
The surgical technique and protocol for dynamic sentinel node biopsy for penile cancer at a Southeast Asian regional hospital
Highlight box
Key findings
• Dynamic sentinel node biopsy (DSNB) is a minimally-invasive surgical diagnostic procedure that accurately stages lymph node status of penile cancer in situations when inguinal nodes are clinically non-palpable.
What is conventional and what is novel/modified?
• Surgical staging for clinically node-negative penile cancer is conventionally performed using modified or radical inguinal lymph node dissection. DSNB for penile cancer is not yet widely utilized in Southeast Asia.
• DSNB has a lower morbidity compared to traditional invasive staging of inguinal lymph nodes by modified or radical inguinal lymph node dissection. Adopting the procedure from a supra-regional centre in UK, our institution has implemented DSNB with the following characteristics: (I) omission of pre-operative ultrasound and fine needle aspiration cytology; (II) delayed DSNB after primary tumour surgery; (III) utilization of a 1-day DSNB protocol; and (IV) use of methylene blue for blue dye sentinel node identification.
What is the implication, and what should change now?
• A DSNB protocol can be adopted in regional hospitals where there are existing sentinel node services for breast cancer and melanoma management. The DSNB technique can be transferred to a Urologist through appropriate fellowship training.
Introduction
Background
Penile cancer is an uncommon malignancy in Singapore, accounting for approximately 0.4% of all male cancers with 50–100 cases each year (1). The most significant prognostic factor for survival in patients with penile cancer is the presence of inguinal lymph nodes involvement, therefore warranting thorough investigations and optimal management of inguinal lymph nodes (2). The inguinal lymph nodes are the first site of metastasis in penile cancer, with an estimated occurrence rate of 25–49% in confirmed penile cancer cases; even in clinically non-palpable inguinal lymph node (cN0) disease, micro-metastatic spread to inguinal lymph node can occur up to 25% of the time (2). Given that cN0 patients who undergoes prophylactic lymphadenectomy have a 5-year survival of 83%, compared with 36% for cN0 patients who develop metastasis on surveillance (3), there is a need for active management of inguinal lymph nodes before the opportunity of cure is missed.
Surgical staging of inguinal lymph nodes is a critical component for the management of cN0 penile cancer. Overall, imaging techniques for the detection of inguinal lymph node metastasis for penile cancer remain inadequate. Computed tomography (CT) and magnetic resonance imaging (MRI) are useful for the detection of enlarged lymph nodes of >0.5–1.0 cm with a sensitivity of 36%; however, imaging alone is only able to assess the size of the node and is unable to differentiate between inflammation and metastasis (4). Furthermore, current imaging modalities are limited as they are unable to detect micro-metastatic disease (5). Hybrid F18 fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) imaging remain investigational as it has limited sensitivity for detection of inguinal lymph node involvement in cN0 cases (6). Thus, major guidelines, including the European Association of Urology (EAU), recommend that cN0 patients in the intermediate-risk (pT1a, Grade 2) or high-risk (pT1b or greater) group, undergo invasive surgical staging such as either an invasive bilateral modified inguinal lymph node dissection (ILND) or dynamic sentinel node biopsy (DSNB) (2).
Rationale
DSNB for penile carcinoma was first described by Cabanas in 1977 (7). This is a minimally invasive diagnostic procedure for lymph node staging of penile cancer patients. It is based on the premise that metastatic spread is a step-wise process over several echelons. Metastatic cells are thought to pass through superficial and deep inguinal nodes before they reach pelvic lymph nodes, then retroperitoneal lymph nodes and distant metastasis (8). With the emergence of techniques like lymphoscintigraphy, and accurate detection of the sentinel nodes using methylene blue visualization and gamma probe localization, DSNB has since emerged as the gold standard for investigating cN0 penile cancer (9). An updated meta-analysis found that DSNB has a sensitivity of 0.87 [95% confidence interval (CI): 0.82–0.91]; this corresponds to a false negative rate of 13%, which translates to a low risk of under-detection of metastatic disease that leads to undertreatment (10). Radical ILND carries the risk of debilitating complications and long-term morbidity such as lymphedema, flap necrosis, lymphocele, deep vein thrombosis and wound infection in 42–57% of its patients; even with a modified ILND for surgical staging, the rate of major complications was reportedly 27% (11). On the other hand, with experience and technical advancements, DSNB carries a much lower risk of postoperative complications, with transient complications such as infection, seroma or lymphocele formation, and mild lymph edema occurring approximately in only 5.7% of cases (12).
