Research in pharmacotherapy for erectile dysfunction
Introduction
Penile erection is a neurovascular phenomenon and multiple pathogenic factors, such as vascular risk factors or diseases, neurologic abnormalities, and hormonal disturbances, are associated with erectile dysfunction (ED) (1). Although oral phosphodiesterase-5 (PDE5) inhibitors, which enhance the nitric oxide (NO)-cGMP pathway by inhibiting the breakdown of cGMP, are generally regarded as an effective therapy for ED, men with ED from diabetes or radical prostatectomy respond poorly to these drugs (2). Severe endothelial dysfunction and cavernous nerve damage are mainly responsible for the poor responsiveness of these patients to PDE5 inhibitors (3,4). Because the effects of PDE5 inhibitors rely on endogenous NO formation, PDE5 inhibitors may fail to increase the level of cGMP above the necessary threshold if the endogenous bioavailable NO is insufficient as the result of severe endothelial dysfunction or nerve damage. Moreover, PDE5 inhibitors are not able to reverse the underlying vascular or neural dysfunction associated with ED. It is another limitation that PDE5 inhibitor in combination with any nitrate compound is prohibited because of the risk of hypotensive crisis (5). Therefore, a new treatment strategy that overcomes the pitfalls of currently available oral PDE5 inhibitors is required.
Many researchers have tried to develop novel therapeutics that target alternative molecular pathways. With the introduction of apomorphine hydrochloride (Uproma SL) into ED market, therapeutics with a central mode of action which target dopamine and melanocortin receptors have been developed and their effectiveness on penile erection have been intensively investigated in preclinical models. Meanwhile, a variety of pharmacologic agents with a peripheral mode of action have been also studied. Those specifically target sites upstream of the NO-dependent activation of soluble guanylate cyclase, the Rho-kinase pathway, and the Maxi-K channel.
Recently, restoring NO bioavailability by rejuvenating or regenerating cavernous endothelial cells, cavernous nerve, or inhibiting tissue fibrosis secondary to tissue hypoxia from neurovascular dysfunction, was found to be a promising therapeutic strategy for ED, especially in men with severe ED that is unresponsive to PDE5 inhibitors. A variety of preclinical trials targeting therapeutic angiogenesis or neural regeneration as well as anti-fibrosis have been reported. This article addresses the current therapeutic targets for ED under clinical or preclinical development, including pharmacotherapy, protein therapy, and gene therapy.
Clinical trials to develop ED therapeutics targeting alternative pathway
Although oral PDE5 inhibitors are effective in the majority of patients, they are not satisfactory for all men with ED. Therefore, many researchers have tried to develop new drugs that target different molecular pathways both centrally and peripherally (Table 1).

Full table
The efficacy and safety of melanocortin receptor agonists, such as melanotan II and bremelanotide, have been evaluated in patients with ED. Subcutaneous administration of melanotan II induced penile erection in 17 of 20 men with psychogenic or organic ED. However, a significant proportion of patients experienced adverse events including severe nausea and yawning (6). Intranasal delivery of bremelanotide significantly improved erectile function in men with diabetic ED (7) or in patients with PDE5 inhibitor failure (8). Intranasal administration of bremelanotide also frequently caused nausea and hypertension. No further clinical developments has been made due to the lack of tolerability.
ABT-724 is a selective dopamine D4 receptor agonist, which successfully induced penile erection in a rat model without side effects such as nausea and emesis (13). ABT-670 is known to have better oral bioavailability than ABT-724 while having similar efficacy (14). However, these selective dopamine D4 receptor agonists are no longer in development.
Clavulanic acid is known to have proerectile potential in rats through upregulation of serotonin and dopamine (15). Although clavulanic acid improved erectile function in a phase IIb clinical trial with 39 ED patients (9), additional clinical trials have been suspended.
Peripherally acting agents including soluble guanylate cyclase activators and Rho-kinase inhibitors have been shown to improve erectile function in clinical trials (10,11). However, development has ceased because these drugs did not show superiority to currently available oral PDE5 inhibitors. Opening of potassium channel causes a decrease in intracellular free calcium concentration and induces relaxation of the cavernous smooth muscle (16). Although the first phase I clinical trial result by using calcium-sensitive Maxi-K ion channel gene (hSlo cDNA) was reported 10 years ago with a good safety profile and encouraging results (12), no additional clinical trial has been done.
