
Survival analysis comparing bladder preservation techniques in octogenarians with muscle-invasive bladder cancer
Highlight box
Key findings
• Among bladder preservation strategies, partial cystectomy (PC) and chemoradiation (CRT) had comparable overall survival among the octogenarian population with muscle-invasive urothelial bladder carcinoma
What was recommended and what is new?
• Concurrent CRT is considered an alternative to radical cystectomy, which is the preferred first-line treatment for muscle-invasive bladder cancer.
• Our study using the National Cancer Database observed that PC had comparable survival outcomes to CRT in carefully selected octogenarians with well-defined criteria.
What is the implication, and what should change now?
• Among carefully selected octogenarians concerned about bladder preservation, these findings could encourage and help with patient counseling and shared decision-making. As the overall survival with PC is comparable with CRT, PC may be considered a viable option in carefully selected octogenarians with muscle-invasive bladder cancer, but it needs further exploration.
IntroductionOther Section
In the United States, bladder cancer is ranked as the 6th most common cancer, and globally, it is ranked 10th, accounting for 2% to 3% of all malignancies (1). The median age at diagnosis of bladder cancer is 73 years, and approximately 20% to 30% of patients were identified with muscle-invasive bladder cancer (MIBC) at the time of diagnosis (2,3). About 71.4% of individuals above 65 years are diagnosed with bladder cancer with a higher incidence at 85 years (4). It was estimated that in 2023, approximately 82,300 newly diagnosed cases and 16,700 deaths were secondary to bladder cancer (5). Over decades, the average life expectancy of an individual has been increasing. The life expectancy for older adults above 80 years old is 8 to 10 years and may vary depending on the associated co-morbidities. In the United States, the life expectancy for men and women aged 80 years old is 7 and 9.1 years, respectively (6,7). In recent years, many individuals in their eighties are undergoing major surgeries due to improvements in the healthcare system and increased life expectancy. Therefore, it is crucial to understand the outcomes of various treatments in octogenarians.
Neoadjuvant chemotherapy with radical cystectomy (RC) is the standard of care for MIBC, and its effectiveness has been well-established (8,9). As bladder preservation protocols like chemoradiation (CRT) are gaining popularity, and due to factors like morbidity associated with RC and life expectancy for age, some elderly populations prefer strategies that help preserve bladder function. Guidelines suggest RC as the preferred first-line treatment and concurrent CRT as an alternative in MIBC. Similarly, partial cystectomy (PC) is suggested as a viable option in carefully selected individuals with solitary lesions and no carcinoma in situ (10). Until the mid-1980s, PC was used in good numbers to treat bladder cancer. With the lack of definitive indication and the concern about oncological and survival outcomes, the usage of PC was limited. Studies have compared survival outcomes between RC and PC and observed that PC may have comparable outcomes in carefully selected individuals with structured criteria (11-14). Similarly, studies have shown that CRT and RC may have comparable oncological and survival outcomes (15,16). Therefore, as an option for bladder preservation, it is essential to understand the outcomes of PC and CRT among the elderly population. Our study uses the National Cancer Database (NCDB) to evaluate the survival outcomes among octogenarians treated with PC and CRT. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-24-602/rc).
MethodsOther Section
Patient selection
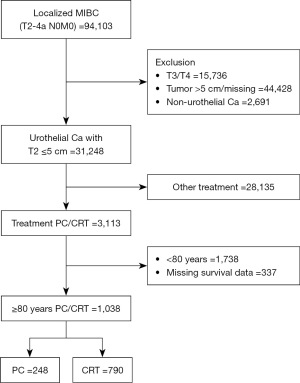
Our retrospective study focused on elderly individuals aged 80 years old and above diagnosed with MIBC and urothelial histology between 2004 and 2018. Our selection criteria included patients having cT2N0M0 with tumor size ≤5 cm and urothelial histology. We gathered data from the National Cancer Database (NCDB), which covers nearly 70% of newly diagnosed cancer cases in the United States (17). The study employed deidentified participant user files, which comply with the Health Insurance Portability and Accountability Act regulations. Quality checks are conducted annually to validate the data. Since the data used are publicly available upon request and are deidentified, re-identification is unlikely, and as the data do not involve direct human subjects, institutional review board approval and informed consent were deemed not required (17,18). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). We divided our primary cohort into two categories based on the intervention: PC and CRT cohorts. The PC cohort included those who underwent PC (coded as PC), and the CRT cohort included individuals who received both chemotherapy and radiation of ≥39.6 Gy within the 90-day timeframe (19). We excluded cases with variant histology, those who underwent cystectomy, carcinoma in situ, hydronephrosis, and those with missing data on chemotherapy, radiotherapy, or follow-up. Our final study population included 3,113 octogenarians who met our inclusion and exclusion criteria (Figure 1).
