Cost consideration in utilization of multiparametric magnetic resonance imaging in prostate cancer
Introduction
Magnetic resonance imaging (MRI) is changing the face of prostate cancer care. Politically, cost of care is under ever increasing focus. Despite legislative attempts to hinder healthcare costs, care expenditures are on track to consume 20% of total United States gross domestic product by 2024 (1). The largest single portion of projected cancer care cost increases are expenditures for care of prostate cancer (2). Concerns have been raised both in the United States and abroad about the cost implications of adding high-technology imaging to the already expensive management of prostate cancer (3). The highest quality prospective trials have shown a survival benefit with PSA based screening, however the major drawback of this approach is the high detection rate of nonlethal prostate cancers and a significant risk of overtreatment (4-6). The major driving force for increased costs in the PSA era is not a consequence of the screening itself, but the resultant costs from treatment (7). Non-imaging based prognostic tools for prostate cancer have expanded in recent years (8-12). This rapid expansion of technology has occurred in the midst of significant controversy with respect to the value of prostate cancer screening (13,14). While the US Preventative Task Force has recommended against PSA screening, the American Urologic Association and American Cancer Society still support its use. Furthermore, recent information about the high contamination rates in the placebo arm of the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) may lead to changes in task force recommendations (15). Regardless, there are still ongoing issues with prostate cancer screening due to the lack of specificity of PSA and the inability of PSA to provide information regarding the clinical significance of prostate cancer (16).
“Prostate MRI” in modern literature generally refers to multiparametric imaging protocols that utilize a combination of dynamic contrasted phases and diffusion weighted imaging (17,18). These phases are evaluated and an aggregate risk for a particular lesion being cancerous is given by the radiologist, commonly as a PI-RADs score (19). Using this score, MRI provides an accurate diagnostic tool in prostate cancer with high specificity for high grade disease (20). The negative predictive value of MRI also provides an opportunity to delay or avoid a biopsy in cases where no lesion is detected. This could reduce both the cost of the biopsy and the potential risk of serious complications, such as sepsis, whose incidence is rising due to increasing rates of quinolone resistance (21-23). Post-biopsy sepsis, while rare, is serious for those patients affected and costly to the healthcare system (24). Many cost studies assume that MRI negative patients would not undergo biopsy, however omission of systematic ultrasound guided biopsies may miss relevant cancers and may not reflect real-world practice (25).
At the same time, providers are moving to less interventional management of low grade prostate cancer, especially in older patients (26). In the midst of these changes, the issue of cost has been an evolving field (27). There is a sparse literature on cost implications of MRI (Table 1) in prostate cancer care. MRI itself is costly, both in initial capital outlay and in cost of ongoing operations. While the added value of MRI in certain clinical circumstances is well established, the requirements for MRI unit, radiologist training, and relative value versus other approaches are all undergoing evaluation (28). Cost considerations in prostate MRI requires knowledge of both the performance characteristics of MRI and the baseline costs of the exam. Furthermore, the paradigm for using the information either to improve or avoid biopsy can impact the overall cost-effectiveness of the approach.
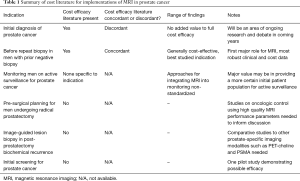
Full table
Baseline prevalence and cost for MRI in various healthcare environments
In cost modeling studies, baseline assumptions and incidence rates can have large impacts on ultimate findings of cost efficacy. One of the most important factors is an accurate estimation of costs which include not only the cost of the equipment but also maintenance, operation (personnel, space) and frequency of use. An expensive machine that is used many times daily has a much lower cost per patient than one used infrequently since the capital costs are fixed and can be distributed among all users.
Another important consideration is, despite the fact that economies are global, that there is regional variability in assumptions regarding cost of MRI. Three different cost analyses of prostate MRI used different costs for MRI. A study from a Dutch group used a cost of MRI at €345, an American study using medicare reimbursement rates of $524 and a Canadian study using hospital expense of $900 (29-31). The determination of baseline costs can result in significantly different conclusions especially if one considers using MRI in every patient with an elevated PSA. Such factors may limit the ultimate conclusion of a cost analysis to its nation of origin. In many healthcare environments, the limiting factor for MRI use may not be cost but availability (32).