Objective
Most urology departments in Singapore have yet to start a DSNB service, and modified or radical ILND remains the preferred staging technique. Notably, the compliance to guidelines is poor with only three of out of thirteen high-risk cN0 patients undergoing ILND in a local case series, with the rest undergoing surveillance (1). In another local case series, we noted that the patients whose cancers progressed appeared to have been undertreated for lymph node metastasis (13). Without DSNB, urologists have to balance the morbidity of modified ILND or radical ILND against the risk of occult inguinal lymph node involvement. In this paper, we describe our surgical technique and protocol for DSNB for the first four DSNB cases in Singapore, and we recommend the adoption of a similar protocol in other regional hospitals. We present this article in accordance with the SUPER reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-681/rc).
Preoperative preparations and requirements
Our lead author (W.L.) underwent a fellowship at a supra-regional tertiary centre for penile cancer at St James’s University Hospital, Leeds Teaching Hospital Trust between 2018 and 2019. The technique for DSNB was transferred from specialist urological surgeon IE through appropriate training and involvement in cases. Our lead author was involved in DSNB for 20 cases or 40 groins during fellowship.
Our hospital is a regional hospital catering to a population of 550,000. The Diagnostic Radiology Department, involving Principal Radiographer J.C.B.L., and Nuclear Medicine Radiologists S.Z.A. and A.K. had sufficient experience with the performance of lymphoscintigraphy for other conditions including melanoma and breast cancer. The pathology department had experience handling sentinel lymph nodes from breast cancer.
As per guidelines recommendation, cN0 patients in the intermediate-risk (pT1a, Grade 2) or high-risk (pT1b or greater) group were offered DSNB. Additionally, cN0 patients with low-to-intermediate risk primary tumor (pT1a) were offered surveillance or DSNB. Both low-to-intermediate risk (pT1a) patients in our series underwent DSNB. In this study, we report the protocol and outcome of patients who underwent DSNB our hospital from November 2021 to October 2022. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the National Healthcare Group Domain Specific Review Board (No. 2024/00312), and informed consent was taken from all patients. A summary of the patients’ oncological characteristics and outcome is shown in Table 1.
Table 1
| Case No. | Pathological stage | Treatment to primary tumor | Lympho-scintigraphy findings | Sentinel node identification | Sentinel node histology | Lymph node management | ILND histology |
|---|---|---|---|---|---|---|---|
| 1 | pT2G2 | Partial penectomy | Left: 1 node; right: 1 node | Left: yes; right: yes | Left: positive; right: negative | Completion left radical ILND | Negative (overall: pN1) |
| 2 | pT1bG3 | Circumcision | Left: 1 node; right: not visualized* | Left: yes; right: yes | Left: negative; right: positive | Completion right radical ILND | Positive, 2 nodes with ENE (overall: pN3) |
| 3 | pT1aG1–2 | Circumcision and partial glansectomy | Left: 1 node; right: 1 node | Left: no^; right: yes | Right: negative | Surveillance | – |
| 4 | pT1aG2 | Partial penectomy | Left: 1 node; right: 1 node | Left: yes; right: yes | Left: positive; right: negative | Completion left radical ILND | Negative (overall: pN1) |
*, non-visualization of right sentinel node on lymphoscintigraphy but surgically identified and histologically positive; ^, non-identification of the left sentinel node despite visualization on lymphoscintigraphy and groin exploration. ILND, inguinal lymph node dissection; ENE, extra-nodal extension.