Preclinical studies with biotherapy
Therapeutic angiogenesis
The penis is a richly vascularized organ, and ED is predominantly a vascular disease (17). The vascular endothelium plays an important role in the regulation of vascular tone and blood flow (18). The cavernous endothelium, which is located on the inner surface of the lacunar spaces in the erectile tissue, also has a crucial role in regulating the tone of the underlying smooth muscle and physiologic penile erection (19). Recently, a link between ED and cardiovascular disease was uncovered, and both diseases were shown to share the same risk factors, including hypercholesterolemia, hypertension, diabetes mellitus, and smoking, with endothelial cell dysfunction being the common denominator between these two conditions (20,21). Therefore, the development of a new therapeutic strategy that reestablishes structural and functional microvasculature in the erectile tissue is needed to treat the underlying conditions of ED and to overcome the limitations of oral PDE5 inhibitors.
Many investigators have reported preclinical results of angiogenic growth factor therapy, such as vascular endothelial growth factor (VEGF), angiopoietins, and basic fibroblast growth factor (bFGF) (Table 2). Of those growth factors, VEGF and angiopoietins have been the most extensively studied. Local intracavernous delivery of VEGF gene or protein has been shown to restore erectile function in rat models of ED induced by castration, diabetes, or dyslipidemia (22-25). However, it was reported that VEGF administration can initiate vessel formation in adult animals, but by itself promotes the formation of leaky, immature and unstable vessels (34), which greatly limits the therapeutic utility of VEGF. In comparison, angiopoietin-1, the ligand of the Tie-2 receptor, is known to generate non-leaky, stable, and mature blood vessels in pre- and postnatal angiogenesis (35). Angiopoietin-1 also counteracts VEGF-induced inflammation in endothelial cells while having an additive effect on vessel formation (36). It was recently demonstrated in a rat model of hypercholesterolemic ED that adenovirus-mediated combined gene delivery of angiopoietin-1 and VEGF into the corpus cavernosum produces an additive effect on erectile function through complete restoration of cavernous endothelial cell content compared with that of either therapy alone (26).
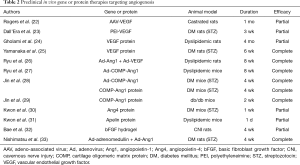
Full table
It was also reported in mouse models of diabetic ED and dyslipidemic ED that a single intracavernous injection of adenovirus-mediated synthetic angiopoietin-1 gene, a soluble and potent angiopoietin-1 variant, or two successive intracavernous injections of synthetic angiopoietin-1 protein significantly increased cavernous endothelial cell proliferation, endothelial nitric oxide synthase (eNOS) phosphorylation, and cGMP expression and decreased the production of reactive oxygen species (ROS), such as superoxide anion and peroxynitrite. These changes restored erectile function up to 4 weeks in diabetic mice and 8 weeks in dyslipidemic mice (27-29). Although relatively long-term recovery of erectile function achieved with angiopoietin-1 protein is noteworthy, difficulty in large-scale production of the recombinant protein limits the clinical application of this therapy.
Angiopoietin-4 also represents a family of angiopoietins and plays a crucial role in angiogenesis (37). It was found that local delivery of angiopoietin-4 protein restores the endogenous Akt-NO pathway through regeneration of cavernous endothelial cells and inhibition of ROS generation, which resulted in recovery of erectile function in diabetic mice. However, the effect of angiopoietin-4 protein on diabetes-induced ED was partial, and the duration of erectile function recovery was relatively short (30).