The US healthcare market is the largest and often most costly in the world (33). At current, the US domestic healthcare expenditures are similar in size to the entire gross domestic product of the United Kingdom (33). In the United States, the total “fair cost” for a prostate biopsy has been stated at $2,600 by one healthcare ratings agency (34). Currently, there is not a defined CPT code for the fusion portion of MRI-ultrasound fusion prostate biopsy, though the radiologist will typically bill for the performance and interpretation of the MRI (35). As fusion devices require significant purchasing outlays, this is a cost hurdle to adoption of the technology; actual prices of fusion devices are not generally released, but some web pages have referred to average purchase prices in the $200,000 range (36). In the United States the majority of costs for PSA-based prostate cancer screening are incurred by the downstream procedural-based portions of the treatment algorithm (37). Cost analyses of MRI in prostate cancer screening typically revolve around the ability to avoid initial biopsy in men with elevated PSA values and the cost savings inherent in these decreased procedural charges. Pre-procedural MRI has been prospectively shown to improve the yield of prostate biopsy and detect more clinically significant prostate cancers (38,39), however the specific role of MRI in managing prostate cancer at various clinical decision points is an ongoing area of research.
Considerations and limitations in prostate MRI literature
In the urologic surgical literature there is a well-recognized effect of surgical approach and surgeon experience on ultimate clinical and oncologic outcomes in surgery. Within the performance of prostate MRI the same considerations impact the utility of the exam, but studies utilizing differing MRI modalities [e.g., 1.5 Tesla MRI without endorectal coil (40) versus 3 Tesla MRI with endorectal coil (41,42)] are often referred to without explicit differentiation. Both coil type and field strength have been shown to impact image quality (43) and more modern, higher field strength scanners are more expensive. The utility of prostate MRI performed without a standardized reporting system and by non-genitourinary specialist radiologists is likely lower than what is generally reported in the literature (44). Other caveats apply to the cost literature in MRI as a whole; costs (27) and availability (45) for MRI vary widely based on healthcare environment and care delivery models. Even in academic medical centers, the performance of prostate MRI varies widely, though the vast majority offer some form of MRI based prostate imaging (45). Various countries around the world have widely differing numbers of per-patient MRI units (46). While cost is often the limiting factor in market-based healthcare models, resource availability and central resource allotment are often more primary drivers in command economy and single-payer models (32).
Most literature in prostate MRI uses a Likert-type score to measure lesion suspicion on imaging. The PI-RADS system is the most widely reported, validated scoring scheme for prostate MRI (19) and its widespread use is contributing to more uniform studies in the field (47). Prostate MRI is significantly more sensitive for larger (0.5 cm or greater) and higher grade tumors, an aspect of its performance sometimes referred to as an asset as it misses many “clinically insignificant” (small, low grade) prostate cancers (48).
The question of cost-efficacy itself depends on each of these performance characteristics and the regional costs of goods and services. To that end, examining each clinical application of prostate MRI with respect to cost can allow for individual decision points to be better understood both with respect to economic implications and with respect to patient impact.
Costs of multi-parametric MRI (mpMRI) fusion biopsy
Costs of MRI as a portion of the prostate cancer treatment algorithm should also be taken in context of competing technologies. Literature surrounding biomarkers for prostate cancer in detection, risk stratification and monitoring roles is robust but no biomarker or genomic test is currently standard of care in the management of men with the disease (49). Urine based markers including PCA3 and TMPRSS2: ERG improve the performance characteristics of PSA somewhat but provide no localizing information (50); the cost for these exams is similar to cost figures used in some European cost studies utilizing prostate MRI (29,51). Other alternatives that may provide this valuable localizing information at a lower cost, including multiparametric ultrasound biopsy, are supported by less robust data and will require additional validation (52).
The purest form of utilizing MRI information in prostate biopsy is performing multiparametric MRI and subsequent in-bore MRI-guided biopsy of suspicious lesions. While this approach demonstrates high quality performance characteristics (53), resource availability will likely limit its widespread use. An intermediate option is the use of MRI-ultrasound fusion hardware and software packages that allow the MRI data to be superimposed over live ultrasound images, guiding the provider during the biopsy. The utility of MRI-fusion software and hardware is itself a point of controversy. In theory a provider could review relevant MRI images and target a region of interest using ultrasound guidance, a practice used elsewhere in the body for the biopsy of metastases. This practice of viewing the MRI and targeting with ultrasound is generally referred to as “cognitive fusion” (54). The fusion approach involves use of software that overlays an MRI image on a “real-time” ultrasound image allowing assessment of the accuracy of the biopsy in relation to the MRI. A 2015 study in an ex vivo model showed greatly improved detection of relevant lesions using MRI machine-based fusion versus cognitive fusion (55), however a prospective, blinded in-human study failed to show a difference in cancer detection between the two modalities (56). Similar results were found in another in-human 2013 prospective study which found no difference in cancer detection between machine-based fusion and cognitive fusion (57).
