
Randomized trial of low intensity shockwave therapy for erectile dysfunction utilizing grayscale ultrasound for analysis of erectile tissue homogeneity/inhomogeneity
Introduction
Background
Erectile dysfunction (ED) is the persistent inability to attain and/or maintain an erection sufficient for satisfactory sexual activity for a duration of at least 3 months (1). The most common etiology of ED is vasculogenic dysfunction (2,3). This may be caused by arterial occlusive disease that reduces cavernosal artery perfusion pressure (4,5) or corporal veno-occlusive dysfunction that results from excess cavernosal connective tissue (fibrosis) and reduced cavernosal smooth muscle, decreasing erectile tissue expandability (6,7). Adequate expandability and functional maintenance of penile erection has been associated with cavernosal smooth muscle content of 39–43% of erectile tissue cross-sectional area (6).
Previous clinical studies have demonstrated that low intensity shockwave therapy (LiSWT) increases cavernosal artery blood flow and improves corporal veno-occlusive function in patients with vasculogenic ED (8,9). Although LiSWT has been utilized to treat ED since 2010 (9), the underlying mechanisms by which LiSWT improves erectile function (EF) remain under active investigation. The exposure of shockwave energy to erectile tissue results in expansion and contraction of this tissue. This leads to mechanotransduction, a process that converts mechanical stimuli into biochemical signals. Resultant biochemical changes include increased density of endogenous mesenchymal stem cells, synthesis of cavernosal smooth muscle, degradation of cavernosal collagen, and release of vasodilating factors such as nitric oxide, thereby improving EF (10).
Rationale and knowledge gap
Multiple prospective, randomized placebo-controlled trials have been published concerning the safety and efficacy of LiSWT for the treatment of ED (11,12). The most commonly used outcome assessments of the efficacy of LiSWT for ED have been a validated patient-reported outcome measure, the International Index of Erectile Function (IIEF) (13), and the objective parameters of peak systolic velocity (PSV) and end diastolic velocity (EDV) (14).
In studies unrelated to shockwave therapy, grayscale ultrasound (GUS) imaging during B mode of the erect penis in the presence of maximal smooth muscle relaxation has been reported to be a measure of cavernosal fibrosis (15-17). In men with ED, GUS imaging reveals hypoechoic inhomogeneous regions within the corpora cavernosa, whereas men without ED are observed to have normoechoic homogenous findings within the corpora cavernosa (15,16). To the best of our knowledge, assessment of grayscale images during B-mode ultrasound of the erect penis has not been previously reported as an outcome measure for LiSWT in men with ED.
Objectives
The primary objective of this single-blind, sham-controlled, randomized, prospective study was to examine the efficacy of LiSWT by examining changes from baseline to follow-up visits using GUS visual grading of erectile tissue homogeneity/inhomogeneity during pharmacologic erection and color duplex Doppler ultrasound (DUS) measurements of cavernosal artery PSV and EDV. A secondary objective was to prospectively document the safety of LiSWT in men with ED. Other secondary objectives were to prospectively assess changes, from baseline to follow-up, in EF and lower urinary tract symptoms (LUTS) utilizing multiple patient-reported outcome measures. We present this article in accordance with the CONSORT reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-24-338/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Aspire Independent Review Board (No. 520190180). The study was registered on clinicaltrials.gov as NCT06600893. This single-blind, sham-controlled, randomized, prospective, study in men with ED naïve to acoustic wave treatment, either shockwave or radial ballistic pressure wave therapy, was conducted at a single sexual medicine facility. Randomization assignments were produced by a computer-generated randomizer prior to the start of the trial (18). All procedures were performed by trained members of the research staff. Each participant provided written informed consent and authorized release of protected health information before any study procedure was conducted. Table 1 describes inclusion and exclusion criteria.
Table 1
| Inclusion criteria | Exclusion criteria |
|---|---|
| Written consent/Health Insurance Portability and Accountability Act authorization | Treated with shockwave and/or radial ballistic pressure waves previously |
| Male | Pacemaker or implanted defibrillator |
| 21–80 years old | Clinically significant findings on physical examination |
| In a relationship with a female partner for at least 3 months | Sciatica or severe back pain |
| Body mass index <37 kg/m2 | Uncontrolled hypertension |
| Diagnosis of erectile dysfunction | Uncontrolled hypogonadism |
| Total testosterone ≥300 ng/dL | Unwilling to maintain testosterone replacement therapy if currently using testosterone to treat hypogonadism |
| Prostate-specific antigen <4.0 ng/mL | Radical prostatectomy surgery |
| Willing to attempt sexual activity at least 4 times during screening period and 4 weeks before each follow-up visit | International Index of Erectile Function erectile function domain scores ≥26 |
| Willing to stop all erectile function aids (e.g., prescription and non-prescription medications, injections, vacuum erection devices, constriction ring) during the screening period and 4 weeks before each follow-up visit | Homogenous corpora cavernosa on grayscale ultrasound |
| Agrees to comply with the study procedures and visits | Lesions or active infections on the penis or perineum |
| Continue to use medications for ongoing medical conditions at the same dose except for erectile dysfunction | Unwilling to remove piercings from the genital region |
| History of substance abuse within 12 months prior, or consuming >14 alcoholic drinks per week | |
| Received an investigational drug within 30 days prior to signing consent | |
| Platelet-rich plasma treatment within 3 months of signing consent | |
| Stem cell treatment within 6 months of signing consent | |
| Any condition or exhibiting behavior indicating to the Principal Investigator that the Participant is unlikely to be compliant with study procedures and visits | |
| Any chronic medical condition or psychologic disorder that, in the opinion of the Principal Investigator, makes the Participant ineligible for the study |
Initial 4-week screening period
At the screening visit, each participant’s demographics, medical history, and concomitant medications were recorded and vital signs were measured by the clinical research coordinator. A physical examination was completed, and baseline GUS and DUS during complete smooth muscle relaxation (6) were performed by a study investigator. At completion of the screening visit, participants were instructed not to use any EF aids and to attempt sexual activity at least 4 times over the next 4 weeks, completing a sexual encounter profile (SEP) diary after each attempt.
