oct 2020 | 331 | 36 | 98 | 243 | 708 | 0.79 | NA | 0.58 | 0.73 | Yes | No | No |
TP, the true positive value; FP, the false positive value; TN, the true negative value; FN, the false negative value; AUC, area under the subject working characteristic curve; Index, whether the inclusion exclusion criteria are depicted in detail; Subject, whether the population to be evaluated is depicted in detail; Predesign, whether it is a prospective study; NA, not applicable.
Quality of studies
For the current study, we evaluated the methodological quality of the included studies using the “Risk of Bias Assessment” tool recommended by the Cochrane Handbook 5.0, which has clear criteria for each judgment, which can reduce the influence of subjective factors on the assessor and ensure the reliability of the assessment results. Finally, we concluded that the included literature’s overall quality was moderate to high. Six studies showed a low risk of subject selection bias; five evaluated diagnostic tests showed a low risk of bias; five “gold standard” studies were at low risk of bias. Furthermore, six studies had a low risk of subject flow and progression bias (Figure 2).
Heterogeneity test
The Spearman correlation coefficient between the log of sensitivity and the log of 1 − specificity using the Metadisc 1.4 medical software analysis package can be derived as 0.321, P=0.48, thus indicating the absence of a threshold effect and plotting the DOR forest (Figure 3). The diagnostic and combined diagnostic ratios are not distributed along the same straight line, and Cochrane-Q =88.27, P<0.05, which indicates the presence of heterogeneity due to non-threshold effects. In order to further investigate the sources of heterogeneity, we used meta-regression to explore the sources of heterogeneity using three covariates: whether the population to be evaluated was described in detail, whether the trial to be evaluated was described in detail, and whether it was a prospective study, and the results suggested that the above three covariates were not the primary sources of heterogeneity (Table 2).
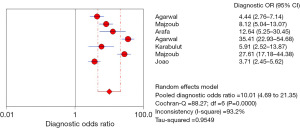
Table 2
| Variable | Coefficient | SE | P value | RDOR | 95% CI |
|---|---|---|---|---|---|
| Cte. | 1.078 | 0.8335 | 0.3250 | – | – |
| S | 0.464 | 0.1690 | 0.1109 | – | – |
| Subject | 0.513 | 0.6407 | 0.5074 | 1.67 | 0.11–26.30 |
| Index | 0.647 | 0.5482 | 0.3592 | 1.91 | 0.18–20.20 |
| Predesign | 0.080 | 0.5239 | 0.8921 | 1.08 | 0.11–10.33 |
SE, standard error; RDOR, relative diagnostic odds ratio; Cte., common table expressions; CI, confidence interval; S, sample standard deviation.
Meta-analysis results
A random-effects model was used for further combined effect sizes given the high heterogeneity among the included literature data, and the combined seven papers had a sensitivity of 0.81 (95% CI: 0.80–0.82); a specificity of 0.66 (95% CI: 0.63–0.69); a PLR of 2.57 (95% CI: 1.89–3.49); an NLR of 0.28 (95% CI: 0.17–0.45); the AUC of SROC was 0.8, and the Q* index was 0.74 (Figures 4-8) (note: the order of authorship is the same as in Table 1).



Publication bias
With the development of evidence-based medicine, the adverse effects of publication bias have long drawn the attention of researchers. At the same time, more and more methods to identify publication bias, such as funnel plots, are commonly used in RCT trials. For the systematic diagnostic evaluation of this study, we used the Deeks method, often used in diagnostic studies, to detect publication bias. The Deeks plot was drawn using Stata 12.0 software showing P=0.33, suggesting that no publication bias was seen in the literature included in the meta-analysis (Figure 9).

Discussion
This is the first evidence-based study to diagnose male infertility by ORP measurement. Infertile men showed higher ORP levels than fertile men. This study demonstrates that ORP has a high diagnostic value in diagnosing male infertility. A new marker for male infertility screening can be used for evaluation, adding more information to the traditional semen analysis.