The ability to perform fusion MRI requires coordination between the radiologist and the urologist. The radiologist needs to identify the suspicious lesions and then “transfer” the MRI images to the ultrasound machine so the urologist cancer fuse the images at time of biopsy. There is currently no mechanism to reimburse either professional for the added time or equipment costs of such a maneuver. This type of cooperation tends to be easier when both urologist and radiologist are in the same institution since it is not currently practical for transferring data from MRI procedures done at “outside” centers.
Cost considerations of mpMRI in the initial diagnosis of prostate cancer
The additional value of initial MRI prior to biopsy in PSA-screened men without a prior negative biopsy is in and of itself a controversial topic. In 2015 a prospective, randomized controlled trial of over 100 men found no difference in all cancers detected and “clinically relevant” cancers detected in men undergoing initial MRI fusion biopsy versus ultrasound guided biopsy using a 3 Tesla scanner and no endorectal coil (58). Similar findings have been noted in a retrospective series of men without prior biopsy using ultrasound guidance versus cognitive fusion and in-bore MRI guided biopsy (59). Other studies have suggested that the initial diagnostic value of MRI may be present in certain population subsets, including those with large prostates (60). Of note a prospective study of MRI and MRI guided biopsy (not ultrasound fusion) demonstrated improved cancer detection in biopsy-naïve men (53). Cost estimates for the addition of MRI to the initial diagnostic algorithm of prostate cancer have ranged widely and often more closely reflect local market forces than actual resource utilization (20,29,30).
The most thorough biopsy method is that of combining transperineal saturation biopsy with MRI fusion biopsy, an approach that has been shown to have index lesion concordance above 95% in patients undergoing subsequent radical prostatectomy (61). As this approach requires general anesthesia, the cost case is less compelling, especially in biopsy-naïve patients. A recent modeling study by Cerantola et al. used a Markov model to assign costs and quality-adjusted life years (QALYs) for two treatment pathways: ultrasound guided prostate biopsy and upfront staging MRI with MR guided biopsy. They concluded that the MRI pathway was more cost-effective at all time points assessed (31). Important caveats about this study include their construction of model which used a 15% rate of active surveillance in the low risk prostate cancer group (which would be more prevalent in the non-MR guided pathway) and favorable cost figures based on estimates for MRI (31). Also, in their model most men developed cancer over the study period which may not reflect real world populations.
An earlier Dutch study examined the cost case of using MRI and in bore MRI-guided biopsy as the primary initial diagnostic modality in the management of prostate cancer and found the approach to be nearly cost-equivalent to current management with a significant improvement in QALYs. A number of assumptions in this study may limit its generalizability including the low costs associated with multiparametric MRI (€300) and MRI-guided prostate biopsy (€800) (29). Another concern with the current models is that they assume that no biopsy is performed on men with negative imaging. The impact of “missed cancer” will need to be assessed in prospective studies. External to the issue of cost is that of value derived by the patient, especially in the indication of initial biopsy; even if an MRI-based initial evaluation of prostate cancer is non-cost effective it may still be desirable as approximately one third of ultrasound biopsies are upgraded when subsequently evaluated with MRI guidance (62).
Cost considerations of mpMRI in patients with a prior negative biopsy
The indication for which there appears to be the best evidence for cost efficacy in prostate MRI is in the man with a negative prior ultrasound-guided prostate biopsy and continued clinical suspicion for prostate cancer (63). In the past clinical nomograms provided information about likelihood of repeated biopsies being positive, but did not provide guidance for localization of repeat biopsy (12). Similarly, additional tests and biomarkers have been shown to improve the performance of PSA in men with prior negative biopsy (64). Studies of MR guided biopsy in men with prior negative ultrasound biopsy have shown an increased rate of detection of high grade tumors, especially in the anterior prostate, a region often poorly sampled in ultrasound-guided biopsy (65). A study from 2015 showed both cost savings in using MRI to inform repeat biopsy and that a large portion of repeat biopsies could be avoided (30). In patients undergoing MR-guided biopsy after negative prior biopsy the possibility of avoiding systematic (non-targeted) biopsies as a cost saving measure has been raised. This approach should be used with caution as it appears that systematic biopsies still add value and detect some clinically relevant cancers in this setting (25). As MRI techniques continue to refine and MRI use in prostate cancer management grows, MRI before repeat prostate biopsy is likely to become increasingly common.