Ultrasound assessments
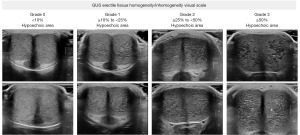
The participant was administered an intracavernosal injection of vasoactive agents, papaverine (30 mg), phentolamine (1–10 mg), prostaglandin E1 (0–60 mcg per 0.1–0.5 mL) to achieve a sustained pharmacologic erection, 3–4 out of 4 on the Erection Hardness Scale (EHS) with redosing as needed (19). Ultrasound was performed using a high-resolution probe (Aixplorer 15.4 mHz transducer). For GUS, three different gain settings 45%, 55%, and 65% with the dynamic range set at 70 dB were used (20,21). Images were captured in the axial plane at the proximal, mid, and distal penile shaft. The GUS erectile tissue homogeneity/inhomogeneity for each area of the penile shaft was visually graded immediately after image acquisition by investigators who were blinded to the treatment status (simulated or active treatment) of the participant (20,21). As shown in Figure 1, GUS visual grade 0 was defined as <10% hypoechoic area; Grade 1 was defined as ≥10% to <25% hypoechoic area; Grade 2 was defined as ≥25% to <50% hypoechoic area; and Grade 3 was defined as ≥50% hypoechoic area.
Additional exploratory analyses of GUS images were performed by using Fiji version 1.53 (22). After conversion of GUS images to 8-bit grayscale, the thresholding tool within Fiji was used to determine the percent of cross-sectional hypoechoic area within the corporal tissue. Computer-assisted image analysis measurements using Fiji were performed independently for the left and right sides (3 measurements per side) and all measurements were averaged to determine the percent hypoechoic area in each penile region (proximal, middle, distal).
DUS was performed on the lower third of the penile shaft in the sagittal plane at B-mode gain 45% with the dynamic range set at 70 dB. PSV and EDV were measured using the right and left cavernosal arteries, avoiding secondary, penetrating branch arteries from the dorsal artery. Since PSV depends on the cosine of the angle of insonation, the angle was kept as close to zero degrees as possible, parallel to the lumen of the artery (23).
LiSWT
After the initial 4-week run-in period, participants still meeting inclusion and exclusion criteria were randomized by the study coordinator to one of two treatment arms, each 1:2 simulated (sham) or active. Simulated or active shockwave treatments were performed using two different protocols, as shown in Figure 2.

Shockwaves were generated by an electrohydraulic shockwave device SoftWave TRT/UroGold 100MTS utilizing the parabolic applicator OP 155. This applicator generates a plasma-driven detonation resulting in a shockwave with a broad and widely-stretched 5 megapascal therapeutic volume. Due to the absence of a concentrated focus, a 14 mm wide shockwave is delivered to the erectile tissue with up to 30 mm penetration (Figure 3). The initial energy flux density setting was 0.12 mJ/mm2. If the participant could not tolerate this energy flux density, it was decreased by increments of 0.01 mJ/mm2. Shockwaves were delivered at a frequency of 4 Hz initially, then at 3 Hz when participants were rolled over from simulated to active treatment. Each treatment had a duration of approximately 30 minutes.
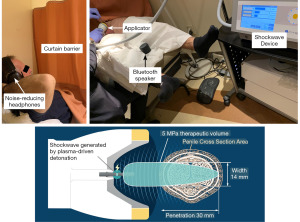
With the participant lying supine, noise-reducing headphones were provided and a curtain drawn, blocking visualization of the shockwave device including the applicator. For simulated treatment, a high-quality recording of shockwave sounds was played through a Bluetooth speaker. Since participants were naïve to acoustic wave therapy, there were no expectations of perceiving sensation from the applicator during simulated (no energy) treatment. The 6 specific penile locations treated are described in Table 2.
Table 2
| Tx Arm | Location | Single blind | Open label | |||
|---|---|---|---|---|---|---|
| # shocks | Frequency (shocks/s) | # shocks | Frequency (shocks/s) | |||
| Arm 1 | Hilum | |||||
| Left | 500 | 4 | 350 | 3 | ||
| Right | 500 | 4 | 350 | 3 | ||
| Penile shaft | ||||||
| Left | 1,000 | 4 | 700 | 3 | ||
| Right | 1,000 | 4 | 700 | 3 | ||
| Penile crus | ||||||
| Left | 1,000 | 4 | 700 | 3 | ||
| Right | 1,000 | 4 | 700 | 3 | ||
| Total | 5,000 | 4 | 3,500 | 3 | ||
| Arm 2 | Hilum | |||||
| Left | 300 | 4 | 200 | 3 | ||
| Right | 300 | 4 | 200 | 3 | ||
| Penile Shaft | ||||||
| Left | 600 | 4 | 400 | 3 | ||
| Right | 600 | 4 | 400 | 3 | ||
| Penile Crus | ||||||
| Left | 600 | 4 | 400 | 3 | ||
| Right | 600 | 4 | 400 | 3 | ||
| Total | 3,000 | 4 | 2,000 | 3 | ||
To treat the right and left hilum and right and left penile shaft, the penile glans were grasped and gently stretched and the applicator moved to each of these regions (Figure 3). The participant was subsequently placed in the lithotomy position in stirrups, the scrotum elevated, and the applicator directed to the right and left penile crura. Treatment was provided to all these regions per protocol (Table 2).
Clinical assessments
At baseline and each follow-up visit, clinical assessments included validated instruments, physical exam, GUS, and DUS. Unblinding occurred at the first follow-up visit, which was scheduled 20 weeks after the first simulated or active shockwave treatment. Participants initially assigned to sham were rolled over to active treatment, as shown in Figure 2. Active treatment was performed at 3 Hz with a decreased number of shocks delivered per treatment, as specified in Table 2, and a second follow-up visit occurred 40 weeks after the first simulated treatment. Participants assigned initially to the active treatment arm received no further treatment and underwent a second follow-up visit at 32 weeks after the first treatment (Figure 2).
Outcome measures
Subjective measures of EF included the EF domain (IIEF-EF) and total score of the IIEF (13), and the SEP diary (24). The two primary objective outcome measures of this study were assessment of GUS erectile tissue homogeneity/inhomogeneity by visual grading and DUS assessment of penile blood flow parameters (PSV and EDV). Changes in GUS erectile tissue homogeneity/inhomogeneity and changes in DUS penile blood flow are reflective of connective tissue activation and improved blood flow, respectively, the indications for which the SoftWave TRT/UroGold 100MTS shockwave device is cleared by the FDA (25). LUTS attributed to benign prostatic hyperplasia (LUTS/BPH) were assessed with the validated International Prostate Symptom Score (I-PSS) questionnaire (26,27). In addition, prostate-specific antigen (PSA) levels were measured. All subjective and objective outcome measures were analyzed by determining the change from baseline at each follow-up visit. Safety was assessed by collecting adverse events at each visit.