Apelin is the endogenous ligand for the G-protein-coupled receptor APJ (38), and apelin and its receptor are expressed in a wide range of tissues, particularly in the endothelial and smooth muscle cells of the heart and blood vessels (39). A significant restoration of erectile function was noted 1 day after injection of apelin protein into the penis of hypercholesterolemic mice. However, erectile function returned to baseline values thereafter. The beneficial effects of apelin on erectile function resulted mainly from activation of eNOS and increase in NO bioavailability through a reduction in ROS-mediated endothelial apoptosis rather than through direct endothelial cell proliferation (31).
bFGF is a potent angiogenic growth factor, and local delivery of hydrogel-mediated bFGF protein into the subcutaneous tissue of penis partially restored erectile function in mice with cavernous nerve injury (CNI) through the restoration of cavernous endothelial and smooth muscle cell contents (32).
Adrenomedullin is a vasoactive peptide that has vasorelaxant and proangiogenic activities (40). Adenovirus-mediated combined gene therapy of adrenomedullin and angiopoietin-1 into the corpus cavernosum of diabetic mice produce an additive effect on erectile function by increasing the expression of vascular endothelial-cadherin and smooth muscle α-actin (33).
Therapeutic neural regeneration
Similar to the premise of therapeutic angiogenesis, neuromodulatory strategies offer the potential to restore normal erectile function. NO released from the cavernous nerve within erectile tissue causes relaxation of smooth muscle and initiates penile erection. CNI is a major cause of ED after radical prostatectomy, and restoration of cavernous nerve function is a final goal for neuroprotective or regenerative strategies. Even with bilateral nerve-sparing approaches, some degree of CNI or neurapraxia is unavoidable owing to an incomplete knowledge of the course of cavernous nerves surrounding the prostate. Advances in the science and technology in the neurobiology field have led to neuromodulatory strategies targeting cavernous nerve protection or regeneration. Several treatment modalities are currently under investigation in animal models, including immunophilin ligands, neurotrophins, sonic hedgehog (SHH), monoclonal antibody to nerve injury-induced protein 1 (Ninjurin-1) or neurotrophic tyrosine kinase receptor type 1 (TrkA), and glial growth factor-2 (GGF-2) (Table 3). Although immunophilin ligands have been shown to induce recovery of erectile function at the preclinical level (49), a clinical trial to evaluate the efficacy of GPI 1485, a nonimmunosuppressive analogue of FK506, on erectile function in men undergoing nerve-sparing radical prostatectomy failed to show the recovery of erectile function (50).
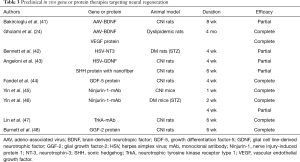
Full table
Neurotrophins, including brain-derived nerve growth factor (BDNF), neurotrophin-3 (NT-3), glial cell line-derived neurotrophic factor (GDNF), and growth differentiation factor-5 (GDF-5), have been shown to restore erectile function through neuroprotection or neural regeneration of the cavernous nerve in animal models of ED (24,41-44).
SHH is known to play an important role in nerve regeneration and improve nerve function in an animal model of diabetic neuropathy (51). SHH delivered to cavernous nerve by peptide amphiphile nanofibers promoted cavernous nerve regeneration and caused a 58% improvement in erectile function in a rat model of CNI (43). The authors suggested that SHH treatment via aligned nanofibers has substantial clinical application to regenerate the cavernous nerve in prostatectomy and diabetic patients.
Ninjurin-1 is known to be involved in neuroinflammatory processes and to be related to vascular regression during the embryonic period (52,53). Recently, it was found that local delivery of monoclonal Ninj1 neutralizing antibody (Ninj1-mAb) significantly increases penile neuronal NOS (nNOS) and neurofilament contents, induced cavernous endothelial proliferation and phosphorylation of Akt and eNOS, and decreased endothelial cell apoptosis in the CNI mice by up-regulating angiopoietin-1 and down-regulating angiopoietin-2. High-dose Ninj1-mAb (2.5 µg/20 µL) induced profound restoration of erectile function in the CNI mice (91% of sham control values), whereas low-dose Ninj1-mAb (1.0 µg/20 µL) elicited partial improvement (45). Intracavernous injection of Ninj1-mAb (2.5 µg/20 µL) also successfully restored erectile function through enhanced angiogenesis and neural regeneration of the penis from STZ-induced diabetic mice, which is highly dependent on angiopoietin-1-Tie2 signaling (46). These findings suggest that dual neurotrophic and angiogenic effects of Ninjurin-1 blockade may provide a good opportunity for treating ED resulting from radical prostatectomy or diabetes mellitus.