The following equipment were required at the operating theatre: a Neoprobe® gamma detection system (Devicor Medical Products, Cincinnati, USA), methylene blue (50 mg in 10 mL, 0.5%) in the form of Proveblue® (Provepharm, Marseille, France), and 26 G needle attached to a 1-mL syringe. The usage of methylene blue was in accordance to the initial implementation of DSNB for penile cancer in UK (14). Compared to isosulfan blue (patent blue V), methylene blue is similarly accurate in sentinel lymph node identification, but is more easily available, cheaper, and has lower allergy risk (15). The radiation risk of sentinel node biopsy to the primary surgeon was previously noted to be low, hence no extra precautions were routinely required (16).
Step-by-step description
Penile lymphoscintigraphy was first performed at the nuclear medicine section of the diagnostic radiology department. Two syringes, each containing 74 Mbq in 0.2 mL volume of technetium-99m (99mTc) nano-albumon, were measured immediately prior to the start of the procedure (17). The first syringe was injected intra-dermally just proximal to the previous tumour site or scar (18), at the 12 o’clock and 3 o’clock position. The second syringe was injected at the 3 o’clock and 9 o’clock position. Where patients had undergone a total penectomy, injection was performed around the pubic scar (18). A dual head gamma camera system with large field of view (FOV) detectors and a Low energy high resolution collimator were used to acquire the images. Immediately after the injection, a 10-minute dynamic study was performed to see the flow of the lymphatic track. Subsequently, 5 minutes of static anterior and lateral images were performed, with a cobalt-57 flood source placed on the posterior side and left lateral side of the patient (Figure 1).
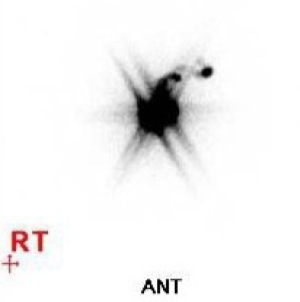
The patient was then handed over to operating theatre, where sentinel node biopsy was undertaken on the same day, within 2 to 4 hours from the initial scan. Following general anaesthetic, the gamma probe was used over the groin skin to mark the approximate sites of the sentinel node with a skin marker (Figure 2). Intravenous cefazolin was administered as prophylactic antibiotic as per consensus practice (19). Subsequently, 1 mL of 0.5% methylene blue solution was injected at 10 sites circumferentially on the shaft of the penis just proximal to the previous tumour site or scar at the intradermal level using a 26 G needle, providing 0.1 mL per site (Figures 3,4). The patient was then cleaned and draped exposing the groins. Direct skin crease incision was performed over the groin where it was previously marked. Combined gamma probe localization (Figure 5) and methylene blue dye identification (Figure 6) were used to identify the sentinel node. Absorbable vicryl 3/0 ties were used to ligate lymphatics associated with the sentinel node (Figure 7). After removing the sentinel node, the groin was scanned for residual radioactivity and palpated for suspicious nodes that may have failed to absorb the tracer (Figure 8). The radioactive count of the excised node is double-checked extracorporeally (Figure 9). Following haemostasis, the wound was closed with absorbable sutures. This process was repeated for the contralateral side. The surgery was around 60 minutes overall, where each groin exploration took around 25 minutes.
A specific complication of the procedure is a low risk of lymphocele formation. This is further reduced by meticulous control of lymphatics during surgical dissection, and lymphatic vessels are ligated with absorbable suture (11).
Postoperative considerations and tasks
The sentinel lymph nodes were processed at the pathology department according to the principles for processing of sentinel lymph nodes. Intraoperative frozen sectioning is not utilized due to the high incidence of micro-metastases that could potentially be missed using this technique (12). Notably, to reduce false negative rates, additional sectioning and immunohistochemical staining of the sentinel node was added to standard hematoxylin and eosin staining to detect micro-metastases (20).
Patients were discharged home the same day with simple oral analgesia. They were seen at 2 weeks for wound and histology review (Figure 10). Those with positive sentinel lymph node(s) would be referred for a completion ILND of the affected side within 4 weeks after DSNB (21). In the event of failure to resect or detect the sentinel lymph node, the patient would be considered eligible for surveillance if the primary disease was low risk (pT1a, Grade 1), surgical staging via modified ILND if the primary disease was intermediate or high risk (≥ pT1b), or repeat DSNB.