Statistical analyses
We analyzed sociodemographic factors like race and ethnicity, gender, insurance, median income, and treatment facility and clinical factors like clinical stage, tumor size, tumor grade, and comorbidity score 30- and 90-day mortality to compare both treatment cohorts. Ethnicity was categorized as Hispanic, non-Hispanic, and others. Race was simplified into white, black, and others. This simplification was performed because of the few patients representing other categories. The clinical and sociodemographic information was evaluated using the Chi-squared test and contingency tables. Propensity score matching with Mahalanobis distance was performed to match both intervention cohorts and to minimize the effect of confounders. Variables like race and ethnicity, gender, comorbidity score, facility type, median income, and tumor grade were used for matching. Kaplan-Meier survival analyses with log-rank test and multivariate Cox regression analyses were used to compare the survival outcomes between both treatment modalities. A P value of <0.05 was defined as a statistically significant difference in all parts of the analyses. We performed the statistical analysis using SAS software, version 9.4.
ResultsOther Section
A total of 671,462 patients were diagnosed with bladder cancer between 2004 and 2018, with 94,103 having localized MIBC and urothelial histology (Figure 1). Among them, 3,113 had T2 tumors ≤5 cm and underwent PC or CRT. Among them, 1,038 were octogenarians who met our selection criteria. In this population, 248 (23.8%) had undergone PC, and 790 (76.2%) underwent CRT (Figure 1). The mean [± standard deviation (SD)] for age of patients treated with PC and CRT was 84.1 (±3.2) and 84.4 (±3.0) years, and the mean (± SD) for follow-up was 40.2 (±32.6) months and 35 (±28.7) months, respectively. After propensity-matching, each cohort had 248 patients. Our analysis (Table 1) showed that the location of tumors in PC and CRT cohorts were 85 (34.3%) and 46 (5.8%) in the dome, 1 (0.4%), and 66 (8.4%) in the trigone, 33 (13.3%) and 174 (22.0%) were in the lateral wall, 19 (7.7%) and 84 (10.6%) were in the posterior wall of the bladder, 26 (10.5%) and 43 (5.4%) were in the anterior wall (P<0.001). The 30-day mortality was 1.2% vs. 0%, and the 90-day mortality was 3.2% vs. 0.6% in patients who underwent PC and CRT, respectively (P<0.001). Among those who underwent PC, 13 (5.2%) received adjuvant radiation, and 34 (13.7%) received adjuvant chemotherapy. There was no statistically significant difference in gender, race, ethnicity, median income, insurance, facility type, comorbidity index, tumor grade, and 30-day readmission.