Cost considerations of mpMRI in the management of men on active surveillance
Active surveillance is rapidly becoming the preferred management pathway for men with low volume, low grade prostate cancer without other adverse risk factors. It appears to offer good oncologic safety with organ preservation possible in a significant portion of men (66). The greatest value of MRI in men on active surveillance may be in providing a more accurate initial staging; the negative predictive value for higher grade tumors appears high and is especially useful in identifying tumors outside of regions of the prostate generally sampled with TRUS biopsy (67).
An argument in support of MRI in active surveillance is that of the high cost of management of post-biopsy sepsis which has been estimated at $7,000 per episode in one study (24). As nearly all current active surveillance protocols involve at least one planned repeat prostate biopsy, this complication is an important focus. Better understanding of the causes of post biopsy sepsis and efforts to reduce its incidence have been frequently published in the urologic literature (23,68,69). Most interventions that reduce the rate of post-biopsy sepsis are themselves cost effective (22). Especially in patients with serious competing medical comorbidity, prostate biopsy may present an unfavorable risk/benefit profile which may require modification of active surveillance protocols in these sicker patients (70). Whether using MRI as a surrogate in these cases is cost-effective is not well studied, but clinical prudence may take precedence even if it is not.
Active surveillance as a cost-saving approach to management of prostate cancer is itself a complex issue. Studies of cost efficacy of active surveillance versus upfront therapy depend heavily on how different health states are valued and what baseline assumptions are used (71). In general, a time-dependent cost balance is recognized where early in therapy active surveillance is clearly superior with respect to cost (as it obviates early need for expensive curative intent surgery or radiation) but may become less cost effective over time due to resources needed for intensive monitoring (72). In general, the driving factor in cost for active surveillance is that of biopsy and so alternative modalities that may provide similar information, such as MRI, may improve its cost profile (73). The largest gap in clinical and cost knowledge is the value and cost efficacy of serial MRI exams in evaluating men on active surveillance. As this approach becomes increasingly used in low-risk disease, the role of MRI will be initially based on the clinical judgement of providers until robust, long term data sets are available.
Cost considerations of mpMRI in surgical planning for prostate cancer
Prostate MRI also has the potential to add value in men already carrying a diagnosis of prostate cancer. Especially in men with a high risk of positive margins at the time of prostatectomy, having a clear pre-surgical map of their tumors could, in theory allow for disease-specific modification of surgical technique that may improve oncologic control. MRI and MRI-guided biopsy prior to prostatectomy have been shown to more accurately correlate with final whole gland pathology than ultrasound guided biopsy (74). In practice, prostate MRI for surgical planning is a more complex issue, especially because many of the multiparametric sequences that most predict high grade prostate tumors (including diffusion mapping) are themselves relatively low resolution compared to standard T1 and T2 images.
In 2015 Rud et al. performed a prospective, randomized trial of MRI prior to robotic prostatectomy to evaluate for the potential to decrease positive surgical margins and found no difference in margin rates over 400 men with PSA-detected prostate cancers (40). Criticisms of this study have included the MRI technique which utilized 1.5 Tesla scanner and no endorectal coil, which has been shown to be an important aspect of high-resolution prostate MRI when performed even on higher field strength machines (42,75). From a cost-perspective, it is difficult to clearly quantify the added value of information regarding clinical stage. In some cases it may guide a decision regarding use of surgery or radiation therapy in conjunction with hormone therapy. This can have considerable cost implications. Furthermore it may impact use of nerve-sparing which can impact functional outcomes with financial implications as well.
In the postsurgical setting MRI has been used with cognitive fusion to biopsy post-prostatectomy recurrences (76); to our knowledge no comparative efficacy studies or cost efficacy studies exist comparing this approach to other prostate imaging modalities such as positron-emission tomography (PET)-choline or prostate-specific membrane antigen (PSMA) scans. If a truly high-resolution imaging option for post-prostatectomy recurrence were available it would be beneficial both from a cost perspective and patient perspective as it would allow for more accurate targeting in secondary interventions and allow for the avoidance of costly salvage techniques like external beam radiotherapy when such local recurrences could be definitively ruled out. The concept of MRI in pre-surgical planning and post-surgical management is still developing rapidly and as MRI technology improves, may prove valuable to patients and providers.
