Friend or foe: role of E-cadherin in prostate cancer metastasis
Alteration of E-cadherin protein level during epithelial-mesenchymal transition (EMT) or mesenchymal-epithelial transition (MET) plays important role in cancer metastasis (1). In a recent paper “Liver Protects Metastatic Prostate Cancer From Induced Death by Activating E-cadherin Signaling” by Ma et al. (2) reported that the interaction between liver cells and metastatic prostate cancer (PCa) cells provokes re-expression of E-cadherin in PCa cells, which protects PCa cells from cell death induced by chemotherapeutic drugs. E-cadherin fabricates this protection via activation of canonical survival signaling pathways, including the extracellular signal-regulated kinases (ERK), protein kinase B (AKT), and the Janus kinase (JAK) signaling. Ma et al. used DU-145, an androgen-receptor (AR)-negative androgen-independent PCa cell line which expresses very low level of E-cadherin on the cell membrane to determine the role of E-cadherin in PCa chemotherapy resistance. The researchers found that either co-culture with primary hepatocytes to mimic liver cell microenvironment or addition of PD153035 (inhibitor suppressing the kinase activity of epidermal growth factor receptor, EGFR) induced the re-expression of E-cadherin in DU-145. These E-cadherin-high DU-145 cells were much more resistant to chemotherapeutic drugs treatment and TNF-related apoptosis-inducing ligand (TRAIL) both in vitro and in vivo.
The finding that elevation of E-cadherin in metastasized DU-145 PCa cells protects PCa cells from chemotherapy is very interesting and may stimulate novel therapies for advanced PCa targeting E-cadherin. However, the difference in AR expression level and phosphatase and tensin homolog (PTEN) status may complicate the effect of E-cadherin on PCa metastasis. In PCa cells, AR modulates the expression of proteins regulating cell cycle, survival and growth. Increase in AR mRNA and protein is observed in castration-resistant prostate cancer (CRPC) as compared to the primary prostate tumors (3). Ligand-activated AR has been reported to bind E-cadherin promoter, to downregulate E-cadherin expression, to activate Snail, to induce EMT, and thus promotes cancer metastasis (4,5). Re-expression of AR in PC-3 cells, another AR-negative PCa cells, increases E-cadherin but represses EMT, migration, and invasion of PC-3 cells in the absence of androgen (6). PTEN is a negative regulator for phosphoinositide 3-kinase (PI3K)-Akt signaling pathway. Deletion of PTEN was observed in 40–70% of PCa patients, resulting in upregulation of PI3K-Akt signaling. Deletion or mutation of PTEN is associated with poor prognosis, cancer metastasis, and progression towards castration-resistant status or PCa (7,8). DU-145 is an AR-negative PCa cell line expressing wild type PTEN. Whether the phenomenon observed in DU-145 is the general case for all PCa cells requires further investigation. It is a good idea to use both LNCaP (AR-positive, mutant PTEN) and PC-3 (AR-negative, PTEN null) for validation. Ma et al. pointed out that E-cadherin promotes protection against chemotherapy drugs partially via activation of Akt1 and Akt2. As Akt3 has been reported to play important role in PCa (9), it will be worthy to examine if Akt3 is involved in the chemotherapeutic protection of E-cadherin.
During the EMT, level of E-cadherin decreases to assist the metastasis of cancer cells. Ma et al. suggested that after PCa cells launch at liver tissue to form metastatic colony, the interaction between PCa cells and hepatocyte cells stimulates the expression of E-cadherin. The elevation of E-cadherin will then protect PCa cells from chemotherapy but also hinder the proliferation of PCa cells. As liver metastasis only counts for 25% of all PCa metastasis, less than the PCa metastasis incidence in lymph node, bone, and lung, it is critical to determine if cells in lymph node, bone, and lung or immune cells may interact with PCa cells to stimulate the expression of E-cadherin.
E-cadherin positive prostate cancer stem cells (CSCs) subpopulation has been reported to express the reprogramming factors SOX2 and OCT3/4 (10). This subpopulation of prostate CSCs is highly invasive and is capable of altering its E-cadherin expression during the process of invasion (10). As CSCs population is well known for being chemotherapy-resistant, we believe that this prostate CSCs population account for most, if not all, of the chemotherapy protective effects being observed by Ma et al. The CSCs population will first decline the expression of E-cadherin to promote cancer metastasis, possibly via epigenetic modification of methylation of the CDH1 promoter (11) and uncoupling of ZEB1 and E-cadherin expression in metastatic PCa (12). At the metastatic lesion, the CSCs population will elevate the expression of E-cadherin. Putzke et al. analyzed 185 PCa metastases and discovered significantly higher E-cadherin expression in bone than in lymph node and soft tissue metastases (12). Using DU-145 sublines with different E-cadherin expression level, Putzke et al. found that DU-145 xenografts positive for E-cadherin exhibit higher aggressiveness and the metastases frequency, suggesting that high E-cadherin expression in metastatic PCa is associated with aggressive tumor growth. This E-cadherin-positive DU-145 cells also demonstrate the highest expression of genes associated with stem cells (12), supporting our hypothesis that CSCs play essential role in E-cadherin-induced chemotherapy resistance.