It was reported that nerve growth factor (NGF) and its receptor TrkA are required for the development and survival of sympathetic neurons in late embryonic and postnatal stages (54,55). Because sympathetic nerves have a role to antagonize penile erection, a specific TrkA monoclonal antibody (TrkA-mAb) might be efficacious for the treatment of ED from CNI by blocking the regeneration of peripheral sympathetic neurons. Local delivery of TrkA-mAb into the major pelvic ganglion or the corpus cavernosum of CNI rats significantly suppressed tyrosine hydroxylase-positive sympathetic nerve fibers in the corpus cavernosum and enhanced nNOS-positive fibers in the dorsal nerve and thereby induced recovery of erectile function (47).
Neuregulins are a family of growth factors related to epidermal growth factor and play a crucial role in axoglial signaling during the development of the peripheral nervous system (56). Intracavernous administration of GGF-2 protein (neuregulin-1β3 type II), a soluble full-length splice variant of the neuregulin-1 gene, promoted axonal integrity and preserved erectile function in a rat model of CNI (48).
Anti-fibrosis
Up-regulation of profibrotic factors and cavernous fibrosis from increased collagen synthesis is also an important cause of ED. Among the various profibrotic factors, Transforming growth factor-β (TGF-β) has been suggested as the most relevant fibrogenic cytokine. TGF-β1 expression is known to be up-regulated in the corpus cavernosum tissues of STZ-induced diabetic rats and in mice with CNI (57,58). Moreover, it was reported that there is an increase in cavernous TGF-β1 expression and collagen synthesis in patients with ED from vascular risk factors or spinal cord injury (59,60). TGF-β1 is known to mediate its fibrotic effects by activating the receptor-associated Smads, including Smad2 and Smad3 (61). TGF-β1 binds to a type I receptor known as activin receptor-like kinase (ALK) 5. The phosphorylation of serine/threonine residues in ALK5 subsequently phosphorylates the major downstream signaling molecules Smad2 and Smad3. When phosphorylated, Smad2/3 form a heteromeric complex with Smad4, which translocates into the nucleus and regulates the transcription of TGF-β1-responsive genes, thereby inducing fibrosis-related changes (61). Moreover, TGF-β-Smad2/3 signal pathway is known to mediate smooth muscle cell apoptosis and to inhibit endothelial cell proliferation. It was recently observed that apoptosis in cavernous smooth muscle and endothelial cells and up-regulation of the TGF-β1-Smad2 pathway in the corpus cavernosum of the mouse begin as early as 3 days after CNI (58). TGF-β1-Smad2/3 signal pathway in the penis was also activated in both diabetic rats and men with spinal cord injury (57,60). These findings suggest that inhibition of TGF-β signaling pathway might be efficacious in the treatment of ED. Preclinical studies targeting anti-fibrosis are summarized in Table 4.

Full table
Smad7 is an inhibitory Smad protein that blocks TGF-β1 signaling through a negative feedback loop. Smad7 protein binds to TGF-β1 type I receptor, which blocks the phosphorylation of the receptor-associated Smads, including Smad2 and Smad3, and eventually inhibits down-stream signaling of TGF-β1 (64). Thus, Smad7 inhibits diverse cellular processes regulated by TGF-β1, such as cell proliferation, apoptosis, and fibrosis (64). It was recently reported that a single injection of an adenovirus encoding the Smad7 gene into the corpus cavernosum of CNI mice significantly decreased the production of extracellular matrix proteins, including PAI-1, fibronectin, collagen I, and collagen IV; decreased endothelial cell apoptosis; and induced eNOS phosphorylation, and that these changes were associated with physiologically relevant changes in erectile function (62).
PT144 peptide is a TGF-β antagonist peptide comprised of amino acids derived from the TGF-β type III receptor and is known to have anti-fibrotic activity in animal models of fibrotic diseases. It was reported in STZ-induced diabetic rats that intraperitoneal administration of PT144 decreased cavernous fibrosis and restored erectile function (63).