Male infertility is one of the most common causes, and the growing prevalence of male infertility worldwide poses a challenge for healthcare professionals and couples preparing to become pregnant (26). Male infertility is the inability of a couple preparing for pregnancy to conceive naturally due to male factors after 1 year of regular intercourse without using any contraception. Currently, there are no specific laboratory indicators for early diagnosis, and determining the infertility outcome of a patient by semen analysis alone often delays the optimal treatment of the patient. Though guidelines and several published predictive models for aiding the early diagnosis and treatment of male infertility exist, diagnostic muddle persists (27,28). A necessary step in early comprehensive treatment is the need for early diagnosis and a thorough and systematic assessment of the severity of the patient’s condition. Numerous studies have searched for the ideal biomarker. However, it is often difficult to apply it clinically, and only inexpensive and easily accessible markers are available, and the ultimate effect needs to be validated by extensive clinical trials. The current preference for male infertility is still for a joint diagnosis, and the specific significance of various biomarkers can only be sought in the clinic for reasonable conclusions. Numerous studies have evaluated the diagnostic value of potential factors in seminal plasma. However, the conventical semen parameters are still the most common and widely used reference in male infertility today—the most studied biomarkers, including miRNA, DNA fragmentation index, etc. (29,30). Popular studies are now proposing that STL may be used as a biomarker to predict the outcome of male infertility and may reflect the severity and pregnancy rate in couples with male infertility factors (31); in addition, exploring the diagnostic relevance of multiple biomarkers when used in combination and conducting work on the development of multi-point detection kits, which can rapidly and reliably detect semen biomarkers, which may have tremendous potential for the diagnosis of male infertility. However, the independent diagnostic value in combined diagnostic indicators should not be neglected. Hence, this study was conducted to systematically estimate the independent diagnostic efficacy of ORP for male infertility, with the hope of providing clinical evidence that ORP can be used in combination with other sensitive biomarkers in diagnosing male infertility.
Treatment of precursors to male infertility may involve pharmacological or surgical interventions or psychological counseling, which can be financially and emotionally expensive for couples preparing for pregnancy. Clinicians use semen analysis to assess a man’s ability to fertilize (32,33). However, it does not assess all sperm functions, and a man’s “true” fertility potential may be underestimated. Despite some progress made with semen analysis techniques, like computer-assisted sperm analysis (CASA), the results of these new techniques are highly variable, the investment in equipment maintenance and manual training is expensive, and the accuracy is not significantly improved compared to traditional manual semen analysis (34-36). In addition, the predictive power of single semen analysis is relatively poor, mainly due to differences in individual semen parameters (37,38). In addition to the vulnerability of semen measurements to laboratory methods and subjective human error (9,15,39), semen parameters are associated with external environmental and lifestyle factors that may change over time in individuals, making single semen analysis an unreliable indicator (40,41). OS also has a critical role in the semen parameters of infertile men. OS is a cascade of pathological effects that occur when the body produces an excess of various reactive molecules, such as ROS, in response to unhelpful stimuli, resulting in an imbalance in the body’s total antioxidant system. There are three sources of ROS in sperm: sperm mitochondria, cytosolic L-amino acid oxidase, and plasma membrane nicotinamide adenine dinucleotide phosphate oxidase. All drive various physiological changes in sperm capacitation by stimulating the cyclic adenosine monophosphate/protein kinase alpha phosphorylation cascade and activating extracellular signal-regulated kinase-like proteins. Excess ROS can disrupt oxidative defense systems and cause OS damage, resulting in impaired sperm function, which is the underlying cause of male infertility.
MiOXSYS is a measurement method based upon electron motion that provides information on the complete redox activity of semen (12,42,43). MiOXSYS requires only a small quantity of samples (about 30 µL of semen) and produces results over a short period based on the patient’s physiological balance of oxidants and antioxidants (44,45). The results are expressed as ORP. MiOXSYS does not affect semen ORP levels, is simple to perform, stable in repeated measurements, and easy to use in clinical practice (13,18). Monitoring ORP is the latest diagnostic method for male infertility in the course of technological development. It can diagnose male infertility and reflect the efficacy of treatment for male infertility (18,36). If the semen analysis parameters are within the normal range and MiOXSYS analysis shows a high positive predictive value for ORP. In this case, clinicians should be vigilant to avoid incorrectly predicting the outcome of male infertility (16,46).
After rigorous screening, in this study, we finally included seven papers, and the overall quality of the literature was moderate to high after assessing literature bias (14). To test for the presence of threshold effects in the literature, we analyzed the Spearman correlation coefficient test between the logarithm of sensitivity and logarithm of (1 − specificity) with the Metadisc 1.4 medical software package, and the threshold effect was P=0.48, so there was no threshold effect in this study. Cochrane-Q =88.27, P<0.05, suggesting the existence of heterogeneity due to non-threshold effects, and based on the high level of heterogeneity, a random effects model was used in this study. To further investigate the sources of heterogeneity due to non-threshold effects, we assigned three covariates: the index (whether the inclusion exclusion criteria are depicted in detail), the subject (whether the population to be evaluated is depicted in detail), the Predesign (whether it is a prospective study) to explore the sources of heterogeneity using meta-regression. The P value was 0.50 for the subject, 0.35 for the index, and 0.89 for Predesign. All the three P values were >0.05, which did not indicate that these three causes were the primary sources of heterogeneity, and further investigation of the sources of heterogeneity would be needed.