Table 1
| Variables | Unmatched population | Matched population | |||||
|---|---|---|---|---|---|---|---|
| PC (N=248) | CRT (N=790) | P value | PC (N=248) | CRT (N=248) | P value | ||
| Sex | 0.87 | 0.99 | |||||
| Male | 177 (71.4) | 568 (71.9) | 177 (71.4) | 177 (71.4) | |||
| Female | 71 (28.6) | 222 (28.1) | 71 (28.6) | 71 (28.6) | |||
| Race | 0.84 | 0.71 | |||||
| White | 228 (91.9) | 724 (91.6) | 228 (91.9) | 232 (93.5) | |||
| Black | 11 (4.4) | 41 (5.2) | 11 (4.4) | 10 (4.0) | |||
| Others | 9 (3.6) | 25 (3.2) | 9 (3.6) | 6 (2.4) | |||
| Ethnicity | 0.45 | 0.80 | |||||
| Non-Hispanic | 238 (96.0) | 742 (93.9) | 238 (96.0) | 237 (95.6) | |||
| Hispanic | 3 (1.2) | 12 (1.5) | 3 (1.2) | 2 (0.8) | |||
| Others | 7 (2.8) | 36 (4.6) | 7 (2.8) | 9 (3.6) | |||
| Insurance | 0.74 | 0.84 | |||||
| Private | 20 (8.1) | 62 (7.8) | 20 (8.1) | 16 (6.5) | |||
| Medicare | 224 (90.3) | 706 (89.4) | 224 (90.3) | 226 (91.1) | |||
| Others | 2 (0.8) | 14 (1.8) | 2 (0.8) | 3 (1.2) | |||
| Unknown | 2 (0.8) | 8 (1.0) | 2 (0.8) | 3 (1.2) | |||
| Median income | 0.88 | 0.84 | |||||
| <$46,277 | 27 (10.9) | 93 (11.8) | 27 (10.9) | 27 (10.9) | |||
| $46,277–$57,856 | 51 (20.6) | 167 (21.1) | 51 (20.6) | 50 (20.2) | |||
| $57,857–$74,062 | 58 (23.4) | 178 (22.5) | 58 (23.4) | 50 (20.2) | |||
| ≥$74,063 | 85 (34.3) | 282 (35.7) | 85 (34.3) | 87 (35.1) | |||
| Unknown | 27 (10.9) | 70 (8.9) | 27 (10.9) | 34 (13.7) | |||
| Facility type | 0.22 | 0.86 | |||||
| Community CP | 21 (8.5) | 75 (9.5) | 21 (8.5) | 16 (6.5) | |||
| Comprehensive CCP | 108 (43.5) | 369 (46.7) | 108 (43.5) | 109 (44.0) | |||
| Academic program | 70 (28.2) | 172 (21.8) | 70 (28.2) | 72 (29.0) | |||
| Integrated network | 49 (19.8) | 174 (22.0) | 49 (19.8) | 51 (20.6) | |||
| Comorbidity index | 0.76 | 0.76 | |||||
| CCI 0 | 155 (62.5) | 498 (63.0) | 155 (62.5) | 165 (66.5) | |||
| CCI 1 | 59 (23.8) | 199 (25.2) | 59 (23.8) | 55 (22.2) | |||
| CCI 2 | 24 (9.7) | 60 (7.6) | 24 (9.7) | 21 (8.5) | |||
| CCI 3 | 10 (4.0) | 33 (4.2) | 10 (4.0) | 7 (2.8) | |||
| 30-days readmission | 0.16 | 0.22 | |||||
| No admission | 224 (90.3) | 738 (93.4) | 224 (90.3) | 236 (95.2) | |||
| Unplanned | 10 (4.0) | 15 (1.9) | 10 (4.0) | 5 (2.0) | |||
| Planned | 7 (2.8) | 13 (1.6) | 7 (2.8) | 4 (1.6) | |||
| Unknown | 7 (2.8) | 24 (3.0) | 7 (2.8) | 3 (1.2) | |||
| Tumor location | <0.001 | <0.001 | |||||
| Trigone | 1 (0.4) | 66 (8.4) | 1 (0.4) | 20 (8.1) | |||
| Dome | 85 (34.3) | 46 (5.8) | 85 (34.3) | 15 (6.0) | |||
| Lateral wall | 33 (13.3) | 174 (22.0) | 33 (13.3) | 54 (21.8) | |||
| Anterior wall | 26 (10.5) | 43 (5.4) | 26 (10.5) | 11 (4.4) | |||
| Posterior wall | 19 (7.7) | 84 (10.6) | 19 (7.7) | 27 (10.9) | |||
| Others | 33 (13.3) | 157 (19.9) | 33 (13.3) | 49 (19.7) | |||
| Bladder NOS | 51 (20.6) | 220 (27.8) | 51 (20.6) | 72 (29.0) | |||
| Tumor grade | 0.70 | 0.41 | |||||
| Grade II | 6 (2.4) | 15 (1.9) | 6 (2.4) | 2 (0.8) | |||
| Grade III | 93 (37.5) | 275 (34.8) | 93 (37.5) | 107 (43.1) | |||
| Grade IV | 130 (52.4) | 422 (53.4) | 130 (52.4) | 121 (48.8) | |||
