
Racial, ethnic, and socioeconomic disparities in rates of stage IV prostate cancer after USPSTF category “D” recommendation against prostate-specific antigen screening: a retrospective cohort study
Highlight box
Key findings
• Significant racial and ethnic disparities exist in metastatic prostate cancer (mPCa) diagnosis at presentation.
• Being insured is associated with decreased odds of mPCa diagnosis at presentation.
• Rates of mPCa diagnosis at presentation increased for all groups post-United States Preventative Services Task Force (USPSTF) downgrade.
• Hispanic and non-Hispanic Black patients had a disproportionately greater increase.
What is known and what is new?
• There are racial and ethnic disparities in PCa diagnosis and treatment.
• The USPSTF downgrade resulted in fewer prostate-specific antigen (PSA) screens performed.
• However, an analysis of the potential differential impact of the USPSTF screening decision on racial and ethnic minorities is not found in the literature.
What is the implication, and what should change now?
• Patients with significant racial, ethnic, insurance status, and income risk factors should receive PSA screening at a higher priority than current USPSTF guidelines.
Introduction
Prostate cancer (PCa) is the second most common cause of cancer death among men in the United States (1). The National Cancer Institute estimates that one in eight men will be diagnosed with PCa during their lifetime (2). Advancements in screening techniques have enabled health care providers (HCP) to diagnose PCa prior to metastasis. The stage at which PCa is diagnosed is a critical determinant for treatment modality and mortality (3).
Randomized controlled trials confirmed that early detection through prostate-specific antigen (PSA) screenings significantly reduced PCa mortality (4). However, HCPs must balance the benefits and harms of PCa screening with PSA, as screening may lead to over-diagnosis and overtreatment with radiation or surgery that can result in deleterious effects on functional parameters (5). Recognizing these potential harms, in 2012, the United States Preventive Services Task Force (USPSTF) published a grade “D” recommendation against PSA screening for men of all ages (6).
Racial and ethnic disparities in metastatic PCa (mPCa) at diagnosis are known; however, the potential differential impact of the USPSTF screening decision on racial and ethnic minorities remains less well understood (7). Black race and Hispanic ethnicity are associated with higher odds of mPCa at presentation and Black men are more likely to be diagnosed with PCa at an earlier age (8,9). Previous studies have investigated social determinants of health including socioeconomic status, geographic location, education level, and health insurance status in relation to PCa incidence, risk, stage at presentation, and survival (10-12). However, a comprehensive contemporary analysis of the potential interactions between age, race, ethnicity, education level, income, insurance status, and the USPSTF “D” screening recommendation with the proportion of mPCa at presentation does not yet exist in the literature.
In this study, we examined risk of presentation with mPCa in the National Cancer Database (NCDB) to evaluate its relationship with race, ethnic, and socioeconomic characteristics. Furthermore, we investigated whether differences in associations between these characteristics and mPCa at presentation are observed as differential rates of change in mPCa prior to and following the USPSTF’s “D” recommendation against PSA screening. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-24-90/rc).
Methods
Data source
Data for patients presenting with mPCa from 2004 to 2017 were obtained from the NCDB, thus the sample size was determined by the total number of cases during the sample period. The NCDB is the largest clinical cancer registry in the world and receives over one million cancer case reports annually. Sourced from hospital registry data, the database represents more than 70 percent of newly diagnosed cancer cases from 1,500 American College of Surgeons Commission on Cancer accredited facilities nationwide (13). The time of the downgrade of the USPSTF recommendation to “D” was set at 2012 (6). The study was exempt from ethical review given minimal risk with the NCDB. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Outcomes and covariates under study
The primary outcome of this population-based cohort study was the percentage of mPCa during the study period, which is defined as distant metastasis of PCa (i.e., beyond regional pelvic lymph nodes) (14). Secondary outcomes included the change in percentage of mPCa within racial/ethnic populations and sociodemographic characteristics that are associated with mPCa.