Data analysis
Due to limited enrollment during the coronavirus disease 2019 (COVID-19) pandemic, study participants randomized to the sham treatment protocols in each treatment arm were combined into a single cohort. Likewise, study participants randomized to the Active Treatment protocols in each treatment arm were also combined. This served to increase the statistical power of the study.
During screening, only one individual with ED was excluded from the study due to homogeneous imaging of corpus cavernosum by GUS. In participants who completed at least one treatment assessment, missing data due to early discontinuation from the study were imputed by the “last observation carried forward” (LOCF) method. Participants who discontinued early prior to any GUS/DUS assessments after shockwave or sham treatment (n=3) were not included in this analysis.
Statistical analysis
Comparisons between baseline and follow-up assessments were analyzed by repeated measures one-way analysis of variance (ANOVA) followed by Dunnett’s post-hoc test for pairwise comparisons to baseline. Blood flow data (PSV and EDV) in left and right cavernosal arteries were analyzed by two-way repeated measures ANOVA with Geisser-Greenhouse correction. Correlations were evaluated by Spearman’s rank correlation analyses. Other analyses were specified with the data presentation. All statistical analyses were performed using Prism 10.2.3 for Windows (GraphPad Software, Boston, MA, USA).
Results
Demographics
The target enrollment for this study was 60 participants. Recruitment, however, was stopped after randomizing 35 participants (13 sham, 22 active) due to the inability to further enroll during the COVID-19 pandemic. Participant enrollment, randomization, and retention are shown in Figure 4, with attrition primarily due to COVID-19. The study was conducted from August 5, 2019 to June 22, 2022, with 29 months of recruitment.
The demographic characteristics of our study cohort are shown in Table 3. Participants had a mean age of 50.1 years with no significant difference between participants in the sham versus active treatment arms (P=0.73). The baseline mean testosterone value in the study cohort (568 ng/dL) was in the mid-tertile of the normal range (175–781 ng/dL) with no statistically significant difference between the sham versus active treatment arms (P=0.36). Participants had a history of multiple medical conditions; consistent with the age and diagnosis of ED, the most common were endocrine/metabolic, musculoskeletal, and cardiovascular disorders. Medications utilized at study entry needed to be continued throughout, with the exception of treatments for ED that needed to be stopped during the 4-week period before baseline and follow-up visits.
Table 3
| Variables | Combined (N=35) | Sham (N=13) | Active Tx (N=22) | P value |
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 50.1 (11.3) | 51.1 (10.7) | 49.7 (11.6) | 0.73 |
| Median (Q1, Q3) | 50.0 (40.5, 60.0) | 48.0 (43.0, 60.0) | 52.5 (38.3, 58.8) | |
| Race/culture, n (%) | ||||
| White | 25 (71.4) | 9 (69.2) | 16 (72.7) | |
| White/Hispanic | 7 (20.0) | 3 (23.1) | 4 (18.2) | |
| Asian | 2 (5.7) | 0 | 2 (9.1) | |
| Mixed | 1 (2.9) | 1 (7.7) | 0 | |
| Testosterone, ng/dL | ||||
| Mean (SD) | 568 (244) | 618 (267) | 538 (231) | 0.36 |
| Median (Q1, Q3) | 489 (406, 632) | 521 (454, 649) | 480 (380, 600) | |
| PSA, ng/mL | ||||
| Mean (SD) | 1.04 (0.78) | 1.4 (1.0) | 0.8 (0.6) | 0.047 |
| Median (Q1, Q3) | 0.78 (0.51, 1.11) | 0.9 (0.6, 2.5) | 0.7 (0.5, 1.0) | |
| History of medical conditions, n (%) | ||||
| Endocrine/metabolic disorders | 25 (71.4) | 9 (69.2) | 16 (72.7) | |
| Musculoskeletal disorders | 16 (45.7) | 2 (15.4) | 14 (63.6) | |
| Cardiovascular disease | 15 (42.9) | 2 (15.4) | 13 (59.1) | |
| Genitourinary conditions | 11 (31.4) | 7 (53.8) | 4 (18.2) | |
| Mood disorders | 8 (22.9) | 3 (23.1) | 5 (22.7) | |
| Malignancy history | 8 (22.9) | 3 (23.1) | 5 (22.7) | |
| Neurological disorders | 7 (20.0) | 5 (38.5) | 2 (9.1) | |
| Gastrointestinal disorders | 5 (14.3) | 0 | 5 (22.7) | |
| History of medications, n (%) | ||||
| Androgen supplementation | 25 (71.4) | 10 (76.9) | 15 (68.2) | |
| Previous PDE5 inhibitor | 23 (65.7) | 10 (76.9) | 13 (59.1) | |
| Anti-hypertensive agents | 10 (28.6) | 6 (46.2) | 4 (18.2) | |
| Previous intracavernosal injection | 9 (25.7) | 3 (23.1) | 6 (27.3) | |
| Seasonal allergy meds | 8 (22.9) | 2 (15.4) | 6 (27.3) | |
| Antidepressants | 7 (20.0) | 2 (15.4) | 5 (22.7) | |
| Diabetes meds | 4 (11.4) | 2 (15.4) | 2 (9.1) | |
| Statins | 4 (11.4) | 1 (7.7) | 3 (13.6) | |
| LUTS/BPH meds | 3 (8.6) | 2 (15.4) | 1 (4.5) | |
| NSAIDs | 3 (8.6) | 0 | 3 (13.6) | |
| Pain meds | 3 (8.6) | 2 (15.4) | 1 (4.5) | |
| Proton pump inhibitors | 3 (8.6) | 0 | 3 (13.6) | |
| Antivirals | 1 (2.9) | 0 | 1 (4.5) | |
| History of alcohol use, n/N (%) | 22/31 (71.0) | 6/11 (54.5) | 16/20 (80.0) | |
| History of tobacco use, n/N (%) | 0/32 | 0/12 | 0/20 |
All patients were diagnosed with erectile dysfunction; this condition is not included in the table. P values refer to comparisons between Sham and Active Tx groups by unpaired t-test. BPH, benign prostatic hyperplasia; LUTS, lower urinary tract symptoms; NSAID, non-steroidal anti-inflammatory drug; PDE, phosphodiesterase inhibitor; PSA, prostate-specific antigen; SD, standard deviation.
