Standards for MRI reporting—the evolution to PI-RADS v 2.0
Introduction
Prostate cancer (PCa) remains a leading cause of death in the United States (1), but the vast majority of men diagnosed with PCa will die from other causes. Thus, the capability of assessing the risk of life-threatening versus indolent PCa has been an important goal of management, with heavy reliance on serum prostate-specific antigen (PSA), which is reasonably sensitive but not specific for PCa. Benign pathologies, such as benign prostatic hyperplasia (BPH) can raise PSA levels, and normal PSA levels cannot exclude the presence of clinically significant PCa (2). Transrectal ultrasound (TRUS)-guided biopsy is another diagnostic test that has traditionally played a major role, but it can miss cancers or underestimate Gleason score in approximately 30% of cases (3). In addition, TRUS is generally considered insufficient for the local staging of PCa (4).
For several decades, conventional magnetic resonance imaging (MRI) has had a niche for staging of some biopsy proven PCas. However, detection and characterization of clinically significant cancer within the prostate gland was not reliable because T2-weighted (T2W) imaging, the mainstay for conventional prostate MRI, was fundamentally limited by its poor specificity and high inter- and intraobserver variability. The emergence of multiparametric MRI (mpMRI) with its combination of anatomical and functional pulse sequences, including diffusion weighted imaging (DWI) and dynamic contrast-enhanced MRI (DCE-MRI), has resulted in measureable improvements, and it has been shown to be useful for identification and guidance of targeted biopsy of suspicious lesions (5). However, wide variations and lack of standardization of MRI data acquisition, interpretation, and reporting have hampered rigorous scientific assessment and comparison of single institution results, and it has been acknowledged as one of the critical impediments to widespread clinical adaptation. Several investigators, including Dickinson et al. (6), attempted to develop criteria for uniform technical parameters and interpretation of mpMRI, but reliable implementation into daily practice remained problematic, as such criteria were hard to define and there was significant disagreement amongst experts in the rapidly evolving field.
PI-RADS v1
In order to promote a greater level of standardization and consistency and facilitate multi-center clinical evaluation and implementation of mpMRI for assessment of PCa, the AdMeTech Foundation International Prostate MRI Working Group, with support of a grant from the U.S. Army Medical Research & Materiel Command (USAMRMC), recommended the development of a set of consensus guidelines, called Prostate Imaging and Reporting and Data System (PI-RADS), that would be based on evidence from published data and consensus expert opinion. The European Society of Urogenital Radiology (ESUR) published the first version of this document, which included clinical indications for prostate mpMRI, minimal and optimal imaging acquisition protocols, and a structured category assessment system now known as PI-RADS version 1 (PI-RADS v1) (7).
Since its publication in 2012, the benefits of using a standardized assessment system such as PI-RADS v1 was validated in several clinical and research scenarios (8-10), and it provided a scaffold on which less experienced radiologists could base their interpretations. However, it was quickly recognized that it suffered from several major limitations, including unclear recommendations for scoring each of the mpMRI parameters. In addition, PI-RADS v1 did not delineate how the scores assigned in each of the pulse sequences would contribute to the determination of the final overall assessment. For example, while some radiologists were simply adding individual scores together to obtain the final score ranging from 1 to 15 (or from 1 to 20 when using MR spectroscopy), others were attempting to subjectively determine the overall score on a scale from 1 to 5. This variability and subjectivity in scoring were confusing to radiologists, referring clinicians, and patients. Given the lack of a clearly defined scheme for assigning an overall assessment, PI-RADS v1 had difficulty in achieving consistency in clinical practice, and it was not widely adopted, especially in the United States.
PI-RADS v2
In an effort to update and improve upon the original version of PI-RADS and establish a single international standard, a joint steering committee of the American College of Radiology (ACR), ESUR and the AdMeTech Foundation collaborated to develop PI-RADS Version-2 (PI-RADS v2), which was released in 2015 (11). Among some of its specific goals were to simplify and standardize the terminology and content of mpMRI reports, develop assessment categories that outline levels of suspicion of having significant PCa, reduce variability in imaging interpretations, and establish acceptable technical parameters for data acquisition. It reflected the contemporary level of knowledge and has become the standard of care in prostate mpMRI.