Covariates included age, race, ethnicity, geographic location, county type, education level median household income, academic medical center and insurance status. Race and Hispanic ethnicity were combined into a single, four-category variable: known Hispanic, non-Hispanic (NH) White, NH Black, and other/unknown race/ethnicity. Geographic location was defined using the U.S. Census division categories of Northeast, South/Southeast, Midwest, and West (15). County type was defined as metropolitan (>250,000 residents/county), urban (2,500–250,000 residents/county), and rural (<2,500 residents/county). Education level was based on the percentage of adults within a zip code that did not graduate high school based on U.S. Census Bureau American Community Survey data (16). Likewise, median household income was defined by zip code and derived from U.S. Census Bureau. Academic medical center was defined as a hospital with an academic cancer program with more than 4 postgraduate medical education programs and more than 500 diagnosed cancer cases per year. Insurance status was defined by the patient’s primary insurance carrier at the time of diagnosis, patients with unknown insurance status were grouped with uninsured patients and reported separately.
Statistical analysis
Available data on all patients with PCa diagnosis of any stage were obtained from the NCDB. Frequencies and percentages of PCa stages and covariates of interest were computed. Relationships between socioeconomic characteristics and presentation with mPCa were examined using logistic regression models. Two-way interactions between race/ethnicity and other characteristics were fitted to examine potential differential relationships between characteristics and mPCa among race/ethnicity groups. Model selection was performed using backwards elimination beginning with a model that included all main effects and their two-way interactions. Factors were eliminated one-by-one in a hierarchical framework, starting with interaction terms and continuing to main effects if all interactions involving them had been eliminated. Because the large sample size made for increased precision, only factors significant at the 0.01 alpha level (or main effects involved in a significant interaction) were retained in the final model. Linear trends in percentage of mPCa at presentation over time, overall and by race/ethnicity, were examined using generalized linear regression models assuming an underlying binomial distribution. Lines were fitted through annualized rates of mPCa with an interaction for years 2012–2017 to evaluate changes in slopes over the post PSA screening era.
Results
We identified 1,275,410 PCa cases in the NCDB from 2004–2017, of which 88,987 (7%) were metastatic at presentation. Table 1 shows sociodemographic characteristics of the study population overall, as well as the frequency and percentage of mPCa cases at presentation by sociodemographic characteristics. We observed that 7% of NH Whites, 9% of NH Blacks, and 10% of Hispanics presented with mPCa. Percentages of mPCa at presentation varied by insurance status as 19% of uninsured patients, 16% of Medicaid patients, and 4% of privately insured patients presented with metastatic disease.
Table 1
| Characteristics | Total | Metastatic PCa |
|---|---|---|
| Age category (years) | ||
| <59 | 329,553 (26%) | 15,280 (5%) |
| 60–65 | 314,203 (25%) | 15,107 (5%) |
| 66–70 | 308,632 (24%) | 16,021 (5%) |
| >71 | 323,022 (25%) | 42,579 (13%) |
| Race | ||
| White | 1,034,324 (81%) | 68,411 (7%) |
| Black | 185,413 (15%) | 16,573 (9%) |
| Other | 37,487 (3%) | 3,061 (8%) |
| Unknown | 18,186 (1%) | 942 (5%) |
| Ethnicity | ||
| Hispanic | 55,773 (4%) | 5,470 (10%) |
| Non-Hispanic | 1,143,786 (90%) | 79,344 (7%) |
| Unknown | 75,851 (6%) | 4,173 (6%) |
| Race/ethnicity combined | ||
| Hispanic (any race, including unknown) | 55,773 (4%) | 5,470 (10%) |
| Non-Hispanic White | 985,707 (77%) | 63,636 (6%) |
| Non-Hispanic Black | 183,516 (14%) | 16,378 (9%) |
| Other/unknown | 50,414 (4%) | 3,503 (7%) |
| Geographic location | ||
| Northeast | 291,028 (23%) | 20,225 (7%) |
| South/Southeast | 362,026 (28%) | 22,492 (6%) |
| Midwest | 423,272 (33%) | 30,737 (7%) |
| West | 198,355 (16%) | 15,468 (8%) |