The combined sensitivity and specificity were 0.81 (95% CI: 0.80–0.82) and 0.66 (95% CI: 0.63–0.69), respectively. As we know, the higher the sensitivity, the less the leakage rate, which is the ability of the diagnostic test to distinguish patients with the target disease, and the higher the specificity, the lower the misdiagnosis rate, which is the ability of the diagnostic test to distinguish non-target patients. Suppose the specificity of the diagnostic test used for differential diagnosis reaches 85% or more. In that case, it can be called a diagnostic test with high specificity and can be used for a definite diagnosis to determine the disease. In the present study, the combined sensitivity was generally good. However, it did not reach the point of clinical expectation. However, we cannot deny its pointing role in clinical diagnosis, and the value of ORP sensitivity needs to be judged in the context of clinical specifics. PLR was 2.57 (95% CI: 1.89–3.49); NLR was 0.28 (95% CI: 0.17–0.45); the PLR is the ratio of the true positive rate to the true negative rate, and a larger PLR indicates a lower rate of misdiagnosis of the diagnostic test and a higher likelihood of switching to the target disease; NLR is the ratio of the false negative rate to the true negative rate, and a lower NLR indicates a lower rate of missed diagnostic tests and a lower likelihood of having the target disease. Because of the large difference in the threshold values, we plotted the SROC curve, a curve based on ROC independent of heterogeneity and threshold. We integrated the information of sensitivity and specificity, which can comprehensively evaluate the accuracy of diagnostic experiments. The AUC of SROC in this meta-analysis was 0.8, and the Q* index was 0.74.
The study still showed several limitations: (I) we did not distinguish between the population of male infertility patients, including the group with proven infertility and the group with abnormal semen. In future studies, we can increase the sample size and discuss each type separately more carefully to further eliminate heterogeneity; (II) for the ORP testing environment; we cannot exclude the differences caused by the testing population’s techniques, usage methods, and surroundings; although the quality of the literature is good, we cannot ignore the impact of the small amount of literature and limited information.
Conclusions
OS leads to elevated levels of ROS in male infertility patients. More clinical attention should be given to the combined assessment of semen OS and semen parameters in male infertility patients. The MiOXSYS system’s measurement of ORP is a direct measurement of OS in semen, expressing the balance between all oxidants and all available antioxidants in the sample, with the advantages of simplicity of operation and stability of repeated measurements. There is substantial evidence that ORP can be a valid and accurate diagnostic marker for male infertility patients. We do not deny the significance of semen analysis in male infertility. We usually combine ORP with semen analysis and other biomarkers in clinical practice. We expect to find combined diagnostic indicators for clinical application, such as combined tests with Sperm DNA fragmentation index and miRNA, to improve the diagnostic sensitivity of male infertility and assess the extent of the condition. Considering the study’s limitations, we still need to expand the sample size to confirm its clinical diagnostic value.