| Unknown | 19 (7.7) | 78 (9.9) | 19 (7.7) | 18 (7.3) | |||
| 30-day mortality | <0.001 | 0.049 | |||||
| Alive | 245 (98.8) | 787 (99.6) | 245 (98.8) | 247 (99.6) | |||
| Dead | 3 (1.2) | 0 (0.0) | 3 (1.2) | 0 (0.0) | |||
| Missing | 0 (0) | 3 (0.4) | 0 (0) | 1 (0.4) | |||
| 90-day mortality | <0.001 | 0.01 | |||||
| Alive | 240 (96.8) | 783 (99.1) | 240 (96.8) | 246 (99.2) | |||
| Dead | 8 (3.2) | 5 (0.6) | 8 (3.2) | 1 (0.4) | |||
| Missing | 0 (0) | 2 (0.3) | 0 (0) | 1 (0.4) | |||
Data are presented as n (%). PC, partial cystectomy; CRT, chemoradiation; CP, Cancer Program; CCP, Community Cancer Program; NOS, not specified; CCI, Charlson-Deyo Comorbidity Index.
Kaplan-Meier survival analysis showed the median overall survival (OS) for the unmatched population of PC and CRT cohorts (Figure 2A) was 38.3 [95% confidence interval (CI): 31.6–46.7] and 33.5 (95% CI: 29.9–38.6) months (P=0.40), and the matched population (Figure 2B) was 38.3 (95% CI: 31.6–46.7) and 32.9 (95% CI: 28.8–43.3) months (P=0.66), respectively. Multivariate Cox regression analysis for the matched population (Table 2) showed there was no significant difference in the risk of mortality between PC and CRT cohorts [hazard ratio =1.07 (95% CI: 0.82–1.39)] (P=0.63). Similarly, there was no significant difference in mortality risk based on tumor location. However, females had a reduced risk of mortality compared to males, and the mortality risk increased with an increase in the comorbidity index. No significant difference in the mortality risk was seen in race, ethnicity, insurance, facility type, tumor location, and tumor grade.

Table 2
| Parameter | Hazard ratio (95% CI) | P value |
|---|---|---|
| Treatment (vs. PC) | ||
| CRT | 1.07 (0.82–1.39) | 0.63 |
| Sex (vs. male) | ||
| Female | 0.70 (0.53–0.91) | 0.008 |
| Race (vs. White) | ||
| Black | 0.82 (0.46–1.45) | 0.49 |
| Unknown | 0.56 (0.24–1.28) | 0.17 |
| Ethnicity (vs. non-Hispanic) | ||
| Hispanic | 2.01 (0.73–5.51) | 0.18 |
| Unknown | 1.33 (0.72–2.46) | 0.36 |
| Insurance (vs. private) | ||
| Medicare | 0.86 (0.54–1.37) | 0.53 |
| Others | 0.52 (0.09–2.90) | 0.45 |
| Unknown | 1.82 (0.51–6.54) | 0.36 |
| Facility type (vs. community CP) | ||
| Comprehensive CCP | 0.83 (0.53–1.30) | 0.42 |
| Academic program | 0.66 (0.41–1.06) | 0.09 |
| Integrated network | 0.83 (0.50–1.37) | 0.46 |
| Comorbidity index (vs. CCI 0) | ||
| CCI 1 | 1.12 (0.83–1.52) | 0.44 |
| CCI 2 | 1.97 (1.32–2.94) | <0.001 |
| CCI 3 | 3.09 (1.64–5.83) | <0.001 |
| Tumor location (vs. trigone) | ||
| Dome | 0.92 (0.45–1.86) | 0.81 |
| Lateral wall | 0.96 (0.49–1.87) | 0.90 |
| Anterior wall | 1.13 (0.53–2.39) | 0.76 |
| Posterior wall | 0.65 (0.31–1.35) | 0.25 |
| Other | 0.90 (0.44–1.84) | 0.77 |
| Bladder NOS | 1.06 (0.55–2.03) | 0.87 |
| Tumor grade (vs. grade IV) | ||
| Grade II | 0.23 (0.01–4.55) | 0.34 |
| Grade III | 0.85 (0.66–1.09) | 0.20 |
| Unknown | 0.75 (0.48–1.19) | 0.22 |
PC, partial cystectomy; CRT, chemoradiation; CI, confidence interval; CP, Community Program; CCP, Cancer Community Program; NOS, not specified; CCI, Charlson-Deyo Comorbidity Index.