| Not specified | 729 (0.06%) | 65 (9%) |
| County type | ||
| Metro | 1,034,340 (81%) | 73,243 (7%) |
| Urban | 172,028 (13%) | 11,721 (7%) |
| Rural | 23,717 (2%) | 1,670 (7%) |
| Unknown | 45,325 (4%) | 2,353 (5%) |
| Education level of population (% no high school diploma) | ||
| ≥29% | 193,422 (15%) | 17,045 (9%) |
| 20–28.9% | 274,098 (21%) | 20,549 (7%) |
| 14–19.9% | 297,975 (23%) | 20,557 (7%) |
| <14% | 509,915 (40%) | 30,836 (6%) |
| Median household income | ||
| <$30,000 | 156,578 (12%) | 13,936 (9%) |
| $30,000–$34,999 | 209,239 (16%) | 15,950 (8%) |
| $35,000–$45,999 | 341,405 (27%) | 24,514 (7%) |
| $46,000 or more | 568,188 (45%) | 34,587 (6%) |
| Insurance status | ||
| Not insured | 21,081 (2%) | 4,045 (19%) |
| Private | 573,758 (45%) | 22,903 (4%) |
| Medicaid | 35,429 (3%) | 5,817 (16%) |
| Medicare or other government based | 617,976 (48%) | 53,960 (9%) |
| Not specified | 27,166 (2%) | 2,262 (8%) |
NCDB, National Cancer Database; PCa, prostate cancer.
Summary results of backward variable selection (Chi-square statistics and P values) for logistic regression models to predict mPCa status at presentation are shown in Tables S1,S2. All two-way interactions between sociodemographic factors were considered for model inclusion. Interactions that remained in the final model, ranked from largest to smallest ratio of chi-square statistic to degrees-of-freedom (DF), included: age with insurance status (χ2/DF 205.1); race/ethnicity with PSA testing era (30.4); insurance status with academic medical center (28.9); age with education (14.2); academic medical center with education (11.7); academic medical center with income (9.1); race/ethnicity with insurance status (7.8); insurance status with income (6.3); race/ethnicity with income (6.2); and insurance status with education (3.8). Tables S1,S2 give more detail including parameter estimates.
Figure 1 depicts the annual percentages of patients presenting with mPCa by year of diagnosis beginning in 2004 through 2017, overall and stratified by race/ethnicity group. Prevalence of mPCa at presentation is stable for years 2004 through 2011, which coincides with the USPSTF pre-grade “D” recommendation era. Beginning in 2012 percentages of mPCa at presentation begin to increase in a trend that continues through 2017. Hispanic and NH Black groups presented with highest percentages of mPCa for both pre- and post-USPSTF “D” recommendation eras with pre-USPSTF percentages among Hispanics ranging from 6.8% to 10% per year, 7.8% to 8.8% per year among NH Blacks, 4.5% to 7% per year among NH Whites, and 4.5% to 8.1% per year among other/unknown race/ethnicities. Post-USPSTF percentages ranged from 11.6% to 14.7% per year among Hispanics; 10.4% to 12.5% per year among NH Blacks, 8% to 10.2% per year among NH Whites, and 9.2% to 10.8% among other/unknown race/ethnicities. This increasing trend for the post-grade “D” recommendation era was consistent across all race/ethnicity groups. Analysis of differences in slopes of the lines for pre- versus post-grade “D” eras is statistically significant (P<0.001) for each race/ethnicity group (Table 2) with each exhibiting significantly positive slopes in the post grade “D” era (P<0.001 for each slope).

Table 2
| Race/ethnicity group | Testing era | Slope (Δ/year) | 95% CI | P value (difference between eras) |
|---|---|---|---|---|
| Hispanic | PSA testing guideline 2004–2011 | 0.0021 | 0.0008, 0.0034 | <0.001 |
| No PSA testing guideline 2012–2017 | 0.0092 | 0.0068, 0.0116 | ||
| Non-Hispanic White | PSA testing guideline 2004–2011 | 0.0012 | 0.0010, 0.0014 | <0.001 |
| No PSA testing guideline 2012–2017 | 0.0070 | 0.0065, 0.0075 | ||
| Non-Hispanic Black | PSA testing guideline 2004–2011 | −0.0005 | −0.0012, 0.0002 | <0.001 |
| No PSA testing guideline 2012–2017 | 0.0073 | 0.0061, 0.0086 | ||
| Non-Hispanic other or unknown | PSA testing guideline 2004–2011 | 0.0016 | 0.0005, 0.0027 | <0.001 |
| No PSA testing guideline 2012–2017 | 0.0064 | 0.0021, 0.0073 | ||
| All races combined | PSA testing guideline 2004–2011 | 0.0011 | 0.0009, 0.0013 | <0.001 |
| No PSA testing guideline 2012–2017 | 0.0072 | 0.0067, 0.0076 |
PSA, prostate-specific antigen; CI, confidence interval.