Cost considerations of mpMRI as a screening modality for prostate cancer
In 2016 a pilot study using prostate MRI as a cancer screening modality was published (77). The cohort included 47 volunteer Canadian men without family history of prostate cancer aged 50–75. The authors utilized a 3T non-endorectal coil MRI and biopsied all patients regardless of PSA. Eighteen of 47 men (38%) were identified with prostate cancer and 3 of the 18 had tumors identified only on MRI-targeted biopsies. At all points in the receiver operating characteristic curve mpMRI was superior to PSA level for prediction of prostate cancers. The cost implications of utilizing upfront MRI as a screening modality are likely in excess of what is feasible, even in the most resource-rich healthcare environments. However, if a clinical risk stratification tool can identify a subset of patients with high likelihood of mortality from prostate cancer who are poorly predicted by PSA levels, it may be reasonable approach in these patients.
Conclusions
The utilization of MRI in the management of prostate cancer is growing rapidly and is changing the practice of urology. With more men being managed with non-curative intent treatments, accurate staging and prognostication of tumors is ever more valuable. Managing the cost of these additional tests will represent a healthcare challenge in the coming years. By optimizing management pathways, evaluating where MRI adds true value and using prudence in which men to screen and biopsy for prostate cancer, providers can balance the needs of their patients with the needs of society as a whole.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Keehan SP, Cuckler GA, Sisko AM, et al. National health expenditure projections, 2014-24: spending growth faster than recent trends. Health Aff (Millwood) 2015;34:1407-17. [Crossref] [PubMed]
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 2011;103:117-28. [Crossref] [PubMed]
- Emberton M. Is prostate magnetic resonance imaging going to break the bank? Eur Urol 2014;66:437-8. [Crossref] [PubMed]
- Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol 2010;11:725-32. [Crossref] [PubMed]
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-8. [Crossref] [PubMed]
- Gosselaar C, Roobol MJ, Schröder FH. Prevalence and characteristics of screen-detected prostate carcinomas at low prostate-specific antigen levels: aggressive or insignificant? BJU Int 2005;95:231-7. [Crossref] [PubMed]
- Heijnsdijk EA, der Kinderen A, Wever EM, et al. Overdetection, overtreatment and costs in prostate-specific antigen screening for prostate cancer. Br J Cancer 2009;101:1833-8. [Crossref] [PubMed]
- Knezevic D, Goddard AD, Natraj N, et al. Analytical validation of the Oncotype DX prostate cancer assay—a clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genomics 2013;14:690. [Crossref] [PubMed]
- Gittelman MC, Hertzman B, Bailen J, et al. PCA3 molecular urine test as a predictor of repeat prostate biopsy outcome in men with previous negative biopsies: a prospective multicenter clinical study. J Urol 2013;190:64-9. [Crossref] [PubMed]
- Filella X, Giménez N. Evaluation of [-2] proPSA and Prostate Health Index (phi) for the detection of prostate cancer: a systematic review and meta-analysis. Clin Chem Lab Med 2013;51:729-39. [PubMed]
- Aubry W, Lieberthal R, Willis A, et al. Budget impact model: epigenetic assay can help avoid unnecessary repeated prostate biopsies and reduce healthcare spending. Am Health Drug Benefits 2013;6:15-24. [PubMed]
- Lopez-Corona E, Ohori M, Scardino PT, et al. A nomogram for predicting a positive repeat prostate biopsy in patients with a previous negative biopsy session. J Urol 2003;170:1184-8; discussion 1188. [Crossref] [PubMed]
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol 2013;190:419-26. [Crossref] [PubMed]
- Moyer VA. U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:120-34. [Crossref] [PubMed]
- Shoag JE, Mittal S, Hu JC. Reevaluating PSA Testing Rates in the PLCO Trial. N Engl J Med 2016;374:1795-6. [Crossref] [PubMed]