These findings suggest that an anti-fibrotic strategy through inhibition of the TGF-β signaling pathway may represent a promising therapeutic strategy for ED from CNI or diabetes.
Conclusions
In spite of numerous clinical trials with small molecules that target central pathway (melanocortin or dopamine receptor agonists) or peripheral pathway (soluble guanylate cyclase activators, Rho-kinase inhibitors, and maxi-K channel), these agents have yet to reach the market. The lack of superiority over PDE5 inhibitors in terms of efficacy or side effect, especially for centrally acting agents, may be a major obstacle for further clinical application.
A variety of preclinical studies have so far demonstrated promising results by way of biotherapies to regenerate cavernous endothelial cells and cavernous nerves and to inhibit cavernous fibrosis. However, we are still not yet at the point of clinical trials mainly because more sophisticated protein engineering or gene-delivery system are needed in the development of biotherapies to be applicable in humans. Moreover, considering multiple pathogenic mechanisms are involved in human ED, it may be an attractive strategy to apply a combination of biotherapies, i.e., promotion of angiogenesis and nerve regeneration, as much as any single therapy for better outcomes. We believe that the current preclinical data and advances in technology will translate into effective pharmacotherapies or biotherapies for ED in the very near future.
Acknowledgements
Funding: This research was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Ji-Kan Ryu, H15C0508).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lue TF. Erectile dysfunction. N Engl J Med 2000;342:1802-13. [Crossref] [PubMed]
- Martínez-Jabaloyas JM, Gil-Salom M, Villamón-Fort R, et al. Prognostic factors for response to sildenafil in patients with erectile dysfunction. Eur Urol 2001;40:641-6. [Crossref] [PubMed]
- Musicki B, Burnett AL. Endothelial dysfunction in diabetic erectile dysfunction. Int J Impot Res 2007;19:129-38. [Crossref] [PubMed]
- Angulo J, González-Corrochano R, Cuevas P, et al. Diabetes exacerbates the functional deficiency of NO/cGMP pathway associated with erectile dysfunction in human corpus cavernosum and penile arteries. J Sex Med 2010;7:758-68. [Crossref] [PubMed]
- Webb DJ, Muirhead GJ, Wulff M. Sildenafil citrate potentiates the hypotensive effects of nitric oxide donor drugs in male patients with stable angina. J Am Coll Cardiol 2000;36:25-31. [Crossref] [PubMed]
- Wessells H, Levine N, Hadley ME, et al. Melanocortin receptor agonists, penile erection, and sexual motivation: human studies with Melanotan II. Int J Impot Res 2000;12:S74-9. [Crossref] [PubMed]
- Hellstrom W, Gittelman M, Zinner N, et al. Randomized, double-blind, placebo-controlled, at-home study to evaluate the efficacy and safety of intranasal bremelanotide in men with erectile dysfunction with and without diabetes mellitus. 9th Congress of the European Society for Sexual Medicine. Vienna, Austria. J Sex Med 2006:117.
- Safarinejad MR, Hosseini SY. Salvage of sildenafil failures with bremelanotide: a randomized, double-blind, placebo controlled study. J Urol 2008;179:1066-71. [Crossref] [PubMed]
- Rexahn Pharmaceuticals, Inc. Efficacy Study of RX-10100 to Treat Erectile Dysfunction (ED). National Library of Medicine (US). ClinicalTrials.gov; Bethesda (MD): 2009.
- Bayer Healthcare AG. A prospective, randomized, double-blind, double-dummy, placebo and active controlled, multicenter study assessing the efficacy and safety of the combination BAY 604552/vardenafil compared to vardenafil (20 mg) for the treatment of erectile dysfunction not sufficiently responsive to standard therapy with PDE5 inhibitors. National Library of Medicine (US), ClinicalTrials.gov; Bethesda (MD): 2014.