Visual depiction of interaction between race/ethnicity and income, and race/ethnicity and insurance status are shown in Figures 2,3, respectively. Among NH Black men, those earning <$30,000 compared to >$46,000/year had 22% greater odds of a diagnosis of mPCa at diagnosis [odds ratio (OR) 1.22, 95% confidence interval (CI), 1.14–1.30]. NH Black men earning <$30,000 versus those earning $35,000–$45,999 had 17% greater odds of mPCa at presentation (OR 1.17, 95% CI: 1.10–1.23), and NH Black men earning <$30,000 versus those earning $30,000–$34,999 had 8% greater odds (OR 1.08, 95% CI: 1.02–1.14). For other race/ethnicity groups, the relationships between median household income and mPCa at presentation were not as disparate as for NH Black males (Figure 2).

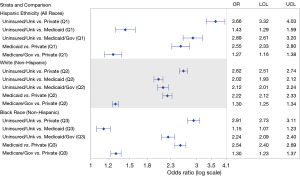
Relationships between insurance status and mPCa at presentation differed significantly between race/ethnicity groups (two-way interaction, P value <0.001 for all), as shown in Figure 3. Uninsured NH Whites had twice the odds of presenting with mPCa compared to those with Medicaid insurance (OR 2.02; 95% CI: 1.93–2.12). Notably, this magnitude of difference between the uninsured and those with Medicaid was not as large among Hispanic (OR 1.43, 95% CI: 1.29–1.59) and NH Black (OR 1.15, 95% CI: 1.07–1.23) patients.
Discussion
In this study, we used the NCDB to analyze factors that are associated with increasing presentation of mPCa and temporal effects of USPSTF’s “D” recommendation for PSA screening in the United States. We observed an increasing percentage of mPCa at diagnosis in all racial/ethnic subgroups under study after the 2012 grade “D” recommendation. Increased access to health insurance was associated with decreased risk of presentation of mPCa, with a differential impact by race. When comparing the uninsured to those with Medicaid, White patients had higher odds of presenting with mPCa compared to Hispanic or NH Black patients, suggesting Medicaid may have had a greater mitigating influence on the percentage of mPCa among Whites than racial minorities. These observations may have implications for Medicaid expansion policy.
Our model suggests that being insured is associated with a decreasing odds of presenting with mPCa. Private insurance was the largest protective factor for NH Black, Hispanic, and NH White populations (Figure 3). Following private insurance, Medicaid and Medicare statuses were associated with decreased odds of mPCa presentation. Uninsured status versus private insurance contributed to greatest risk of presentation with mPCa for NH Black, Hispanic, and NH White men. Our study demonstrated uninsured Hispanics may be most vulnerable with more than a three-fold increased risk of mPCa at diagnosis compared to those with private insurance. Uninsured individuals are disproportionately likely to be Black or Hispanic (17). Previous work illustrated uninsured adults are less likely to receive preventive care and screening services (18). Increased access to health insurance for Black and Hispanic individuals is associated with decreased risk of presentation of mPCa.