DiscussionOther Section
In our study comparing the survival outcomes of PC and CRT in T2 ≤5 cm tumors among octogenarians, we observed there was no significant difference in survival between patients undergoing PC and CRT. Tumors in the dome and anterior wall had predominantly undergone PC, while tumors in the lateral and posterior walls underwent CRT. The 30- and 90-day mortality rates were higher in PC compared to CRT. Patients with an increase in the comorbidity index had an increased mortality risk. However, there was no difference in mortality risk based on race, ethnicity, facility type, tumor location, and tumor grade.
Studies have evaluated the effectiveness of PC in MIBC with different settings. Peak et al. observed that in patients with bladder cancer, 7% to 10% underwent PC, and 2.8% of them had MIBC. They stated that only 5–10% of patients with MIBC were suitable for PC, and selecting more appropriate patients with timely radiotherapy and chemotherapy could yield better outcomes (20). A study by Kassouf et al. observed that 5-year disease-specific survival (DSS) and OS for PC were 87% and 67%, respectively, and superficial tumors, clinical T-stage, and adjuvant chemotherapy were associated with higher recurrence-free survival (RFS) (21). Knoedler et al. noted no significant difference between PC and RC with regard to 10-year cancer-specific survival (CSS) (58% vs. 63%, P=0.63), but the retreatment rate for PC was 18.6% (22). In a study by Zhang et al. on localized MIBC, the 5-year OS for PC with intravesical chemotherapy was 44.6% and stated that PC with intravesical chemotherapy could achieve a comparable tumor control to RC in patients with solitary lesions (23). These studies highlight that PC may offer survival benefits with structured selection criteria. We studied T2 ≤5 cm tumors among octogenarians and observed that OS for PC was non-inferior to CRT, suggesting PC could offer beneficial survival outcomes in carefully selected individuals with well-defined criteria.
In our study, we observed that tumors primarily located in the lateral and posterior walls predominantly underwent CRT, while those in the dome of the bladder underwent PC, which could have been a potential bias. However, there was no difference in the mortality risk based on tumor location. Many studies have reported that CRT has considerable oncological and survival outcomes. de Ruiter et al. observed that CRT is a safe and effective treatment in nonmetastatic MIBC, with an initial cystoscopy showing a 90% complete response and a 5-year DSS and OS in 50% of patients (24). Jiang et al., in their study on neoadjuvant chemotherapy with CRT, observed that cisplatin-tolerating patients had better 2-year DSS and OS (25). A study by Kotha et al. on elderly with MIBC with urothelial histology from the US Veteran Affairs National Database observed that CRT is effective with a 6-year OS rate of 25.7% (26). In our study on octogenarians, we observed that CRT had a 5-year OS of 32.2%. Studies have shown that low-volume solitary lesions, T2 tumors, no carcinoma in situ, and nodal metastasis are suitable for bladder-sparing and improving survival outcomes (27). In our research on bladder preservation, PC and CRT both had comparable survival outcomes. Therefore, the lack of well-structured selection criteria and concerns about oncological and survival outcomes could have possibly limited the utilization of PC.