Although PI-RADS v2 is built on the foundation of PI-RADS v1, there are many important differences. For PI-RADS v1, the focus was on clinical applications of prostate mpMRI, patient management, and assessment of extraprostatic extension (EPE)/staging. PI-RADS v2 instead focuses on lesion detection and characterization (including benign findings), as well as interpretation and reporting. It includes detailed explanations, caveats, and explicit instructions on measuring and mapping PCa. It also includes images that illustrate assessment criteria and an extensive lexicon of relevant terminology. Other important differences are shown in Table 1 and will be highlighted in the remainder of this article, which details the criteria employed in PI-RADS v2 for the assessment of PCa on mpMRI examinations.

Full table
Background
For PI-RADS v2, PCa is divided into clinically significant and insignificant disease. Clinically significant PCa’s are defined as those with a Gleason score ≥7 (including 3+4 with prominent but not predominant Gleason 4 component), and/or volume ≥0.5 cc, and/or EPE (12).
PI-RADS scoring system
PI-RADS v2 assesses the likelihood (probability) of clinically significant PCa for each lesion using a 5-point scale, which takes into account findings from three pulse sequences that comprise a standard mpMRI exam: T2W, DWI, and DCE.
The following final Assessment Categories are used:
- PI-RADS 1—Very low (clinically significant cancer is highly unlikely to be present);
- PI-RADS 2—Low (clinically significant cancer is unlikely to be present);
- PI-RADS 3—Intermediate (the presence of clinically significant cancer is equivocal);
- PI-RADS 4—High (clinically significant cancer is likely to be present);
- PI-RADS 5—Very high (clinically significant cancer is highly likely to be present).
To arrive at one of these five PI-RADS v2 Assessment Categories for each suspicious finding in the prostate, T2W and DWI are individually evaluated and scored using a 5 point scale, while DCE is classified as either positive or negative. The corresponding PI-RADS v2 table for either the peripheral zone (PZ) or transition zone (TZ) is then referenced to integrate all three parameters (T2W, DWI, and DCE) and assign a PI-RADS v2 Assessment Category (PI-RADS 1–5) for each lesion, indicating its likelihood of representing clinically significant cancer. One of the key differences between PI-RADS v1 and v2 is that the T2W, DWI, and DCE scores are not summated to assign an overall PI-RADS category. Rather, the scores are used in a simple hierarchical scheme which varies depending on whether the suspicious finding is predominantly in the PZ or the TZ. However, every pulse sequence that comprises the mpMRI exam (T1W, T2W, DWI, DCE) should be thoroughly interrogated in every case as they may provide clues to the location and nature of a lesion, even if they are not used to determine the PI-RADS v2 Assessment Category for a specific finding.
DWI scoring
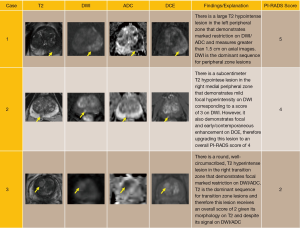
A score of 1 to 5 is assigned on DWI by comparing the signal intensity in a lesion to the average signal of “normal” prostate tissue in the histologic zone in which it is located. Table 2 provides the criteria for assigning a score from 1 through 5 based on findings from DWI. Note that these criteria take into consideration: (I) lesion shape and margins; (II) signal intensity; (III) size; and (IV) observations from both the high b-value images and the ADC map. See Figure 1 for examples.

Full table
In the PZ, DWI is considered to be the “dominant” sequence. In other words, assignment of a PI-RADS v2 Assessment Category for a lesion in the PZ is based predominantly on the DWI score (Table 3). For example, if the DWI score is 4 then the PI-RADS Assessment Category should also be 4, irrespective of the scores on T2W or DCE sequences. The only exception to this direct relationship of the DWI score and PI-RADS Assessment Category in the PZ is when a DWI score of 3 is upgraded to a final PI-RADS Assessment Category of 4 because of a positive (+) DCE score. (See “DCE scoring” section for definitions of positive and negative DCE scores.) For all other DWI scores in the PZ (i.e., 1,2,4,5), the final PI-RADS Assessment Category is based solely on the DWI score and is independent of both the DWI score and T2W score.