- Sanofi-Avenis. Randomized, double-blind, placebo and active controlled study of the activity of SAR407899 single-dose on the ability to increase duration of penile rigidity, under experimental condition, in patients with mild to moderate erectile dysfunction. National Library of Medicine: ClinicalTrials.gov, Bethesda (MD): 2009.
- Melman A, Bar-Chama N, McCullough A, et al. hMaxi-K gene transfer in males with erectile dysfunction: results of the first human trial. Hum Gene Ther 2006;17:1165-76. [Crossref] [PubMed]
- Cowart M, Latshaw SP, Bhatia P, et al. Discovery of 2-(4-pyridin-2-ylpiperazin-1-ylmethyl)-1H-benzimidazole (ABT-724), a dopaminergic agent with a novel mode of action for the potential treatment of erectile dysfunction. J Med Chem 2004;47:3853-64. [Crossref] [PubMed]
- Patel MV, Kolasa T, Mortell K, et al. Discovery of 3-methyl-N-(1-oxy-3’,4’,5’,6’-tetrahydro-2’H-[2,4’-bipyridine]-1’-ylmethyl)benzamide(ABT-670), an orally bioavailable dopamine D4 agonist for the treatment of erectile dysfunction. J Med Chem 2006;49:7450-65. [Crossref] [PubMed]
- Chan JS, Kim DJ, Ahn CH, et al. Clavulanic acid stimulates sexual behaviour in male rats. Eur J Pharmacol 2009;609:69-73. [Crossref] [PubMed]
- Christ GJ, Spray DC, Brink PR. Characterization of K currents in cultured human corporal smooth muscle cells. J Androl 1993;14:319-28. [PubMed]
- Kendirci M, Nowfar S, Hellstrom WJ. The impact of vascular risk factors on erectile function. Drugs Today (Barc) 2005;41:65-74. [Crossref] [PubMed]
- Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation 2003;108:2054-9. [Crossref] [PubMed]
- Bivalacqua TJ, Usta MF, Champion HC, et al. Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl 2003;24:S17-37. [Crossref] [PubMed]
- Thompson IM, Tangen CM, Goodman PJ, et al. Erectile dysfunction and subsequent cardiovascular disease. JAMA 2005;294:2996-3002. [Crossref] [PubMed]
- Solomon H, Man JW, Jackson G. Erectile dysfunction and the cardiovascular patient: endothelial dysfunction is the common denominator. Heart 2003;89:251-3. [Crossref] [PubMed]
- Rogers RS, Graziottin TM, Lin CS, et al. Intracavernosal vascular endothelial growth factor (VEGF) injection and adeno-associated virus-mediated VEGF gene therapy prevent and reverse venogenic erectile dysfunction in rats. Int J Impot Res 2003;15:26-37. [Crossref] [PubMed]
- Dall'Era JE, Meacham RB, Mills JN, et al. Vascular endothelial growth factor (VEGF) gene therapy using a nonviral gene delivery system improves erectile function in a diabetic rat model. Int J Impot Res 2008;20:307-14. [Crossref] [PubMed]
- Gholami SS, Rogers R, Chang J, et al. The effect of vascular endothelial growth factor and adeno-associated virus mediated brain derived neurotrophic factor on neurogenic and vasculogenic erectile dysfunction induced by hyperlipidemia. J Urol 2003;169:1577-81. [Crossref] [PubMed]
- Yamanaka M, Shirai M, Shiina H, et al. Vascular endothelial growth factor restores erectile function through inhibition of apoptosis in diabetic rat penile crura. J Urol 2005;173:318-23. [Crossref] [PubMed]
- Ryu JK, Cho CH, Shin HY, et al. Combined angiopoietin-1 and vascular endothelial growth factor gene transfer restores cavernous angiogenesis and erectile function in a rat model of hypercholesterolemia. Mol Ther 2006;13:705-15. [Crossref] [PubMed]
- Ryu JK, Kim WJ, Koh YJ, et al. Designed angiopoietin-1 variant, COMP-angiopoietin-1, rescues erectile function through healthy cavernous angiogenesis in a hypercholesterolemic mouse. Sci Rep 2015;5:9222. [Crossref] [PubMed]
- Jin HR, Kim WJ, Song JS, et al. Intracavernous delivery of a designed angiopoietin-1 variant rescues erectile function by enhancing endothelial regeneration in the streptozotocin-induced diabetic mouse. Diabetes 2011;60:969-80. [Crossref] [PubMed]
- Jin HR, Kim WJ, Song JS, et al. Intracavernous delivery of synthetic angiopoietin-1 protein as a novel therapeutic strategy for erectile dysfunction in the type II diabetic db/db mouse. J Sex Med 2010;7:3635-46. [Crossref] [PubMed]