Acknowledgments
We thank Mr. X.L., Centre for Evidence-Based Medicine, Lanzhou University, for his assistance in checking the English language.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-24-32/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-24-32/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-24-32/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cioppi F, Rosta V, Krausz C. Genetics of Azoospermia. Int J Mol Sci 2021;22:3264. [Crossref] [PubMed]
- Bergsma AT, Li HT, Eliveld J, et al. Local and Systemic Oxidative Stress Biomarkers for Male Infertility: The ORION Study. Antioxidants (Basel) 2022;11:1045. [Crossref] [PubMed]
- Gualtieri R, Kalthur G, Barbato V, et al. Sperm Oxidative Stress during In Vitro Manipulation and Its Effects on Sperm Function and Embryo Development. Antioxidants (Basel) 2021;10:1025. [Crossref] [PubMed]
- Smits RM, Mackenzie-Proctor R, Yazdani A, et al. Antioxidants for male subfertility. Cochrane Database Syst Rev 2019;3:CD007411. [PubMed]
- Barati E, Nikzad H, Karimian M. Oxidative stress and male infertility: current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell Mol Life Sci 2020;77:93-113. [Crossref] [PubMed]
- Uribe P, Meriño J, Matus CE, et al. Autophagy is activated in human spermatozoa subjected to oxidative stress and its inhibition impairs sperm quality and promotes cell death. Hum Reprod 2022;37:680-95. [Crossref] [PubMed]
- Zhang G, Jiang F, Chen Q, et al. Associations of ambient air pollutant exposure with seminal plasma MDA, sperm mtDNA copy number, and mtDNA integrity. Environ Int 2020;136:105483. [Crossref] [PubMed]
- Kavoussi PK, Gilkey MS, Machen GL, et al. Varicocele Repair Improves Static Oxidation Reduction Potential as a Measure of Seminal Oxidative Stress Levels in Infertile Men: A Prospective Clinical Trial Using the MiOXSYS System. Urology 2022;165:193-7. [Crossref] [PubMed]
- Symeonidis EN, Evgeni E, Palapelas V, et al. Redox Balance in Male Infertility: Excellence through Moderation-"Mέτρον ἄριστον". Antioxidants (Basel) 2021;10:1534. [Crossref] [PubMed]
- Moazamian A, Gharagozloo P, Aitken RJ, et al. Oxidative stress and reproductive function: Sperm telomeres, oxidative stress, and infertility. Reproduction 2022;164:F125-33. [Crossref] [PubMed]
- Silva R, Carrageta DF, Alves MG, et al. Antioxidants and Male Infertility. Antioxidants (Basel) 2022;11:1152. [Crossref] [PubMed]
- Douglas C, Parekh N, Kahn LG, et al. A Novel Approach to Improving the Reliability of Manual Semen Analysis: A Paradigm Shift in the Workup of Infertile Men. World J Mens Health 2021;39:172-85. [Crossref] [PubMed]
- Agarwal A, Sharma R, Roychoudhury S, et al. MiOXSYS: a novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertil Steril 2016;106:566-573.e10. [Crossref] [PubMed]
- Vassiliou A, Martin CH, Homa ST, et al. Redox potential in human semen: Validation and qualification of the MiOX(sys) assay. Andrologia 2021;53:e13938. [Crossref] [PubMed]
- Cicek OSY, Kaya G, Alyuruk B, et al. The association of seminal oxidation reduction potential with sperm parameters in patients with unexplained and male factor ınfertility. Int Braz J Urol 2021;47:112-9. [Crossref] [PubMed]
- Agarwal A, Arafa M, Chandrakumar R, et al. A multicenter study to evaluate oxidative stress by oxidation-reduction potential, a reliable and reproducible method. Andrology 2017;5:939-45. [Crossref] [PubMed]
- Karabulut S, Korkmaz O, Yılmaz E, et al. Seminal oxidation-reduction potential as a possible indicator of impaired sperm parameters in Turkish population. Andrologia 2021;53:e13956. [Crossref] [PubMed]
- Martins AD, Agarwal A. Oxidation reduction potential: a new biomarker of male infertility. Panminerva Med 2019;61:108-17. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Ge L, Tian JH, Li YN, et al. Association between prospective registration and overall reporting and methodological quality of systematic reviews: a meta-epidemiological study. J Clin Epidemiol 2018;93:45-55. [Crossref] [PubMed]
- Majzoub A, Arafa M, Mahdi M, et al. Oxidation-reduction potential and sperm DNA fragmentation, and their associations with sperm morphological anomalies amongst fertile and infertile men. Arab J Urol 2018;16:87-95. [Crossref] [PubMed]
- Arafa M, Agarwal A, Al Said S, et al. Semen quality and infertility status can be identified through measures of oxidation-reduction potential. Andrologia 2018;50:e12881. [Crossref] [PubMed]
- Agarwal A, Panner Selvam MK, Arafa M, et al. Multi-center evaluation of oxidation-reduction potential by the MiOXSYS in males with abnormal semen. Asian J Androl 2019;21:565-9. [Crossref] [PubMed]