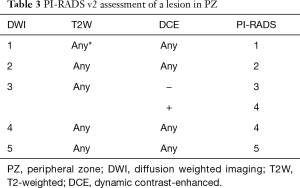
Full table
It is important to remember that certain benign conditions display focal hypointense ADC signal as well. Familiarity with these conditions and their typical MR appearance is essential for making the appropriate PI-RADS assessment. For example, while fibrosis, calculi, and hemorrhage may all be hypointense on both T2W and ADC due to insufficient signal, they will also be markedly hypointense on all DWI images, essentially excluding clinically significant disease. Benign prostatic hypertrophy presents a greater challenge. Encapsulated, circumscribed, and round nodules in the TZ or PZ generally represent BPH or extruded BPH, respectively, regardless of their ADC/DWI signal. However, not uncommonly, BPH nodules may lack some or all of their benign morphologic features and demonstrate marked ADC hypointensity, making assessment difficult. This remains a recognized limitation of mpMRI diagnosis and usually requires great expertise and experience on the part of the reader.
T2W scoring
In the TZ, T2W is the dominant sequence for assigning the overall PI-RADS assessment category. In other words, the T2W score directly corresponds to the overall assessment score. For example, if the T2W score for a TZ lesion is 2, the overall assessment score will also be 2, irrespective of scores on DWI and DCE. The only exception occurs with a T2W score of 3, in which case the DWI score serves as a tiebreaker (Table 4).
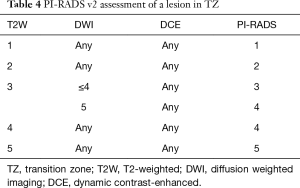
Full table
Scoring of T2W also utilizes a 5 point scale, but the definitions of each score slightly differ between the PZ and TZ (Tables 5,6), reinforcing the importance of correctly determining the lesion location. Areas where distinguishing PZ and TZ may be especially problematic include the interface of the central zone (CZ) and PZ at the base of the gland and the interface of the anterior horn of the PZ with TZ at the anterior fibromuscular stroma (AS). In addition, both PZ and TZ cancers may extend across anatomical boundaries (i.e., exhibit an invasive behavior), further confounding assessment.

Full table

Full table
While many benign conditions may complicate evaluation of the PZ as mentioned above, detecting malignancy in the TZ is even more challenging. BPH is extremely common (13) and when present it is composed of variable amounts of glandular (T2-hyperintense) and stromal (T2-hypointense) tissue. Identifying T2 hypointense cancer amongst this very heterogeneous tissue requires significant concentration and experience. Typical T2W features of TZ tumors that may prove useful include ill-defined moderate hypointensity (“erased charcoal” or “smudgy fingerprint” appearance), spiculated margins, lenticular shape, absence of a complete hypointense capsule, and invasion of the urethral sphincter and AS. The more features present, the higher the likelihood of a clinically significant TZ cancer.
DCE scoring
DCE is considered “positive” when a lesion confirmed on T2 and/or DWI demonstrates earlier or contemporaneous enhancement in relation to adjacent normal prostatic tissue. While typically this enhancement occurs within 10 seconds of contrast appearing within the femoral arteries, the exact timing varies. Some of the factors that play a role include contrast injection rate, cardiac output, and temporal resolution used to acquire the images. Negative and positive DCE scores are defined in Table 7.

Full table
Most published data show that the added value of DCE over and above the combination of T2W and DWI is modest (14). The main problem with relying on DCE is the variable enhancement kinetics of PCa. Not every prostate neoplasm enhances or washes out early, and, conversely, enhancement alone is not definitive for clinically significant cancer. Moreover, some benign processes exhibit early enhancement and should be interpreted in the context of the entire scan, as is often seen with BPH nodules. In addition, the enhancement analysis itself remains subjective thus contributing to inconsistency of results.
As seen in the scoring scheme described above, DCE only plays a secondary role in determination of PI-RADS v2 Assessment Category. In fact, it does not contribute to the overall assessment at all when the finding in the PZ has a low (PI-RADS 1 or 2) or high (PI-RADS 4 or 5) likelihood of clinically significant cancer. DCE only comes into play when upgrading a lesion in the PZ with a DWI score of 3 to the final PI-RADS assessment of 4, if scored as positive. Overall, when T2W and DWI are of diagnostic quality, DCE plays a minor role in determining PI-RADS Assessment Category, which constitutes a major difference from the original version of PI-RADS. See Figure 2 for examples.