- Kwon MH, Ryu JK, Kim WJ, et al. Effect of intracavernous administration of angiopoietin-4 on erectile function in the streptozotocin-induced diabetic mouse. J Sex Med 2013;10:2912-27. [Crossref] [PubMed]
- Kwon MH, Tuvshintur B, Kim WJ, et al. Expression of the apelin-APJ pathway and effects on erectile function in a mouse model of vasculogenic erectile dysfunction. J Sex Med 2013;10:2928-41. [Crossref] [PubMed]
- Bae JH, Shrestha KR, Park YH, et al. Comparison between subcutaneous injection of basic fibroblast growth factor-hydrogel and intracavernous injection of adipose-derived stem cells in a rat model of cavernous nerve injury. Urology 2014;84:1248.e1-7. [Crossref] [PubMed]
- Nishimatsu H, Suzuki E, Nomiya A, et al. Adrenomedullin and angiopoietin-1 additively restore erectile function in diabetic rats: Comparison with the combination therapy of vascular endothelial growth factor and angiopoietin-1. J Sex Med 2013;10:1707-19. [Crossref] [PubMed]
- Lee RJ, Springer ML, Blanco-Bose WE, et al. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation 2000;102:898-901. [Crossref] [PubMed]
- Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 1996;87:1161-9. [Crossref] [PubMed]
- Chae JK, Kim I, Lim ST, et al. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol 2000;20:2573-8. [Crossref] [PubMed]
- Valenzuela DM, Griffiths JA, Rojas J, et al. Angiopoetins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci USA 1999;96:1904-9. [Crossref] [PubMed]
- Tatemoto K, Hosoya M, Habata Y, et al. Isolation and Characterization of a Novel Endogenous Peptide Ligand for the Human APJ Receptor. Biochem Biophys Res Commun 1998;251:471-6. [Crossref] [PubMed]
- Kleinz MJ, Skepper JN, Davenport AP. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul Pept 2005;126:233-40. [Crossref] [PubMed]
- Kim W, Moon SO, Sung MJ, et al. Angiogenic role of adrenomedullin through activation of Akt, mitogen-activated protein kinase, and focal adhesion kinase in endothelial cells. FASEB J 2003;17:1937-9. [PubMed]
- Bakircioglu ME, Lin CS, Fan P, et al. The effect of adeno-associated virus mediated brain derived neurotrophic factor in an animal model of neurogenic impotence. J Urol 2001;165:2103-9. [Crossref] [PubMed]
- Bennett NE, Kim JH, Wolfe DP, et al. Improvement in erectile dysfunction after neurotrophic factor gene therapy in diabetic rats. J Urol 2005;173:1820-4. [Crossref] [PubMed]
- Angeloni NL, Bond CW, Tang Y, et al. Regeneration of the cavernous nerve by Sonic hedgehog using aligned peptide amphiphile nanofibers. Biomaterials 2011;32:1091-101. [Crossref] [PubMed]
- Fandel TM, Bella AJ, Lin G, et al. Intracavernous growth differentiation factor-5 therapy enhances the recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med 2008;5:1866-75. [Crossref] [PubMed]
- Yin GN, Kim WJ, Jin HR, et al. Nerve injury-induced protein 1 (Ninjurin-1) is a novel therapeutic target for cavernous nerve injury-induced erectile dysfunction in mice. J Sex Med 2013;10:1488-501. [Crossref] [PubMed]
- Yin GN, Choi MJ, Kim WJ, et al. Inhibition of Ninjurin 1 restores erectile function through dual angiogenic and neurotrophic effects in the diabetic mouse. Proc Natl Acad Sci U S A 2014;111:E2731-40. [Crossref] [PubMed]
- Lin G, Li H, Zhang X, et al. Novel therapeutic approach for neurogenic erectile dysfunction: effect of neurotrophic tyrosine kinase receptor type 1 monoclonal antibody. Eur Urol 2015;67:716-26. [Crossref] [PubMed]
- Burnett AL, Sezen SF, Hoke A, et al. GGF2 is neuroprotective in a rat model of cavernous nerve injury-induced erectile dysfunction. J Sex Med 2015;12:897-905. [Crossref] [PubMed]
- Bella AJ, Lin G, Cagiannos I, et al. Emerging neuromodulatory molecules for the treatment of neurogenic erectile dysfunction caused by cavernous nerve injury. Asian J Androl 2008;10:54-9. [Crossref] [PubMed]
- Burnett AL, McCullough AR, Smith JA Jr, et al. Neuromodulation to preserve erectile function after radical prostatectomy: results from the GPI 1485 neuroimmunophilin ligand clinical trial. J Urol 2007;177:383-4.