LiSWT safety data
Treatment-related adverse events were limited to transient pain/discomfort at the site of the applicator during the procedure. During application of shockwave, one participant found the energy flux density of 0.12 mJ/mm2 intolerable, requiring the energy to be decreased throughout the study (range, 0.10–0.11 mJ/mm2), and two participants required a decrease to 0.11 mJ/mm2 on one visit only. All other adverse events were considered unrelated.
Subjective EF assessments (IIEF and SEP)
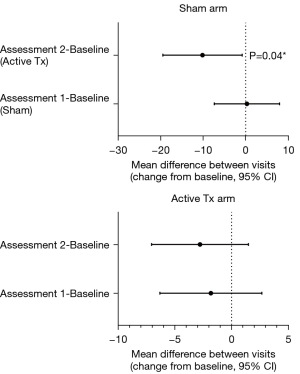
Mean baseline IIEF total scores were 32.2±10.4 in the Sham arm and 36.0±7.8 in the Active treatment arm, while mean baseline IIEF-EF scores were 11.5±5.7 and 12.0±5.6 in the Sham and Active treatment arms respectively. LiSWT consistently improved the mean total and EF domain scores of the IIEF in both arms after active treatment (Figure 5). For total IIEF score, these changes were statistically significant at Assessment 2 after participants in the Sham arm had completed active treatment (Week 40) or participants in the Active treatment arm had completed the study (Week 32). For IIEF-EF, participants in the Sham and Active treatment arms had increased scores that approached but did not reach statistical significance (Figure 5).

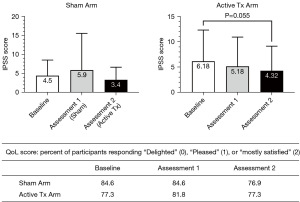
LiSWT also consistently increased success rates for SEP 2, 3, 4, and 5 in both arms after active treatment (Figure 6). In the Sham treatment arm, the mean success rate for SEP 3 (reflecting hardness for successful intercourse) increased from 11.7% at baseline to 45.5% after conversion to active treatment and approached statistical significance (P=0.08). In the Active Treatment Arm, the mean success rate for SEP 3 significantly increased (P=0.049) to 56.1% relative to baseline (22.7%). Success rates for SEP 2, 4 and 5 in the Active Treatment Arm approached but did not reach statistical significance.

Objective EF assessments
GUS erectile tissue homogeneity/inhomogeneity
At baseline, GUS erectile tissue homogeneity/inhomogeneity of study participants had a range of visual grades with grade 2 being the most prevalent (Figure 7A). Within participants, the mean coefficient of variation for visual grades between proximal, middle and distal regions of the penile shaft was 24.5%. Mean baseline visual grade scores for all participants ranged from 1.7±0.7 to 2.0±0.7 in each penile shaft region and the mean baseline visual grade total score (sum of 3 regions) was 5.4±1.7. To validate the use of visual grading as a clinically relevant parameter, visual grade total scores were compared to IIEF-EF scores and a statistically significant negative correlation (P=0.002) was found between these two assessments (Figure 7B).

Sham treatment did not show changes in visual grading of overall penile shaft erectile tissue homogeneity/inhomogeneity (total score) at 20 weeks (Assessment 1) relative to baseline (Figure 8). In contrast, participants in the Active Treatment Arm manifested statistically significant improvements relative to baseline for visual grading of overall penile shaft erectile tissue homogeneity/inhomogeneity at 20 (Assessment 1) and 32 weeks (Assessment 2). Representative examples of GUS images at baseline and after treatment are shown in Figure 9.


When examining the specific regions of the penile shaft, simulated treatment resulted in no significant changes from baseline in visual grading (data not shown). However, active treatment resulted in significant improvement in change from baseline in the proximal region at both follow-up assessments (Figure 10). Irrespective of statistically significant changes from baseline in tissue homogeneity/inhomogeneity, there was an increased percentage of participants with improved visual grading of GUS erectile tissue homogeneity/inhomogeneity in the proximal region after LiSWT in both treatment arms, while sham treatment did not cause such an increase (Figure 11).


Exploratory computer-assisted image analysis of GUS erectile tissue homogeneity/inhomogeneity
GUS erectile tissue homogeneity/inhomogeneity was also assessed using computer-assisted image analysis. To validate this method, independent measurements of hypoechoic area within the left and right corporal bodies in GUS images were compared in all participants and found to be significantly correlated (P<0.001) (Figure 12A). Visual grading of the same images was significantly correlated with the percentage of hypoechoic area determined by computer-assisted image analysis (Spearman’s r=0.427, 95% CI: 0.298–0.541; P<0.001) and the mean differences in hypoechoic area between each grade were significantly different as shown in Figure 12B. Finally, hypoechoic area had a statistically significant negative correlation with IIEF-EF score and this correlation maintained statistical significance when hypoechoic area was stratified by penile shaft region (Figure 13). Using this method, change from baseline in hypoechoic area was consistent with visual grading. As shown in Figure 14, simulated treatment did not change hypoechoic area, whereas active LiSWT reduced hypoechoic area with statistically significant changes for participants in the Sham arm that were converted to Active treatment (Assessment 2).


Duplex Doppler PSV and EDV measurements
Mean PSV and EDV values at baseline for the entire study cohort were 28.7±11.7 and 5.1±5.5 cm/s, respectively. Sham treatment did not significantly change PSV or EDV values from baseline (data not shown). However, while there were no significant differences in PSV, participants in the Active treatment arm had reduced mean EDV values from baseline that reached statistical significance for the left cavernosal artery at both follow-up assessments (Figure 15).

IPSS/QOL/PSA
The mean total IPSS score at baseline for the entire study cohort was 6.0±5.9, indicating mild symptoms of LUTS/BPH. There was no significant change in IPSS score in any treatment arm (Figure 16), although there was a small trend for total IPSS scores to decrease after LiSWT and this approached statistical significance in the Active treatment arm at Assessment 2. Concerning quality of life (QoL) scores related to urinary function, a majority of participants reported a high value at baseline with no significant change throughout the study (Figure 16).