Su et al., in their Surveillance, Epidemiology, and End Results (SEER) database study, compared PC and trimodality therapy as bladder preservation strategies and observed that PC had better survival outcomes than the trimodality approach with a median OS of 41 and 21 months, respectively (28). Our study on the octogenarians who underwent PC with stringent selection criteria like T2 tumors ≤5 cm with no carcinoma in situ had comparable survival outcomes to CRT, suggesting PC could be considered in carefully selected individuals. In a tetra modality treatment protocol by Kijima et al., patients treated with induction CRT and consolidative PC had a 5-year CSS rate of 83% and OS of 77% (29). In another study on tetra modality treatment on elderly aged 75 and above, they observed that 5-year RFS and CSS were 98% and 95%, respectively (30). In our study, limited data was available on patients treated with adjuvant therapies following PC. Therefore, additional survival benefits that may be achieved by combining PC with adjuvant chemotherapy and radiation need further exploration.
This study had several limitations, including its retrospective design. A propensity-score matched analysis was performed to limit the influence of possible confounding factors and selection bias, but they cannot be eliminated entirely. The findings from a study on databases need careful interpretation. The study’s results underscore the necessity for further evaluation of the impact of PC on carefully selected individuals in this population subset. Oncological outcomes and chemotherapy information, like RFS, CSS, chemotherapy dose, and regimen, and also information on focality, and urine cytology were unavailable. Due to the availability of limited information on the adjuvant therapies following PC in this patient population, further studies evaluating the impact of radiation and chemotherapy are required. Details on salvage cystectomy and depth of transurethral resection were also not available. Future studies combining these treatment modalities with well-structured selection criteria to evaluate the oncological outcomes and their effectiveness could help us understand the role of PC in bladder preservation strategy among elderly individuals. These findings could help encourage collaborative decision-making among the healthier elderly population concerned about preserving bladder function.
ConclusionsOther Section
In our study on PC and CRT among octogenarians with T2 tumors ≤5 cm, we observed PC and CRT had comparable OS. Therefore, PC can be considered a viable option in a carefully selected octogenarian population. PC as a bladder preservation strategy with adjuvant chemotherapy and radiation may provide additional survival benefits, which need further exploration.
AcknowledgmentsOther Section
We thank Miami Cancer Institute and Baptist Hospital for allowing us to conduct the study.
FootnoteOther Section
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-24-602/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-24-602/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-24-602/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are responsible for all aspects of the work, and questions about the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Being a study from a publicly available database as de-identified data, ethical approval, and participant consent was not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Fang W, Yang ZY, Chen TY, et al. Ethnicity and survival in bladder cancer: a population-based study based on the SEER database. J Transl Med 2020;18:145. [Crossref] [PubMed]
- Hanna N, Trinh QD, Seisen T, et al. Effectiveness of Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer in the Current Real World Setting in the USA. Eur Urol Oncol 2018;1:83-90. [Crossref] [PubMed]
- Bizzarri FP, Scarciglia E, Russo P, et al. Elderly and bladder cancer: The role of radical cystectomy and orthotopic urinary diversion. Urologia 2024;91:500-4. [Crossref] [PubMed]
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Manton KG, Vaupel JW. Survival after the age of 80 in the United States, Sweden, France, England, and Japan. N Engl J Med 1995;333:1232-5. [Crossref] [PubMed]
- Thinggaard M, McGue M, Jeune B, et al. Survival Prognosis in Very Old Adults. J Am Geriatr Soc 2016;64:81-8. [Crossref] [PubMed]
- Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol 2021;79:82-104. [Crossref] [PubMed]