PI-RADS assessment category “X”
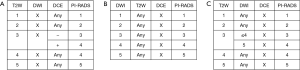
Certain technical and/or patient factors may significantly limit mpMRI examination. One or more of the components of the mpMRI (T1W, T2W, DWI, DCE) may be suboptimal or absent, necessitating a different scoring scheme. The most common scenario is inadequate DWI and/or DCE. If both are inadequate or missing, the assessment should be used only for staging for determination of EPE. If one is inadequate or missing, it should be assigned a PI-RADS Assessment Category “X” for that component and the lesion should be scored according to the alternate scheme (see Figure 3).
Reporting
Communicating results of an mpMRI examination in a clear, concise, and structured fashion is as essential as detecting the pertinent abnormality. With PI-RADS v1, the lack of standardized terminology and reporting was an impediment to the widespread adaptation of mpMRI. PI-RADS v2 sought to correct for this deficiency by making a number of recommendations to reduce variability in image interpretation, simplify terminology and standardize content.
Each report should include a calculation of prostate volume, which is determined using the formula for a prolate ellipse: (maximum AP diameter) × (maximum transverse diameter) × (maximum longitudinal diameter) × 0.52. This information may be used to determine PSA density (PSA/prostate volume) and influence various management decisions.
The pre-existing methods of measuring lesions themselves suffered from underestimation of tumor volume and extent compared to histology (15). As a result, the proper technique for measuring lesions has been a subject of debate. In hopes of avoiding confusion and better approximating true histologic volumes, PI-RADS v2 incorporated guidelines for tumor measurements:
- A lesion in the PZ should be measured on ADC (the “dominant” sequence in the PZ) and a lesion in the TZ should be measured on T2W (the “dominant” sequence in the TZ). If lesion measurement is difficult or compromised on ADC (for PZ) or T2W (for TZ), measurement should be made on the sequence that shows the suspicious finding best;
- The minimum requirement is to report the largest dimension of a lesion on an axial image. If the largest dimension of a suspicious finding is on sagittal and/or coronal images, this measurement and imaging plane should also be reported. If a lesion is not clearly delineated on an axial image, the measurement on the plane which best depicts the finding should be reported;
- The MRI report should clearly state the image number and sequence used to obtain the measurement;
- Alternatively, the lesion volume (rather than single or two dimensions) may be documented;
- Up to four most suspicious lesions should be reported with the “index” lesion clearly marked.
The index lesion is defined as one that is most likely to yield the highest Gleason score, contribute to extraprostatic extension, or produce positive margins at surgery. This should correspond to the lesion with the highest PI-RADS v2 Assessment Category. If the highest PI-RADS v2 Assessment Category is assigned to two or more lesions, the index lesion should be the one that shows extraprostatic extension. Thus, a smaller lesion with EPE should be defined as the index lesion despite the presence of a larger tumor with the identical PI-RADS Assessment Category. If none of the lesions demonstrates extraprostatic extension, the largest of the tumors with the highest PI-RADS v2 Assessment Category should be considered the index lesion.
While reporting of benign findings (e.g., cyst) is optional, it may assist with biopsy guidance and follow-up imaging by providing anatomic landmarks. Regardless, each reported lesion should be assigned to a prostate sector(s) on a Sector Map (16) consisting of 39 sectors: 36 for the prostate, 2 for the seminal vesicles (SV) and 1 for the external urethral sphincter:
- The prostate is divided into right/left on axial sections by a vertical line drawn through the center (indicated by the prostatic urethra) and into anterior/posterior by a horizontal line through the middle of the gland;
- The right and PZ at prostate base, midgland, and apex are each subdivided into three sections: anterior (a), medial posterior (mp), and lateral posterior (lp);
- The right and left TZ at prostate base, midgland, and apex are each subdivided into two sections: anterior (a) and posterior (p);
- The AS is divided into right/left at the prostate base, midgland, and apex;
- The SV are divided into right/left.
Division of the prostate and associated structures into sectors standardizes reporting and facilitates precise localization for MR-targeted biopsy and therapy, pathological correlation, and research. This Sector Map should be attached to the radiology report (either in electronic or hardcopy format) with identified suspicious findings clearly marked. If a suspicious finding extends beyond the boundaries of one sector, all involved neighboring sectors should be indicated on the map (as a single lesion).