- Joao F, Duval C, Bélanger MC, et al. Reassessing the interpretation of oxidation-reduction potential in male infertility. Reprod Fertil 2022;3:67-76. [Crossref] [PubMed]
- Majzoub A, Arafa M, El Ansari W, et al. Correlation of oxidation reduction potential and total motile sperm count: its utility in the evaluation of male fertility potential. Asian J Androl 2020;22:317-22. [Crossref] [PubMed]
- Heng FW, Shorey S. Experiences of endometriosis-associated infertility among women and their partners: A qualitative systematic review. J Clin Nurs 2022;31:2706-15. [Crossref] [PubMed]
- Khodabandelu S, Basirat Z, Khaleghi S, et al. Developing machine learning-based models to predict intrauterine insemination (IUI) success by address modeling challenges in imbalanced data and providing modification solutions for them. BMC Med Inform Decis Mak 2022;22:228. [Crossref] [PubMed]
- Pang WK, Amjad S, Ryu DY, et al. Establishment of a male fertility prediction model with sperm RNA markers in pigs as a translational animal model. J Anim Sci Biotechnol 2022;13:84. [Crossref] [PubMed]
- Blaseg E, Von Wald T, Hansen KA. Vitamin D levels and human sperm DNA fragmentation: a prospective, cohort study. Basic Clin Androl 2022;32:14. [Crossref] [PubMed]
- Pantos K, Grigoriadis S, Tomara P, et al. Investigating the Role of the microRNA-34/449 Family in Male Infertility: A Critical Analysis and Review of the Literature. Front Endocrinol (Lausanne) 2021;12:709943. [Crossref] [PubMed]
- Gentiluomo M, Luddi A, Cingolani A, et al. Telomere Length and Male Fertility. Int J Mol Sci 2021;22:3959. [Crossref] [PubMed]
- Wang H, McGoldrick LL, Chung JJ. Sperm ion channels and transporters in male fertility and infertility. Nat Rev Urol 2021;18:46-66. [Crossref] [PubMed]
- Szczykutowicz J, Kałuża A, Kaźmierowska-Niemczuk M, et al. The Potential Role of Seminal Plasma in the Fertilization Outcomes. Biomed Res Int 2019;2019:5397804. [Crossref] [PubMed]
- Finelli R, Leisegang K, Tumallapalli S, et al. The validity and reliability of computer-aided semen analyzers in performing semen analysis: a systematic review. Transl Androl Urol 2021;10:3069-79. [Crossref] [PubMed]
- Vij SC, Agarwal A. Editorial on "An automated smartphone-based diagnostic assay for point-of-care semen analysis". Ann Transl Med 2017;5:507. [Crossref] [PubMed]
- Gill K, Kups M, Harasny P, et al. The Negative Impact of Varicocele on Basic Semen Parameters, Sperm Nuclear DNA Dispersion and Oxidation-Reduction Potential in Semen. Int J Environ Res Public Health 2021;18:5977. [Crossref] [PubMed]
- Ghayda RA, Cannarella R, Calogero AE, et al. Artificial Intelligence in Andrology: From Semen Analysis to Image Diagnostics. World J Mens Health 2024;42:39-61. [Crossref] [PubMed]
- Panner Selvam MK, Moharana AK, Baskaran S, et al. Current Updates on Involvement of Artificial Intelligence and Machine Learning in Semen Analysis. Medicina (Kaunas) 2024;60:279. [Crossref] [PubMed]
- Elbardisi H, Finelli R, Agarwal A, et al. Predictive value of oxidative stress testing in semen for sperm DNA fragmentation assessed by sperm chromatin dispersion test. Andrology 2020;8:610-7. [Crossref] [PubMed]
- Boeri L, Pozzi E, Capogrosso P, et al. Infertile men with semen parameters above WHO reference limits at first assessment may deserve a second semen analysis: Challenging the guidelines in the real-life scenario. PLoS One 2023;18:e0280519. [Crossref] [PubMed]
- Xu R, Zhong Y, Li R, et al. Association between exposure to ambient air pollution and semen quality: A systematic review and meta-analysis. Sci Total Environ 2023;870:161892. [Crossref] [PubMed]
- Agarwal A, Roychoudhury S, Sharma R, et al. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: clinical utility in male factor infertility. Reprod Biomed Online 2017;34:48-57. [Crossref] [PubMed]
- Galimov SN, Gromenko JY, Bulygin KV, et al. The level of secondary messengers and the redox state of NAD(+)/NADH are associated with sperm quality in infertility. J Reprod Immunol 2021;148:103383. [Crossref] [PubMed]
- Balló A, Czétány P, Busznyákné KS, et al. Oxido-Reduction Potential as a Method to Determine Oxidative Stress in Semen Samples. Int J Mol Sci 2023;24:11981. [Crossref] [PubMed]
- Castleton PE, Deluao JC, Sharkey DJ, et al. Measuring Reactive Oxygen Species in Semen for Male Preconception Care: A Scientist Perspective. Antioxidants (Basel) 2022;11:264. [Crossref] [PubMed]
- Castleton P, Gyawali P, Mathews N, et al. MiOXSYS(®) and OxiSperm(®) II assays appear to provide no clinical utility for determining oxidative stress in human sperm-results from repeated semen collections. Andrology 2023;11:1566-78. [Crossref] [PubMed]