There was no complication attributable to DSNB in the series. Case 1 had a lymphocele following completion left ILND that resolved with percutaneous drainage and instillation of iodine sclerosant. Patients were reviewed every 3-monthly for groin examination, and 6-monthly with CT scan of abdomen and pelvis. The median follow-up of the four patients was 15.5 months (range, 12 to 22 months). There was no incidence of false negative DSNB during follow-up.
Tips and pearls
Two situations that we encountered in case 2 and case 3 serve as important considerations for subsequent adopters of DSNB. Appropriate management of these situations can improve the reliability of the procedure and reduce the false negative rate (12), which is defined as the occurrence of groin lymph node metastasis after a negative DSNB within a 12-month period from the DSNB (10).
Non-visualization on lymphoscintigraphy
For case 2, initial lymphoscintigraphy showed non-visualization of the right groin sentinel node (Figure 1). However, further on-table assessment of the right groin using the gamma probe, palpation, and right groin exploration found the histologically metastatic right inguinal sentinel node and necessitated the patient for a subsequent right groin ILND.
Unilateral non-visualization of sentinel node during lymphoscintigraphy can happen in 12–44% of penile cancer cohorts; this may be due to altered lymphatic drainage from prior surgery, or preferential drainage of lymphatic channels to one side (22). A more concerning reason for non-visualization is the inhibition of tracer accumulation due to tumor infiltration in the sentinel lymph node (23). Non-visualization on the initial lymphoscintigraphy does not exclude the presence of a sentinel lymph node, and exploration of the non-visualized groin could still reveal blue stained lymphatic vessels leading to a poorly radioactive lymph node (24). In case 2, the sentinel node was not visualized on lymphoscintigraphy due to tumor infiltration (Figure 1), and the combination of on-table wound palpation, gamma probe localization and blue dye identification led to the discovery of the metastatic sentinel node and subsequent ILND. Alternative management of non-visualization include repeat DSNB, modified ILND, or clinical surveillance where the primary disease is clinically low-risk (pT1a, Grade 1) (22).
Failure to identify sentinel node despite positive lymphoscintigraphy
For case 3, initial lymphoscintigraphy showed visualized bilateral groin sentinel nodes, although the left lymphoscintigraphy tracer focus was faint. The radioactive count rate was low and close to background during gamma probe assessment of the left groin, and no sentinel node could be identified over the reported hot spot seen on lymphoscintigraphy. Due to the low-to-intermediate risk primary tumor (pT1a, Grade 1–2), the patient elected for surveillance of this groin.
Even in large penile cancer DSNB case series, a small percentage of hot-spots or presumed sentinel nodes were not dissected, either because the radioactive count was very low, or the hot spots were thought to be pelvic secondary nodes (21). Overall, this occurred in 1 of 7 visualized hot-spots among our four patients (case 3). Again, the management of such groins needs to be risk stratified, and modified ILND needs to be considered if the primary disease was intermediate or high risk (≥ pT1b).
Discussion
Surgical highlights
DSNB involves the excision of the first lymph nodes that receive the lymphatic drainage of a peri-tumoral region. The technique for DSNB has undergone refinement since the initial description in 1977; these technique refinements include the use of intradermal blue dye injections to improve lymphatic mapping, and the innovative use of hand-held gamma-ray detector to radio-localize the sentinel node after injection of a radiolabeled technetium (99mTc)-nanocolloid tracer (25). In 1994, the Netherlands Cancer Institute (NCI) first described the technique of performing pre-operative lymphoscintigraphy, which was followed by the combination of intraoperative patent blue dye injection and gamma ray probe localization (26). Subsequent adoption of DSNB for penile cancer in the UK was based on the same technique (14).
We implemented DSNB based on the delayed DSNB protocol at tertiary institutions in the UK that accept tertiary referrals who had already undergone primary surgery at the regional hospital; this protocol was shown to be similarly accurate to primary DSNB with primary tumour in situ (18). We also followed a 1-day protocol for DSNB, which eliminated the requirement for inpatient stay, and had a logistical benefit of a single-day booking for lymphoscintigraphy and gamma probe detector in the operating theatre. The 1-day protocol may potentially detect a greater number of sentinel nodes per groin due higher retained gamma counts, although the implication for this finding on false negative rate needs to be further evaluated (27).