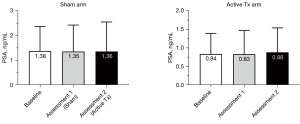
The mean baseline PSA value was 1.04 ng/mL. While mean PSA values were lower in the active treatment arm compared to the sham group (P=0.047), within each treatment arm, PSA levels remained stable throughout the study with no significant increases in either treatment arm (Figure 17).
Discussion
Key findings
The age and vascular risk factors of our study cohort were similar to those reported in other epidemiologic studies of men with ED secondary to cardiovascular disease and generalized atherosclerosis, conditions associated with cavernosal fibrosis and corporal veno-occlusive dysfunction (15,28-31). Sham treatment was simulation only and delivered no energy to the penis as the applicator was not activated. Sham treatment showed no statistically significant difference from baseline for any measure of erectile or urinary function, including assessments by IIEF, SEP, GUS visual grading of erectile tissue homogeneity/inhomogeneity, PSV, EDV, and IPSS.
Active LiSWT consistently improved subjective measures of EF, significantly increasing mean IIEF total scores and mean success rates for SEP 3 over baseline and increasing mean IIEF-EF scores to an extent approaching statistical significance. Active LiSWT also significantly improved mean visual grades of GUS and significantly reduced EDV. An EDV value approaching zero is consistent with effective subtunical venule compression against the tunica, and normal corporal veno-occlusive function. Lastly, active LiSWT improved LUTS while PSA levels remained stable throughout the study.
Although some assessments did not reach statistical significance, trends toward improvement after active LiSWT were consistent. Premature termination of enrollment due to the COVID-19 pandemic limited the overall number of participants and reduced statistical power. Nevertheless, participants in the Active Treatment Arm continued to improve after the 20-week follow-up assessment even though they received no further shockwave treatment through the end of the study (32 weeks after initiation of LiSWT). This observation is consistent with the fact that shockwave-induced effects of mechanotransduction that lead to biochemical signal changes in tissue are progressive, extending over months after LiSWT. Such a continuing improvement of tissue health was noted by Holfeld et al. up to 1-year post-treatment (32).
Strengths and limitations
One strength of this sham-controlled, prospective LiSWT study was that it used objective ultrasound-based primary outcome measures. In addition to DUS-based PSV and EDV values, visual grading of GUS images was used for the first time, supported by statistically significant negative correlation with IIEF-EF scores and statistically significant positive correlation with percentage of hypoechoic area as determined by computer-assisted image analysis. Another strength is the use of a true sham, and not just a low setting of energy for the applicator. An additional strength is the assessment of LUTS/BPH changes following LiSWT in these participants with ED. The major limitation is the small sample size due to recruitment being stopped during the COVID-19 pandemic.
Comparison with similar research
Since 2010, multiple sham-controlled prospective trials of LiSWT have been performed in men with ED. Subjective patient-reported outcomes such as the IIEF have demonstrated significant improvement after LiSWT. Objective hemodynamic testing measuring PSV and EDV values obtained during erect penile DUS studies have also shown significant improvement. In addition to improvements in subjective and objective hemodynamic parameters after LiSWT, we observed decreased erectile tissue inhomogeneity with GUS, consistent with improved EF. The mechanisms by which LiSWT changes erectile physiology have been well described in animal models.
Animal models
LiSWT to the penis has resulted in improved EF in animal models of diabetes (33-35), obesity (36), hypertension (37), nerve injury (38), and aging (39), conditions associated with compromised EF. Mechanistic insights on the benefits of LiSWT have been gained from studies demonstrating significantly increased in situ penile progenitor cell activation within erectile tissues (40), significantly increased endogenous mesenchymal stem cell density, cavernosal neuronal nitric oxide synthase expression, and cavernosal smooth muscle content in treated versus untreated animals (34). LiSWT has also been shown to significantly improve intensity of stromal cell-derived factor-1 expression and increase cavernosal α-smooth muscle actin concentration (33). In other animal studies, LiSWT has been shown to increase cavernosal smooth muscle content and improve cavernosal smooth muscle/collagen ratio compared to controls (36,37,41). It is thus hypothesized that these progenitor stem cells differentiate into more specialized cells, including cavernosal smooth muscle and endothelial cells, and increase cavernosal collagen degradation, resulting in improved veno-occlusive function and vasodilation in the corpora cavernosa (36,37,41).
Similar changes have been noted in other realms of cardiovascular research. In animal models of acute and chronic ischemic heart failure, extensive LiSWT studies using biopsied myocardial tissue examined changes in cardiac muscle function (42-44). These models showed that cardiac LiSWT resulted in secretion of angiogenic cytokines and growth factors improving angiogenesis and arteriogenesis in the border zone of the ischemic myocardium, thereby improving cardiac muscle function (42-44).
Prospective LiSWT clinical trials for ED
A meta-analysis of 7 prospective clinical trials with a total of 607 participants suggested that LiSWT improves EF in individuals with ED, particularly of vasculogenic etiology, and appears to be a safe and effective nonpharmacologic disease modifying treatment option (45). In this meta-analysis, the mean IIEF-EF score at 1-month post-treatment showed a statistically significant increase in the active versus sham groups. No significant adverse events were reported (45). In a more recent sham-controlled study, LiSWT significantly improved EF when assessed by the Sexual Health Inventory for Men and EHS with sustained improvement up to 6 months after treatment (46). In a double-blind, randomized, sham-controlled trial of men with documented vasculogenic ED, mean PSV was statistically significantly increased in the LiSWT versus sham groups 3 months after treatment (47). In a different clinical trial, mean baseline EDV was significantly lowered after LiSWT (48).