A decrease in the utilization of PSA screening is attributed to the grade “D” recommendations against PSA screening for men of all ages published in 2012 by the USPSTF (19,20). Contributing factors to end PSA screening included risks of treatment, costs of screening, PCa specific mortality benefit, and over-diagnosis of PCa (21). Percentage of mPCa increased 2.75% in 2012 following the USPSTF recommendation and is expected to increase through 2025 (22). On initial observation, the rise in mPCa depicted in Figure 2 begins from 2011–2012, around the same time as the 2012 USPSTF recommendation. However, a study by Sammon et al. (23) demonstrated a 5% decrease in PSA testing from 2010–2013 which may contribute to the rise in mPCa prior to the 2012 recommendation. Furthermore, Abdollah et al. (24) found a rapid decrease in PSA testing from 34.9% to 31.9% from 2011 to 2013 which was corroborated by Jemal et al. (25) who found a similar 7% decrease in PSA screening during a similar time period, suggesting a rapid acceptance of the 2012 USPSTF recommendation.
Our analysis illustrated that from 2004 through 2017, NH Black and Hispanic patients were the most likely to present with mPCa (Figure 1). Notably, NH Black and Hispanic populations sustained the largest increases in yearly change of mPCa at presentation in comparison of the pre- and post-grade “D” eras (Table 2). Overall increases in the percentage of presentation with mPCa may be explained by decreased PSA testing following the grade “D” recommendation against screening. However, significantly increased percentages of presentation of mPCa for NH Blacks and Hispanics highlight disparities within racial and ethnic minority communities. Racial and ethnic differences in presentation of mPCa may reflect systemic barriers to early diagnosis with possible contributing factors being social and economic disparities.
Lower economic status, as measured by quartile of median household income in residential ZIP code, was identified as a risk factor for presentation with mPCa. According to our analysis, residing in an area with a higher median household income is a protective factor against mPCa for NH Black and NH White populations. In 2019, an analysis by the National Cancer Institute found that low income is linked to advanced stage of PCa for all races (26). Interpretation of income as a variable in isolation is less helpful than considering how income may affect access to health insurance. In the United States, most adults under 65 receive health insurance through an employer-based plan. From 1999 to 2014, fewer individuals in the workforce were offered insurance through their employer. Decreases in coverage rates have disproportionately affected families with low incomes (27). Health insurance and income, combined may perpetuate racial and ethnic differences in mPCa at presentation.
Additional elements related to social determinants of health include education, neighborhood factors, and proximity to a metropolitan area. In our model, we found few differences in mPCa at presentation in metropolitan versus rural counties. Small but significant increased risk was noted for metropolitan versus rural NH Black (OR 1.18, 95% CI: 1.01–1.38) and NH White populations (OR 1.08, 95% CI: 1.03–1.15). Education analysis by zip codes where ≥29% of individuals had less than high school degree versus <14%, demonstrated significantly increased risk for all NH Whites, NH Blacks, and Hispanics. These results demonstrate that further research with detailed neighborhood factors such as school rankings, population density, public transportation, assessment of food dessert status, and access to public parks, is needed to understand the association of mPCa risk.
The study conclusions are limited by the shortcomings of the NCDB database. First, race and ethnicity were limited to Black, White, Hispanic, and “Other”, which does not properly represent the full complexity of patient identity. Second, the method by which the NCDB collects data introduces bias into its sample population. The NCDB is a hospital-based registry, meaning that only patients who receive cancer diagnosis or treatment at a hospital accredited by the American College of Surgeons Commission on Cancer are included (28). Mallin et al. found that between 2012 and 2014, only 58% of PCa diagnoses were captured by the NCDB (29). Due to this sampling technique, the NCDB database has previously been found to underrepresent low-grade PCa as well as racial and ethnic minorities (30). Other study limitations include its retrospective design, which limits extrapolation to current patient demographics, and limits the ability to establish causal relationships between the variables. The study is also limited by the inability to control for changes in patient comorbidities during the study period.
Other study limitations include its retrospective design, which limits extrapolation to current patient demographics, and limits the ability to establish causal relationships between the variables. Our study is also limited by the inability to control for changes in patient comorbidities during the study period. However, our findings concerning the relationship between “D” recommendation and increased mPCa rates for NH White and Black men are consistent with previous work that used the more inclusive Surveillance, Epidemiology, and End Results (SEER) database (31). Another limitation is that measures of education and income were based on aggregated data from ZIP code of residence, rather than being ascertained from individual patients. Although ZIP code aggregation is known to incorporate bias, this statistical method is frequently used in urology literature when an alternative is not available (32-34).