Strengths and limitations
Identification of patients with micro-metastasis using a DSNB procedure limits the radical ILND procedure to those with positive lymph nodes. As only 20–25% of patients with cN0 harbour micro-metastasis, routine modified ILND or radical ILND would overtreat 75–80% of patients, potentially causing complications in up to 50% of these patients (21,25). Our experience showed no complication due to DSNB, which is consistent with low rates of complication in international studies (12,21).
A key limitation of the DSNB technique is the false negative rate. The most significant factor to reduce the risks of false negatives in DSNB is the optimal selection of patient preoperatively. A thorough physical examination is key to ensure selection of penile cancer patients with impalpable lymph nodes. We demonstrated that the surgeon can also reduce the risk of false negatives in DSNB intraoperatively by palpating the wound to identify poorly stained and non-radioactive lymph nodes that are clinically suspicious for metastasis to reduce the risk of non-detection in the DSNB procedure (24). The NCI group described a modification to their original technique by the addition of preoperative ultrasound of all cN0 groins with fine needle aspiration cytology (FNAC) of suspicious nodes that brought about a reduction of false negative rates to 4.8% (12). In our practice, we performed ultrasound guided FNAC only for cases with palpable lymph nodes but not for cN0 cases. This was because it would require special training for Radiologists for ultrasound identification of suspicious nodes. The group from Denmark, which also did not perform ultrasound guided FNAC for cN0 cases, was able to achieve a false negative rate of 10.8% with a median follow-up of 73 months (21).
Comparison with other techniques and researches
Video-endoscopic ILND (VEIL) and robot-assisted video-endoscopic ILND (RAVEIL) have been introduced for management of penile cancer patients in both cN0 and clinically node-positive settings. These procedures were reported to have nodal yields comparable with open ILND, and with a potential to decrease morbidity and length of stay (28). More specifically, VEIL/RAVEIL can decrease skin-related complications like skin necrosis and wound complications, and lymphatic complications like lymphedema compared to open ILND (29). Due to the heterogeneity of indications, surgical technique, and outcomes monitoring, the role of VEIL/RAVEIL remain unclear in guidelines (29). More practically, these procedures have a significant learning curve (29), and the availability of RAVEIL may be limited in regional hospital settings. On the contrary, sentinel node services have been well-established in most regional hospitals, particularly for breast cancer management.
Implications and actions recommended
The DSNB procedure is transferrable through appropriate training. Previously, it was reported that DSNB can be adopted without a learning curve, such that hospitals with a smaller volume of patients can also perform this procedure (30). To optimize patient outcomes and ensure proper operative standards, both a trained surgeon and an experienced and prepared team are vital. Our lead surgeon gained fellowship experience at a supra-regional tertiary referral institution for penile cancer in UK, and developed the protocol with the support of the nuclear medicine, operating theatre, and pathology departments. Due to our regional hospital’s prior experience in sentinel node biopsy for breast cancer, no additional investment in equipment was necessary. From the patient’s perspective, the additional cost of performing lymphoscintigraphy was offset by a lower surgical table code for lymph node biopsy.
The implementation of DSNB resulted in 5 of 8 groins (62.5%) avoiding ILND in the cN0 setting. In cases 1 and 4, DSNB showed a single positive left inguinal lymph node, and subsequent ipsilateral ILND showed no other inguinal involvement (pN1). This suggested that the DSNB approach correctly identified and excised the only positive sentinel node. In these two cases, DSNB helped to avoid a contralateral ILND, which reduced morbidity for the two patients. Alternatively, if case 4 (pT1aG2 primary) had elected for a surveillance protocol, he would have delayed the diagnosis of node positive disease, which would have resulted in a poorer prognosis (2).