LiSWT and B-mode GUS imaging
Corporal erectile tissue fibrosis, a significant pathophysiology of ED, is secondary to corporal veno-occlusive dysfunction, and results from atherosclerotic vascular disease (6,31). Poor erectile tissue expandability from excess corporal erectile tissue fibrosis prevents closure of the subtunical space and effective compression of subtunical venules. The gold standard for hemodynamic assessment of EF is DUS, measuring PSV and EDV. GUS, which can be performed concomitantly with DUS (23) using high resolution sonographic equipment, has been reported to detect cavernosal erectile tissue fibrosis (49). This is particularly relevant as atherosclerosis-associated cavernosal erectile tissue fibrosis in the penile shaft is usually diffuse and not palpable on physical examination. Since diagnosis of ED secondary to corporal veno-occlusive dysfunction is currently based on elevated EDV values on DUS, a false negative test may occur in the presence of complete smooth muscle relaxation by maximal use of smooth muscle relaxation agents, resulting in low EDV values despite the presence of corporal erectile tissue fibrosis (50). Objective assessment of erectile tissue homogeneity/inhomogeneity by GUS may represent an additional, more sensitive measure of the integrity of corporal veno-occlusive function. Preliminary findings from a small study reported that erectile tissue homogeneity/inhomogeneity on GUS was correlated to cavernosal smooth muscle and connective tissue content based on erectile tissue biopsy; those men with elevated visual grades of erectile tissue inhomogeneity were noted to have a high percentage of corporal erectile tissue fibrosis while those with low visual grades had a low percentage of corporal erectile tissue fibrosis (51). Thus, performing GUS with objective assessment of visual grades of erectile tissue homogeneity/inhomogeneity before and after LiSWT may be a new strategy for assessing efficacy of shockwave therapy for ED.
LiSWT for LUTS/BPH symptoms
To the best of our knowledge, there are no reports examining the changes in LUTS/BPH symptoms after LiSWT treatment for ED. Zhang et al. utilized perineal LiSWT to treat LUTS/BPH symptoms in men poorly responsive to medical therapy and not considered candidates for surgery (52). They reported that LiSWT resulted in statistically significant improvements in IPSS, QOL and IIEF that were sustained at 3 months (52). They hypothesized that LiSWT increased synthesis of nitric oxide, resulting in prostate smooth muscle relaxation and improvement in LUTS/BPH symptoms. Since there is a high association between ED and LUTS/BPH, future LiSWT clinical trials for ED should consider including urinary outcome measures (53).
LiSWT and PSA
There are also no reports examining changes in PSA blood test values after LiSWT treatment for ED. Kim et al., however, reported on the changes in PSA blood test values with perineal LiSWT in a population of men with chronic pelvic pain syndrome (CPPS) (54). At the 4-week follow-up, there was no statistically significant difference in PSA between the LiSWT and sham group. Zimmermann et al. similarly studied perineal LiSWT in men with CPPS and again found no statistically significant difference in PSA before and after the LiSWT (55).
Explanations of findings
In individuals with ED, LiSWT to the penis has been hypothesized to induce numerous downstream biochemical signaling events within the erectile tissue through the process of mechanotransduction, as supported by animal studies (56-58). In these animal studies, it was observed that the ratio of cavernosal smooth muscle to connective tissue increased (59). Similarly, the ratio of cavernosal smooth muscle to connective tissue may have been improved by LiSWT in our study cohort, as demonstrated by statistically significant changes in erectile tissue homogeneity on GUS in individuals with ED.
The concept of LiSWT improving smooth muscle function and/or increasing tissue content of smooth muscle is further supported by the observation of clinical function improvement in patients with ischemic heart failure undergoing cardiac LiSWT. Holfeld et al. performed a single-blind, parallel-group, sham-controlled trial involving direct cardiac LiSWT in addition to coronary artery bypass surgery in patients with ischemic heart failure (32). They found a statistically significant improvement in left ventricular ejection fraction at 1 year follow-up in the direct cardiac LiSWT group (n=30) versus sham (n=28). These findings are consistent with our study, which similarly shows improved smooth muscle function and tissue composition following penile LiSWT up to 32 weeks post-treatment. We conclude that LiSWT is a disease modifying strategy that improves muscle health and decreases tissue fibrosis in both the heart and the penis (Figure 18), thereby improving the function of both organs.

Conclusions
This is a single-blind, sham-controlled, randomized, prospective, Institutional Review Board (IRB)-approved study in men with ED naïve to shockwave and/or radial ballistic pressure wave therapy conducted at a single sexual medicine facility and terminated prematurely because of COVID-19. This clinical trial with LiSWT utilized traditional, widely-used subjective and objective outcome parameters, but to our knowledge, it is the first to utilize visual grading of GUS imaging as one of the primary outcome measures to assess changes in corporal veno-occlusive function and EF. The improvement in erectile tissue homogeneity after LiSWT is a novel finding, consistent with the hypothesis that LiSWT-induced mechanotransduction results in numerous beneficial cellular and subcellular changes within the cavernosal erectile tissue. LiSWT appears to be safe and efficacious for ED. Additionally, LiSWT appears to improve LUTS without affecting PSA. Future research exploring LiSWT for ED should consider utilizing GUS imaging to better assess cavernosal tissue changes responsible for the improvement in EF, as well as BPH/LUTS.
Acknowledgments
The authors would like to thank JoAnna Williams, CCRC as well as sub-investigators Catherine Gagnon, FNP-BC and Julea Minton, NP-C for their time, dedication and professionalism working on this trial.
Funding: This research did not receive any funding; however, participants were asked to pay a nominal fee to support the IRB costs and laboratory fees.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-24-338/rc
Trial Protocol: Available at https://tau.amegroups.com/article/view/10.21037/tau-24-338/tp
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-24-338/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-24-338/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-24-338/coif). S.W.G. serves on the advisory board of Softwave TRT. I.G. serves on the advisory board of Softwave TRT, on the speaker’s bureau, and is named on a pending patent. These conflicts of interest occurred after completion of the research. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Aspire Independent Review Board (No. 520190180) and informed consent was taken from all the participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA 1993;270:83-90. [Crossref] [PubMed]
- Jackson G. Erectile dysfunction and cardiovascular disease. Int J Clin Pract 1999;53:363-8. [Crossref] [PubMed]
- Rosen RC, Miner M, Burnett AL, et al. Proceedings of PRINCETON IV: PDE5 inhibitors and cardiac health symposium. Sex Med Rev 2024;12:681-709.