It is important to note that in 2018 the USPSTF revised its 2012 guidance to make PSA screening for men ages 55 to 69 years a category “C” recommendation, with PSA screening for men over 70 remained a category “D” recommendation (35). This screening recommendation revision is outside the bounds of our study period, but represents an important topic of future analysis.
Conclusions
Significant racial, ethnic, and socioeconomic disparities exist for patients who are diagnosed with mPCa at presentation. Since the USPSTF grade “D” recommendation against PSA screening, the percentage of mPCa at diagnosis has increased disproportionately in NH Black and Hispanic populations.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-24-90/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-24-90/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-24-90/coif). A.K.H. serves as an unpaid editorial board member of Translational Andrology and Urology from November 2023 to October 2025. G.A.B. has a leadership role for the American Board of Urology Residency Review Committee and the Sexual Medicine Society. D.D.T. received a research grant from Grail, receives travel support from and has a leadership role at the South-East Section of the American Urology Association, and has a leadership role at the Florida Urological Society. T.D.L. receives consulting fees from Bristol Myers Squibb, ImmunityBio, and Urogen Pharma. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was exempt from ethical review given minimal risk with the NCDB.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pernar CH, Ebot EM, Wilson KM, et al. The Epidemiology of Prostate Cancer. Cold Spring Harb Perspect Med 2018;8:a030361. [Crossref] [PubMed]
- American Cancer Society. Key Statistics for Prostate Cancer. Available online: https://www.cancer.org/cancer/types/prostate-cancer/about/key-statistics.html#:~:text=Deaths%20from%20prostate%20cancer,-Prostate%20cancer%20is&text=About%201%20man%20in%2041,point%20are%20still%20alive%20today
- Hawkes N. Cancer survival data emphasise importance of early diagnosis. BMJ 2019;364:l408. [Crossref] [PubMed]
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the Effects of Screening on Prostate Cancer Mortality in the ERSPC and PLCO Trials. Ann Intern Med 2017;167:449-55. [Crossref] [PubMed]
- Barry MJ, Simmons LH. Prevention of Prostate Cancer Morbidity and Mortality: Primary Prevention and Early Detection. Med Clin North Am 2017;101:787-806. [Crossref] [PubMed]
- Moyer VA. U.S. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:120-34. [Crossref] [PubMed]
- Rodriguez S, Sparks AD, Zhou H, et al. Racial disparities in late-stage prostate cancer: a SEER analysis 2005-2015. Can J Urol 2019;26:9946-51. [PubMed]
- Weiner AB, Matulewicz RS, Tosoian JJ, et al. The effect of socioeconomic status, race, and insurance type on newly diagnosed metastatic prostate cancer in the United States (2004-2013). Urol Oncol 2018;36:91.e1-6. [Crossref] [PubMed]
- Smith ZL, Eggener SE, Murphy AB. African-American Prostate Cancer Disparities. Curr Urol Rep 2017;18:81. [Crossref] [PubMed]
- Aghdam N, McGunigal M, Wang H, et al. Ethnicity and insurance status predict metastatic disease presentation in prostate, breast, and non-small cell lung cancer. Cancer Med 2020;9:5362-80. [Crossref] [PubMed]
- DeRouen MC, Schupp CW, Yang J, et al. Impact of individual and neighborhood factors on socioeconomic disparities in localized and advanced prostate cancer risk. Cancer Causes Control 2018;29:951-66. [Crossref] [PubMed]
- Coughlin SS. A review of social determinants of prostate cancer risk, stage, and survival. Prostate Int 2020;8:49-54. [Crossref] [PubMed]
- Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-90. [Crossref] [PubMed]
- American College of Surgeons. Standards for Oncology Registry Entry. Chicago, IL, 2024. Available online: https://www.facs.org/media/fjrdyjoj/store-2024-final-01232024.pdf
- U.S. Census Bureau. Statistical Abstract of the United States: 1995 (115th edition). Washington, DC, 1995.