Based on the epidemiology of penile cancer in Singapore and the region that our hospital covers, we manage 5–10 cases of penile cancer per year. For countries with a low number of penile cancers a year such as Singapore, considerations to improve the utilization of DSNB should include centralization of penile cancer lymph node management, especially when delayed DSNB after primary tumour surgery has been shown to be as effective as primary DSNB.
Conclusions
In this report, we described the protocol and surgical technique for DSNB for cN0 penile cancer in Singapore, which was adopted from a tertiary referral centre in UK after appropriate subspecialty training. Our initial experience demonstrated the ability to correctly excise the sentinel node, and appropriately stratify the groins that can undergo surveillance. With DSNB, patients are offered an option of having lower risks of long-term morbidity, without compromising the detection of lymph node metastases. While further follow-up is still required to properly assess the long-term benefits and morbidity for the patients, we believe that this is a valuable addition to the management plan of appropriately selected penile cancer patients in both Singapore and other Asian hospitals with a urology service.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-681/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-681/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-681/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the National Healthcare Group Domain Specific Review Board (No. 2024/00312), and informed consent was taken from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tan TW, Chia SJ, Chong KT. Management of penile cancer in a Singapore tertiary hospital. Arab J Urol 2017;15:123-30. [Crossref] [PubMed]
- Hakenberg OW, Compérat EM, Minhas S, et al. EAU guidelines on penile cancer: 2014 update. Eur Urol 2015;67:142-50. [Crossref] [PubMed]
- McDougal WS, Kirchner FK Jr, Edwards RH, et al. Treatment of carcinoma of the penis: the case for primary lymphadenectomy. J Urol 1986;136:38-41. [Crossref] [PubMed]
- Barski D, Georgas E, Gerullis H, et al. Metastatic penile carcinoma - an update on the current diagnosis and treatment options. Cent European J Urol 2014;67:126-32. [Crossref] [PubMed]
- Mueller-Lisse UG, Scher B, Scherr MK, et al. Functional imaging in penile cancer: PET/computed tomography, MRI, and sentinel lymph node biopsy. Curr Opin Urol 2008;18:105-10. [Crossref] [PubMed]
- Sadeghi R, Gholami H, Zakavi SR, et al. Accuracy of 18F-FDG PET/CT for diagnosing inguinal lymph node involvement in penile squamous cell carcinoma: systematic review and meta-analysis of the literature. Clin Nucl Med 2012;37:436-41. [Crossref] [PubMed]
- Cabanas RM. An approach for the treatment of penile carcinoma. Cancer 1977;39:456-66. [Crossref] [PubMed]
- Mehralivand S, van der Poel H, Winter A, et al. Sentinel lymph node imaging in urologic oncology. Transl Androl Urol 2018;7:887-902. [Crossref] [PubMed]
- Teh J, Duncan C, Qu L, et al. Inguinal lymph node dissection for penile cancer: a contemporary review. Transl Androl Urol 2020;9:3210-8. [Crossref] [PubMed]
- Fallara G, Pozzi E, Onur Cakir O, et al. Diagnostic Accuracy of Dynamic Sentinel Lymph Node Biopsy for Penile Cancer: A Systematic Review and Meta-analysis. Eur Urol Focus 2023;9:500-12. [Crossref] [PubMed]
- Spiess PE, Hernandez MS, Pettaway CA. Contemporary inguinal lymph node dissection: minimizing complications. World J Urol 2009;27:205-12. [Crossref] [PubMed]
- Leijte JA, Kroon BK, Valdés Olmos RA, et al. Reliability and safety of current dynamic sentinel node biopsy for penile carcinoma. Eur Urol 2007;52:170-7. [Crossref] [PubMed]