- Levine FJ, Greenfield AJ, Goldstein I. Arteriographically determined occlusive disease within the hypogastric-cavernous bed in impotent patients following blunt perineal and pelvic trauma. J Urol 1990;144:1147-53. [Crossref] [PubMed]
- Azadzoi KM, Goldstein I. Erectile dysfunction due to atherosclerotic vascular disease: the development of an animal model. J Urol 1992;147:1675-81. [Crossref] [PubMed]
- Nehra A, Goldstein I, Pabby A, et al. Mechanisms of venous leakage: a prospective clinicopathological correlation of corporeal function and structure. J Urol 1996;156:1320-9. [Crossref] [PubMed]
- Nehra A, Azadzoi KM, Moreland RB, et al. Cavernosal expandability is an erectile tissue mechanical property which predicts trabecular histology in an animal model of vasculogenic erectile dysfunction. J Urol 1998;159:2229-36. [Crossref] [PubMed]
- Narasimman M, Sandler M, Bernstein A, et al. A primer on the restorative therapies for erectile dysfunction: a narrative review. Sex Med Rev 2024;12:505-12. [Crossref] [PubMed]
- Vardi Y, Appel B, Jacob G, et al. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol 2010;58:243-8. [Crossref] [PubMed]
- de Oliveira PS, Ziegelmann MJ. Low-intensity shock wave therapy for the treatment of vasculogenic erectile dysfunction: a narrative review of technical considerations and treatment outcomes. Transl Androl Urol 2021;10:2617-28. [Crossref] [PubMed]
- Sokolakis I, Dimitriadis F, Teo P, et al. The Basic Science Behind Low-Intensity Extracorporeal Shockwave Therapy for Erectile Dysfunction: A Systematic Scoping Review of Pre-Clinical Studies. J Sex Med 2019;16:168-94. [Crossref] [PubMed]
- Kalyvianakis D, Mykoniatis I, Pyrgidis N, et al. The Effect of Low-Intensity Shock Wave Therapy on Moderate Erectile Dysfunction: A Double-Blind, Randomized, Sham-Controlled Clinical Trial. J Urol 2022;208:388-95. [Crossref] [PubMed]
- Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822-30. [Crossref] [PubMed]
- Lue TF, Hricak H, Marich KW, et al. Vasculogenic impotence evaluated by high-resolution ultrasonography and pulsed Doppler spectrum analysis. Radiology 1985;155:777-81. [Crossref] [PubMed]
- Kim J, Drury R, Morenas R, et al. Pathophysiology and Grayscale Ultrasonography of Penile Corporal Fibrosis. Sex Med Rev 2022;10:99-107. [Crossref] [PubMed]
- Bertolotto M, Calderan L, Cova MA. Imaging of penile traumas--therapeutic implications. Eur Radiol 2005;15:2475-82. [Crossref] [PubMed]
- Connolly JA, Borirakchanyavat S, Lue TF. Ultrasound evaluation of the penis for assessment of impotence. J Clin Ultrasound 1996;24:481-6. [Crossref] [PubMed]
- Dotmatics. Randomly assign subjects to treatment groups. GraphPad Software. Accessed September 20, 2019. Available online: https://www.graphpad.com/quickcalcs/randomize1.cfm
- Mulhall JP, Abdel-Moneim A, Abobakr R, et al. Improving the accuracy of vascular testing in impotent men: correcting hemodynamic alterations using a vasoactive medication re-dosing schedule. J Urol 2001;166:923-6. [Crossref] [PubMed]
- Goldstein S, Goldstein I, Kim N, et al. Interim Analysis of a Sham-Controlled Randomized, Prospective Study Using Low Intensity Shockwave Therapy (LiSWT) for Improvement of Erectile Function. J Sex Med 2023;20:qdad060.124.
- Winter A, Rubin R, Goldstein I. Improving Diagnostic Capture of Organic ED on Ultrasound: Corporal Heterogeneity Grading to Assess Penile Fibrosis. J Sex Med 2018;15:S82. [Crossref]
- Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012;9:676-82. [Crossref] [PubMed]
- Sikka SC, Hellstrom WJ, Brock G, et al. Standardization of vascular assessment of erectile dysfunction: standard operating procedures for duplex ultrasound. J Sex Med 2013;10:120-9. [Crossref] [PubMed]
- Araujo AB, Allen KR, Ni X, et al. Minimal clinically important differences in the vaginal insertion and successful intercourse items of the sexual encounter profile. J Sex Med 2012;9:169-79. [Crossref] [PubMed]
- SoftWave Tissue Regeneration Technologies Receives FDA Clearance for Blood Circulation Increase, Minor Aches and Pains Relief and Connective Tissue Activation. m1y 4, 2021, 2021. Accessed April 7. 2024. Available online: https://softwavetrt.com/softwave-tissue-regeneration-technologies-receives-fda-clearance-for-blood-circulation-increase-minor-aches-and-pains-relief-and-connective-tissue-activation/
- el Din KE, Koch WF, de Wildt MJ, et al. Reliability of the International Prostate Symptom Score in the assessment of patients with lower urinary tract symptoms and/or benign prostatic hyperplasia. J Urol 1996;155:1959-64. [Crossref] [PubMed]
- van Venrooij GE, Boon TA, de Gier RP. International prostate symptom score and quality of life assessment versus urodynamic parameters in men with benign prostatic hyperplasia symptoms. J Urol 1995;153:1516-9. [Crossref] [PubMed]
- Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54-61. [Crossref] [PubMed]
- Johannes CB, Araujo AB, Feldman HA, et al. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol 2000;163:460-3. [Crossref] [PubMed]
- Allen MS, Tostes RC. Cigarette smoking and erectile dysfunction: an updated review with a focus on pathophysiology, e-cigarettes, and smoking cessation. Sex Med Rev 2023;11:61-73. [Crossref]
- Sáenz de Tejada I, Angulo J, Cellek S, et al. Pathophysiology of erectile dysfunction. J Sex Med 2005;2:26-39. [Crossref] [PubMed]
- Holfeld J, Nägele F, Pölzl L, et al. Cardiac shockwave therapy in addition to coronary bypass surgery improves myocardial function in ischaemic heart failure: the CAST-HF trial. Eur Heart J 2024;45:2634-43. [Crossref] [PubMed]