PI-RADS v2 limitations
Although PI-RADS v2 represents a major step in the integration of mpMRI into clinical practice, it still suffers from several limitations that will need to be addressed in the future versions. Scoring of DCE serves as one such example and was discussed earlier. Also, despite serving as critical factors in assigning the overall assessment score, there are no strict definitions for what constitutes mild, moderate, or marked restriction on DWI or hypointensity on T2W, and they can vary depending on technical parameters. Similarly, other terms in the PI-RADS v2 lexicon are highly subjective and open to interpretation, such as “focal shape” and “definite invasive behavior” .
Applying the PI-RADS v2 assessment system in certain anatomical zones may also be problematic. The CZ often displays a level of diffusion restriction that is similar to significant PCa, and other characteristics not delineated in the scoring system play more important roles in distinguishing CZ from PCa, such as symmetry and location.
Locations of certain lesions may be uncertain altogether, as is the case at the apex where differentiation between TZ and PZ is often challenging, yet PI-RADS v2 assessment scores can vary greatly depending on whether TZ or PZ criteria are applied.
Conclusions
Building on the important work resulting in PI-RADS v1, several key changes were incorporated into PI-RADS v2. This article has highlighted some of the more vital modifications: introduction of the concept of a “dominant sequence” depending on the lesion location, relegation of DCE to the secondary role, conversion to a 5-point scale of assessment, and elimination of MR spectroscopy from the scoring scheme.
As it stands, PI-RADS v2 currently represents the most up-to-date information on how to acquire, interpret, and report mpMRI of the prostate. It is a step forward in simplifying the initial efforts of standardization made in PI-RADS v1, but will undoubtedly require more modifications as research continues, experience accrues, and technology evolves.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med 2004;350:2239-46. [Crossref] [PubMed]
- Kvåle R, Møller B, Wahlqvist R, et al. Concordance between Gleason scores of needle biopsies and radical prostatectomy specimens: a population-based study. BJU Int 2009;103:1647-54. [Crossref] [PubMed]
- Terris MK, Stamey TA. Determination of prostate volume by transrectal ultrasound. J Urol 1991;145:984-7. [PubMed]
- Turkbey B, Merino MJ, Gallardo EC, et al. Comparison of endorectal coil and nonendorectal coil T2W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: correlation with whole-mount histopathology. J Magn Reson Imaging 2014;39:1443-8. [Crossref] [PubMed]
- Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol 2011;59:477-94. [Crossref] [PubMed]
- Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012;22:746-57. [Crossref] [PubMed]
- Rosenkrantz AB, Kim S, Lim RP, et al. Prostate cancer localization using multiparametric MR imaging: comparison of Prostate Imaging Reporting and Data System (PI-RADS) and Likert scales. Radiology 2013;269:482-92. [Crossref] [PubMed]
- de Rooij M, Hamoen EH, Fütterer JJ, et al. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR Am J Roentgenol 2014;202:343-51. [Crossref] [PubMed]
- Arumainayagam N, Ahmed HU, Moore CM, et al. Multiparametric MR imaging for detection of clinically significant prostate cancer: a validation cohort study with transperineal template prostate mapping as the reference standard. Radiology 2013;268:761-9. [Crossref] [PubMed]
- Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 2016;69:16-40. [Crossref] [PubMed]
- Ploussard G, Epstein JI, Montironi R, et al. The contemporary concept of significant versus insignificant prostate cancer. Eur Urol 2011;60:291-303. [Crossref] [PubMed]
- McNicholas T, Swallow D. Benign prostatic hyperplasia. Surgery (Oxford) 2011;29:282-6. [Crossref] [PubMed]
- Hansford BG, Peng Y, Jiang Y, et al. Dynamic Contrast-enhanced MR Imaging Curve-type Analysis: Is It Helpful in the Differentiation of Prostate Cancer from Healthy Peripheral Zone? Radiology 2015;275:448-57. [Crossref] [PubMed]
- Le Nobin J, Orczyk C, Deng FM, et al. Prostate tumour volumes: evaluation of the agreement between magnetic resonance imaging and histology using novel co-registration software. BJU Int 2014;114:E105-12. [Crossref] [PubMed]
- Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol 2011;59:477-94. [Crossref] [PubMed]