- Lau WD, Ong CH, Lim TP, et al. Penile cancer: a local case series and literature review. Singapore Med J 2015;56:637-40. [Crossref] [PubMed]
- Hadway P, Smith Y, Corbishley C, et al. Evaluation of dynamic lymphoscintigraphy and sentinel lymph-node biopsy for detecting occult metastases in patients with penile squamous cell carcinoma. BJU Int 2007;100:561-5. [Crossref] [PubMed]
- Varghese P, Abdel-Rahman AT, Akberali S, et al. Methylene blue dye--a safe and effective alternative for sentinel lymph node localization. Breast J 2008;14:61-7. [Crossref] [PubMed]
- Saha S, Jacklin R, Siddika A, et al. Safety of radioactive sentinel node biopsy for breast cancer and the pregnant surgeon - A review. Int J Surg 2016;36:298-304. [Crossref] [PubMed]
- Valdés Olmos RA, Tanis PJ, Hoefnagel CA, et al. Penile lymphoscintigraphy for sentinel node identification. Eur J Nucl Med 2001;28:581-5. [Crossref] [PubMed]
- Omorphos S, Saad Z, Arya M, et al. Feasibility of performing dynamic sentinel lymph node biopsy as a delayed procedure in penile cancer. World J Urol 2016;34:329-35. [Crossref] [PubMed]
- Fankhauser CD, Ayres BE, Issa A, et al. Practice Patterns Among Penile Cancer Surgeons Performing Dynamic Sentinel Lymph Node Biopsy and Radical Inguinal Lymph Node Dissection in Men with Penile Cancer: A eUROGEN Survey. Eur Urol Open Sci 2021;24:39-42. [Crossref] [PubMed]
- Jensen JB, Jensen KM, Ulhøi BP, et al. Sentinel lymph-node biopsy in patients with squamous cell carcinoma of the penis. BJU Int 2009;103:1199-203. [Crossref] [PubMed]
- Jakobsen JK, Krarup KP, Sommer P, et al. DaPeCa-1: diagnostic accuracy of sentinel lymph node biopsy in 222 patients with penile cancer at four tertiary referral centres - a national study from Denmark. BJU Int 2016;117:235-43. [Crossref] [PubMed]
- Sahdev V, Albersen M, Christodoulidou M, et al. Management of non-visualization following dynamic sentinel lymph node biopsy for squamous cell carcinoma of the penis. BJU Int 2017;119:573-8. [Crossref] [PubMed]
- Goyal A, Douglas-Jones AG, Newcombe RG, et al. Effect of lymphatic tumor burden on sentinel lymph node biopsy in breast cancer. Breast J 2005;11:188-94. [Crossref] [PubMed]
- Kroon BK, Horenblas S, Estourgie SH, et al. How to avoid false-negative dynamic sentinel node procedures in penile carcinoma. J Urol 2004;171:2191-4. [Crossref] [PubMed]
- Yeung LL, Brandes SB. Dynamic sentinel lymph node biopsy as the new paradigm for the management of penile cancer. Urol Oncol 2013;31:693-6. [Crossref] [PubMed]
- Kroon BK, Horenblas S, Meinhardt W, et al. Dynamic sentinel node biopsy in penile carcinoma: evaluation of 10 years experience. Eur Urol 2005;47:601-6; discussion 606. [Crossref] [PubMed]
- Dimopoulos P, Christopoulos P, Shilito S, et al. Dynamic sentinel lymph node biopsy for penile cancer: a comparison between 1- and 2-day protocols. BJU Int 2016;117:890-6. [Crossref] [PubMed]
- Russell CM, Salami SS, Niemann A, et al. Minimally Invasive Inguinal Lymphadenectomy in the Management of Penile Carcinoma. Urology 2017;106:113-8. [Crossref] [PubMed]
- Patel KN, Salunke A, Bakshi G, et al. Robotic-Assisted Video-Endoscopic Inguinal Lymphadenectomy (RAVEIL) and Video-Endoscopic Inguinal Lymphadenectomy (VEIL) versus Open Inguinal Lymph-Node Dissection (OILND) in carcinoma of penis: Comparison of perioperative outcomes, complications and oncological outcomes. A systematic review and meta-analysis. Urol Oncol 2022;40:112.e11-22. [Crossref] [PubMed]
- Leijte JA, Hughes B, Graafland NM, et al. Two-center evaluation of dynamic sentinel node biopsy for squamous cell carcinoma of the penis. J Clin Oncol 2009;27:3325-9. [Crossref] [PubMed]