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859-66. [Crossref] [PubMed]
- Witjes JA, Feikema AAH. Organ-Sparing Strategies in Muscle-Invasive Bladder Cancer. Cancer Manag Res 2021;13:7833-9. [Crossref] [PubMed]
- Long G, Hu Z, Liu Z, et al. Partial and radical cystectomy provides equivalent oncologic outcomes in bladder cancer when combined with adequate lymph node dissection: A population-based study. Urol Oncol 2023;41:327.e1-8. [Crossref] [PubMed]
- Leveridge MJ, Siemens DR, Izard JP, et al. Partial cystectomy for urothelial carcinoma of the bladder: Practice patterns and outcomes in the general population. Can Urol Assoc J 2017;11:412-8. [Crossref] [PubMed]
- Chung R, Moran GW, Movassaghi M, et al. Survival outcomes in patients with muscle invasive bladder cancer undergoing radical vs. partial cystectomy. Urol Oncol 2023;41:356.e11-8. [Crossref] [PubMed]
- Pon Avudaiappan A, Prabhakar P, Lusnia C, et al. A comparative study of survival outcomes between partial and radical cystectomy in octogenarians with muscle-invasive bladder cancer. Transl Androl Urol 2024;13:1486-97. [Crossref] [PubMed]
- Zlotta AR, Ballas LK, Niemierko A, et al. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. Lancet Oncol 2023;24:669-81. [Crossref] [PubMed]
- Avudaiappan AP, Prabhakar P, Ganapathi H, et al. Propensity score matched survival analysis of octogenarians with muscle-invasive bladder cancer: chemoradiation compared to radical cystectomy. Can J Urol 2023;30:11686-91. [PubMed]
- Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-90. [Crossref] [PubMed]
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol 2017;3:1722-8. [Crossref] [PubMed]
- Kaushik D, Wang H, Michalek J, et al. Chemoradiation Vs Radical Cystectomy for Muscle-invasive Bladder Cancer: A Propensity Score-weighted Comparative Analysis Using the National Cancer Database. Urology 2019;133:164-74. [Crossref] [PubMed]
- Peak TC, Hemal A. Partial cystectomy for muscle-invasive bladder cancer: a review of the literature. Transl Androl Urol 2020;9:2938-45. [Crossref] [PubMed]
- Kassouf W, Swanson D, Kamat AM, et al. Partial cystectomy for muscle invasive urothelial carcinoma of the bladder: a contemporary review of the M. D. Anderson Cancer Center experience. J Urol 2006;175:2058-62. [Crossref] [PubMed]
- Knoedler JJ, Boorjian SA, Kim SP, et al. Does partial cystectomy compromise oncologic outcomes for patients with bladder cancer compared to radical cystectomy? A matched case-control analysis. J Urol 2012;188:1115-9. [Crossref] [PubMed]
- Zhang B, Liu T, He Y, et al. Clinical application and efficacy analysis of partial cystectomy combined with intravesical chemotherapy in muscle-invasive bladder cancer. BMC Urol 2023;23:91. [Crossref] [PubMed]
- de Ruiter BM, van de Kamp MW, van Steenbergen JPZ, et al. A Multicenter Retrospective Cohort Series of Muscle-invasive Bladder Cancer Patients Treated with Definitive Concurrent Chemoradiotherapy in Daily Practice. Eur Urol Open Sci 2022;39:7-13. [Crossref] [PubMed]
- Jiang DM, Jiang H, Chung PWM, et al. Neoadjuvant Chemotherapy Before Bladder-Sparing Chemoradiotherapy in Patients With Nonmetastatic Muscle-Invasive Bladder Cancer. Clin Genitourin Cancer 2019;17:38-45. [Crossref] [PubMed]
- Kotha NV, Kumar A, Nelson TJ, et al. Treatment Discontinuation in Patients With Muscle-Invasive Bladder Cancer Undergoing Chemoradiation. Adv Radiat Oncol 2021;7:100836. [Crossref] [PubMed]
- Hamad J, McCloskey H, Milowsky MI, et al. Bladder preservation in muscle-invasive bladder cancer: a comprehensive review. Int Braz J Urol 2020;46:169-84. [Crossref] [PubMed]
- Su Q, Gao S, Lu C, et al. Comparing Prognosis Associated with Partial Cystectomy and Trimodal Therapy for Muscle-Invasive Bladder Cancer Patients. Urol Int 2023;107:46-57. [Crossref] [PubMed]
- Kijima T, Tanaka H, Koga F, et al. Selective tetramodal bladder-preservation therapy, incorporating induction chemoradiotherapy and consolidative partial cystectomy with pelvic lymph node dissection for muscle-invasive bladder cancer: oncological and functional outcomes of 107 patients. BJU Int 2019;124:242-50. [Crossref] [PubMed]
- Tanaka H, Fukushima H, Kijima T, et al. Feasibility and outcomes of selective tetramodal bladder-preservation therapy in elderly patients with muscle-invasive bladder cancer. Int J Urol 2020;27:236-43. [Crossref] [PubMed]