- Kusano KF, Allendoerfer KL, Munger W, et al. Sonic hedgehog induces arteriogenesis in diabetic vasa nervorum and restores function in diabetic neuropathy. Arterioscler Thromb Vasc Biol 2004;24:2102-7. [Crossref] [PubMed]
- Ifergan I, Kebir H, Terouz S, et al. Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann Neurol 2011;70:751-63. [Crossref] [PubMed]
- Lee HJ, Ahn BJ, Shin MW, et al. Ninjurin1 mediates macrophage-induced programmed cell death during early ocular development. Cell Death Differ 2009;16:1395-407. [Crossref] [PubMed]
- Crowley C, Spencer SD, Nishimura MC, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 1994;76:1001-11. [Crossref] [PubMed]
- Smeyne RJ, Klein R, Schnapp A, et al. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 1994;368:246-9. [Crossref] [PubMed]
- Falls DL. Neuregulins: Functions, forms, and signaling strategies. Exp Cell Res 2003;284:14-30. [Crossref] [PubMed]
- Zhang LW, Piao S, Choi MJ, et al. Role of increased penile expression of transforming growth factor-beta1 and activation of the Smad signaling pathway in erectile dysfunction in streptozotocin-induced diabetic rats. J Sex Med 2008;5:2318-29. [Crossref] [PubMed]
- Jin HR, Chung YG, Kim WJ, et al. A mouse model of cavernous nerve injury-induced erectile dysfunction: functional and morphological characterization of the corpus cavernosum. J Sex Med 2010;7:3351-64. [Crossref] [PubMed]
- Ryu JK, Han JY, Chu YC, et al. Expression of cavernous transforming growth factor-beta1 and its type II receptor in patients with erectile dysfunction. Int J Androl 2004;27:42-9. [Crossref] [PubMed]
- Shin TY, Ryu JK, Jin HR, et al. Increased cavernous expression of transforming growth factor-β1 and activation of the Smad signaling pathway affects erectile dysfunction in men with spinal cord injury. J Sex Med 2011;8:1454-62. [Crossref] [PubMed]
- Massagué J, Chen YG. Controlling TGF-beta signaling. Genes Dev 2000;14:627-44. [PubMed]
- Song KM, Chung JS, Choi MJ, et al. Effectiveness of intracavernous delivery of adenovirus encoding Smad7 gene on erectile function in a mouse model of cavernous nerve injury. J Sex Med 2014;11:51-63. [Crossref] [PubMed]
- Li WJ, Wang H, Zhou J, et al. P144, a TGF-β1 antagonist peptide, synergizes with sildenafil and enhances erectile response via amelioration of cavernosal fibrosis in diabetic rats. J Sex Med 2013;10:2942-51. [Crossref] [PubMed]
- Kamiya Y, Miyazono K, Miyazawa K. Smad7 inhibits transforming growth factor-beta family type i receptors through two distinct modes of interaction. J Biol Chem 2010;285:30804-13. [Crossref] [PubMed]