- Shin D, Jeon SH, Tian WJ, et al. Extracorporeal shock wave therapy combined with engineered mesenchymal stem cells expressing stromal cell-derived factor-1 can improve erectile dysfunction in streptozotocin-induced diabetic rats. Transl Androl Urol 2021;10:2362-72. [Crossref] [PubMed]
- Qiu X, Lin G, Xin Z, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med 2013;10:738-46. [Crossref] [PubMed]
- Assaly-Kaddoum R, Giuliano F, Laurin M, et al. Low Intensity Extracorporeal Shock Wave Therapy Improves Erectile Function in a Model of Type II Diabetes Independently of NO/cGMP Pathway. J Urol 2016;196:950-6. [Crossref] [PubMed]
- Ruan Y, Zhou J, Kang N, et al. The effect of low-intensity extracorporeal shockwave therapy in an obesity-associated erectile dysfunction rat model. BJU Int 2018;122:133-42. [Crossref] [PubMed]
- Assaly R, Giuliano F, Clement P, et al. Extracorporeal Shock Waves Therapy Delivered by Aries Improves Erectile Dysfunction in Spontaneously Hypertensive Rats Through Penile Tissue Remodeling and Neovascularization. Sex Med 2019;7:441-50. [Crossref] [PubMed]
- Wang HS, Ruan Y, Banie L, et al. Delayed Low-Intensity Extracorporeal Shock Wave Therapy Ameliorates Impaired Penile Hemodynamics in Rats Subjected to Pelvic Neurovascular Injury. J Sex Med 2019;16:17-26. [Crossref] [PubMed]
- Sokolakis I, Dimitriadis F, Psalla D, et al. Effects of low-intensity shock wave therapy (LiST) on the erectile tissue of naturally aged rats. Int J Impot Res 2019;31:162-9. [Crossref] [PubMed]
- Lin G, Reed-Maldonado AB, Wang B, et al. In Situ Activation of Penile Progenitor Cells With Low-Intensity Extracorporeal Shockwave Therapy. J Sex Med 2017;14:493-501. [Crossref] [PubMed]
- Lei H, Xin H, Guan R, et al. Low-intensity Pulsed Ultrasound Improves Erectile Function in Streptozotocin-induced Type I Diabetic Rats. Urology 2015;86:1241.e11-8. [Crossref] [PubMed]
- Gollmann-Tepeköylü C, Lobenwein D, Theurl M, et al. Shock Wave Therapy Improves Cardiac Function in a Model of Chronic Ischemic Heart Failure: Evidence for a Mechanism Involving VEGF Signaling and the Extracellular Matrix. J Am Heart Assoc 2018;7:e010025. [Crossref] [PubMed]
- Holfeld J, Zimpfer D, Albrecht-Schgoer K, et al. Epicardial shock-wave therapy improves ventricular function in a porcine model of ischaemic heart disease. J Tissue Eng Regen Med 2016;10:1057-64. [Crossref] [PubMed]
- Tepeköylü C, Primessnig U, Pölzl L, et al. Shockwaves prevent from heart failure after acute myocardial ischaemia via RNA/protein complexes. J Cell Mol Med 2017;21:791-801. [Crossref] [PubMed]
- Campbell JD, Trock BJ, Oppenheim AR, et al. Meta-analysis of randomized controlled trials that assess the efficacy of low-intensity shockwave therapy for the treatment of erectile dysfunction. Ther Adv Urol 2019;11:1756287219838364. [Crossref] [PubMed]
- Kennady EH, Bryk DJ, Ali MM, et al. Low-intensity shockwave therapy improves baseline erectile function: a randomized sham-controlled crossover trial. Sex Med 2023;11:qfad053. [Crossref] [PubMed]
- Kalyvianakis D, Hatzichristou D. Low-Intensity Shockwave Therapy Improves Hemodynamic Parameters in Patients With Vasculogenic Erectile Dysfunction: A Triplex Ultrasonography-Based Sham-Controlled Trial. J Sex Med 2017;14:891-7. [Crossref] [PubMed]
- De Oliveira PS, De Oliveira TR, Nunes Á, et al. Low-intensity shock wave therapy for erectile dysfunction and the influence of disease duration. Arch Ital Urol Androl 2019;90:276-82. [Crossref] [PubMed]
- Bertolotto M, Perrone R, Bucci S, et al. Comparison of conventional ultrasound and real-time spatial compound imaging in evaluation of patients with severe Peyronie's disease. Acta Radiol 2008;49:596-601. [Crossref] [PubMed]
- Nascimento B, Miranda EP, Terrier JE, et al. A Critical Analysis of Methodology Pitfalls in Duplex Doppler Ultrasound in the Evaluation of Patients With Erectile Dysfunction: Technical and Interpretation Deficiencies. J Sex Med 2020;17:1416-22. [Crossref] [PubMed]
- Kim N, Yee A, Goldstein A, et al. Correlation of Penile Grayscale Pharmaco-Ultrasonography with Erectile Tissue Composition in Men with Erectile Dysfunction. J Urol 2023;209:e366. [Crossref]
- Zhang D, Wang YL, Gong DX, et al. Radial Extracorporeal Shock Wave Therapy as a Novel Agent for Benign Prostatic Hyperplasia Refractory to Current Medical Therapy. Am J Mens Health 2019;13:1557988319831899. [Crossref] [PubMed]
- De Nunzio C, Roehrborn CG, Andersson KE, et al. Erectile Dysfunction and Lower Urinary Tract Symptoms. Eur Urol Focus 2017;3:352-63. [Crossref] [PubMed]
- Kim KS, Choi YS, Bae WJ, et al. Efficacy of Low-Intensity Extracorporeal Shock Wave Therapy for the Treatment of Chronic Pelvic Pain Syndrome IIIb: A Prospective-Randomized, Double-Blind, Placebo-Controlled Study. World J Mens Health 2022;40:473-80. [Crossref] [PubMed]
- Zimmermann R, Cumpanas A, Hoeltl L, et al. Extracorporeal shock-wave therapy for treating chronic pelvic pain syndrome: a feasibility study and the first clinical results. BJU Int 2008;102:976-80. [Crossref] [PubMed]
- Liu T, Shindel AW, Lin G, et al. Cellular signaling pathways modulated by low-intensity extracorporeal shock wave therapy. Int J Impot Res 2019;31:170-6. [Crossref] [PubMed]
- Drury R, Natale C, Hellstrom WJG. Reviewing the evidence for shockwave- and cell-based regenerative therapies in the treatment of erectile dysfunction. Ther Adv Urol 2021;13:17562872211002059. [Crossref] [PubMed]
- d'Agostino MC, Craig K, Tibalt E, et al. Shock wave as biological therapeutic tool: From mechanical stimulation to recovery and healing, through mechanotransduction. Int J Surg 2015;24:147-53. [Crossref] [PubMed]
- Peng D, Yuan H, Liu T, et al. Smooth Muscle Differentiation of Penile Stem/Progenitor Cells Induced by Microenergy Acoustic Pulses In Vitro. J Sex Med 2019;16:1874-84. [Crossref] [PubMed]