- U.S. Census Bureau. American Community Survey Data. 2020. Available online: https://www.census.gov/programs-surveys/acs/data.html
- Finegold K, Conmy A, Chu RC, et al. Trends in the U.S. Uninsured Population, 2010-2020. (Issue Brief No. HP-2021-02). Washington, DC: Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. February 11, 2021.
- Lines LM, Urato M, Halpern MT, et al. Insurance Coverage and Preventive Care Among Adults. Research Triangle Park (NC): RTI Press; 2014.
- Cohn JA, Wang CE, Lakeman JC, et al. Primary care physician PSA screening practices before and after the final U.S. Preventive Services Task Force recommendation. Urol Oncol 2014;32:41.e23-30. [Crossref] [PubMed]
- Li J, Berkowitz Z, Hall IJ. Decrease in Prostate Cancer Testing Following the US Preventive Services Task Force (USPSTF) Recommendations. J Am Board Fam Med 2015;28:491-3. [Crossref] [PubMed]
- Shoag JE, Nyame YA, Gulati R, et al. Reconsidering the Trade-offs of Prostate Cancer Screening. N Engl J Med 2020;382:2465-8. [Crossref] [PubMed]
- Kelly SP, Anderson WF, Rosenberg PS, et al. Past, Current, and Future Incidence Rates and Burden of Metastatic Prostate Cancer in the United States. Eur Urol Focus 2018;4:121-7. [Crossref] [PubMed]
- Sammon JD, Abdollah F, D'Amico A, et al. Predicting Life Expectancy in Men Diagnosed with Prostate Cancer. Eur Urol 2015;68:756-65. [Crossref] [PubMed]
- Abdollah F, Dalela D, Haffner MC, et al. The Role of Biomarkers and Genetics in the Diagnosis of Prostate Cancer. Eur Urol Focus 2015;1:99-108. [Crossref] [PubMed]
- Jemal A, Fedewa SA, Ma J, et al. Prostate Cancer Incidence and PSA Testing Patterns in Relation to USPSTF Screening Recommendations. JAMA 2015;314:2054-61. [Crossref] [PubMed]
- Percy-Laurry A, Altekruse SF, Hossain MB, et al. Association Between Socioeconomic Status and Tumor Grade Among Black Men with Prostate Cancer. J Natl Med Assoc 2018;110:53-7. [Crossref] [PubMed]
- Long M, Claxton G, Mar 21 ADP, et al. Trends in Employer-Sponsored Insurance Offer and Coverage Rates, 1999-2014. KFF 2016; Available online: https://www.kff.org/private-insurance/issue-brief/trends-in-employer-sponsored-insurance-offer-and-coverage-rates-1999-2014/
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol 2017;3:1722-8. [Crossref] [PubMed]
- Mallin K, Browner A, Palis B, et al. Incident Cases Captured in the National Cancer Database Compared with Those in U.S. Population Based Central Cancer Registries in 2012-2014. Ann Surg Oncol 2019;26:1604-12. [Crossref] [PubMed]
- Modi PK, Ward KC, Filson CP. Characteristics of prostate cancer patients captured by facility-based versus geography-based cancer registries. Urol Oncol 2023;41:324.e1-7. [Crossref] [PubMed]
- Desai MM, Cacciamani GE, Gill K, et al. Trends in Incidence of Metastatic Prostate Cancer in the US. JAMA Netw Open 2022;5:e222246. [Crossref] [PubMed]
- Grubesic TH. Zip codes and spatial analysis: Problems and prospects. Socioecon Plann Sci 2008;42:129-49. [Crossref]
- Attalla K, Paulucci DJ, Blum K, et al. Demographic and socioeconomic predictors of treatment delays, pathologic stage, and survival among patients with penile cancer: A report from the National Cancer Database. Urol Oncol 2018;36:14.e17-24. [Crossref] [PubMed]
- Sellke N, Badreddine J, Rhodes S, et al. The Racial and Socioeconomic Characteristics of Men Using Mail-in Semen Testing Kits in the United States. Urology 2023;180:135-9. [Crossref] [PubMed]
- US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018;319:1901-13. [Crossref] [PubMed]