Ma et al. mentioned in the papers that according to the analysis of TCGA, lower E-cadherin level was found in patients with distant metastasis. Our analysis using Oncomine database also indicates that expression of E-cadherin is lower in metastatic prostate tumors as compared to primary tumors (Figure S1). This obviously seems to be contradictory to the chemotherapy-protective role of E-cadherin as suggested by Ma’s paper. We believe that this can be explained by the fact that high E-cadherin expression hampers proliferation of PCa cells at metastatic lesion. Therefore, a mixture of larger population of PCa cells with low E-cadherin expression and a small population of prostate CSCs with high E-cadherin expression forms the primary prostate tumors. During the EMT and cancer metastasis, the E-cadherin decrease in prostate CSCs cells to proceed migration and invasion. At the metastatic site, the CSCs cells regain E-cadherin expression and generate low E-cadherin PCa cells. The average level of E-cadherin of the metastatic prostate tumors may be lower than the primary tumors to accommodate the tough micro-environment at the metastatic lesion (Figure 1).

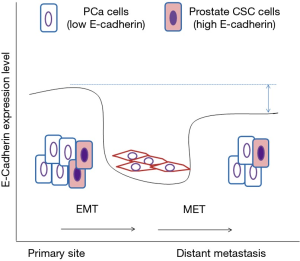
In summary, Ma et al. provided evidence to demonstrate that E-cadherin is not only a tumor suppressor protein, but can also diminish the effects of chemotherapy via activation of ERK, Akt, and JAK signaling pathway. Development of novel therapy for advanced PCa should take E-cadherin and these signaling pathways into consideration.
Acknowledgements
Funding: This study was supported by CS-104-PP-14 from National Health Research Institutes (NHRI) and MOST 105-2923-B-400-001-MY3 from Ministry of Science and Technology (MOST) for CPC in Taiwan. CYC is supported by MOST 105-2811-B-400-031 from MOST in Taiwan.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009;9:265-73. [Crossref] [PubMed]
- Ma B, Wheeler SE, Clark AM, et al. Liver protects metastatic prostate cancer from induced death by activating E-cadherin signaling. Hepatology 2016;64:1725-42. [Crossref] [PubMed]
- Chuu CP, Kokontis JM, Hiipakka RA, et al. Androgens as therapy for androgen receptor-positive castration-resistant prostate cancer. J Biomed Sci 2011;18:63. [Crossref] [PubMed]
- Liu YN, Liu Y, Lee HJ, et al. Activated androgen receptor downregulates E-cadherin gene expression and promotes tumor metastasis. Mol Cell Biol 2008;28:7096-108. [Crossref] [PubMed]
- Zhu ML, Kyprianou N. Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells. FASEB J 2010;24:769-77. [Crossref] [PubMed]
- Huo C, Kao YH, Chuu CP. Androgen receptor inhibits epithelial-mesenchymal transition, migration, and invasion of PC-3 prostate cancer cells. Cancer Lett 2015;369:103-11. [Crossref] [PubMed]
- Bedolla R, Prihoda TJ, Kreisberg JI, et al. Determining risk of biochemical recurrence in prostate cancer by immunohistochemical detection of PTEN expression and Akt activation. Clin Cancer Res 2007;13:3860-7. [Crossref] [PubMed]
- Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997;275:1943-7. [Crossref] [PubMed]
- Lin HP, Lin CY, Huo C, et al. AKT3 promotes prostate cancer proliferation cells through regulation of Akt, B-Raf, and TSC1/TSC2. Oncotarget 2015;6:27097-112. [Crossref] [PubMed]
- Bae KM, Parker NN, Dai Y, et al. E-cadherin plasticity in prostate cancer stem cell invasion. Am J Cancer Res 2011;1:71-84. [PubMed]
- Graff JR, Gabrielson E, Fujii H, et al. Methylation patterns of the E-cadherin 5' CpG island are unstable and reflect the dynamic, heterogeneous loss of E-cadherin expression during metastatic progression. J Biol Chem 2000;275:2727-32. [Crossref] [PubMed]
- Putzke AP, Ventura AP, Bailey AM, et al. Metastatic progression of prostate cancer and e-cadherin regulation by zeb1 and SRC family kinases. Am J Pathol 2011;179:400-10. [Crossref] [PubMed]