- Thompson IM, Ankerst DP, Chi C, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. JAMA 2005;294:66-70. [Crossref] [PubMed]
- Costa DN, Pedrosa I, Roehrborn C, et al. Multiparametric magnetic resonance imaging of the prostate: technical aspects and role in clinical management. Top Magn Reson Imaging 2014;23:243-57. [Crossref] [PubMed]
- Bjurlin MA, Meng X, Le Nobin J, et al. Optimization of prostate biopsy: the role of magnetic resonance imaging targeted biopsy in detection, localization and risk assessment. J Urol 2014;192:648-58. [Crossref] [PubMed]
- Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging-Reporting and Data System:2015,Version 2. Eur Urol 2016;69:16-40. [Crossref] [PubMed]
- de Rooij M, Hamoen EH, Fütterer JJ, et al. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR Am J Roentgenol 2014;202:343-51. [Crossref] [PubMed]
- Adibi M, Hornberger B, Bhat D, et al. Reduction in hospital admission rates due to post-prostate biopsy infections after augmenting standard antibiotic prophylaxis. J Urol 2013;189:535-40. [Crossref] [PubMed]
- Adibi M, Pearle MS, Lotan Y. Cost-effectiveness of standard vs intensive antibiotic regimens for transrectal ultrasonography (TRUS)-guided prostate biopsy prophylaxis. BJU Int 2012;110:E86-91. [Crossref] [PubMed]
- Loeb S, Vellekoop A, Ahmed HU, et al. Systematic review of complications of prostate biopsy. Eur Urol 2013;64:876-92. [Crossref] [PubMed]
- Roth H, Millar JL, Cheng AC, et al. The state of TRUS biopsy sepsis: readmissions to Victorian hospitals with TRUS biopsy-related infection over 5 years. BJU Int 2015;116 Suppl 3:49-53. [Crossref] [PubMed]
- Salami SS, Ben-Levi E, Yaskiv O, et al. In patients with a previous negative prostate biopsy and a suspicious lesion on magnetic resonance imaging, is a 12-core biopsy still necessary in addition to a targeted biopsy? BJU Int 2015;115:562-70. [Crossref] [PubMed]
- Hoffman RM, Shi Y, Freedland SJ, et al. Treatment patterns for older veterans with localized prostate cancer. Cancer Epidemiol 2015;39:769-77. [Crossref] [PubMed]
- Hutchinson RC, Costa DN, Lotan Y. The economic effect of using magnetic resonance imaging and magnetic resonance ultrasound fusion biopsy for prostate cancer diagnosis. Urol Oncol 2016;34:296-302. [Crossref] [PubMed]
- Nassiri N, Natarajan S, Margolis DJ, et al. Targeted Prostate Biopsy: Lessons Learned Midst the Evolution of a Disruptive Technology. Urology 2015;86:432-8. [Crossref] [PubMed]
- de Rooij M, Crienen S, Witjes JA, et al. Cost-effectiveness of magnetic resonance (MR) imaging and MR-guided targeted biopsy versus systematic transrectal ultrasound-guided biopsy in diagnosing prostate cancer: a modelling study from a health care perspective. Eur Urol 2014;66:430-6. [Crossref] [PubMed]
- Lotan Y, Haddad AQ, Costa DN, et al. Decision analysis model comparing cost of multiparametric magnetic resonance imaging vs. repeat biopsy for detection of prostate cancer in men with prior negative findings on biopsy. Urol Oncol 2015;33:266.e9-16. [Crossref] [PubMed]
- Cerantola Y, Dragomir A, Tanguay S, et al. Cost-effectiveness of multiparametric magnetic resonance imaging and targeted biopsy in diagnosing prostate cancer. Urol Oncol 2016;34:119.e1-9. [Crossref] [PubMed]
- Emery DJ, Forster AJ, Shojania KG, et al. Management of MRI wait lists in Canada. Healthc Policy 2009;4:76-86. [PubMed]
- 2015 health care providers outlook United States. Available online: https://www2.deloitte.com/content/dam/Deloitte/us/Documents/life-sciences-health-care/us-2015-global-hc-country-reports-011215.pdf
- Total Fair Price for Prostate Biopsy. Healthcare Bluebook 2015. Available online: https://healthcarebluebook.com/page_ProcedureDetails.aspx?id=92&dataset=MD&g=Prostate+Biopsy
- Rubenstein J. How to Code for Magnetic Resonance Imaging-informed Prostate Biopsies. Rev Urol 2014;16:88-9. [PubMed]
- MRI-Ultrasound Fusion Guided Biopsy. Cancerquest.org 2016. Available online: http://www.cancerquest.org/detection-diagnosis-mri-ultrasound-fusion.html
- Ma X, Wang R, Long JB, et al. The cost implications of prostate cancer screening in the Medicare population. Cancer 2014;120:96-102. [Crossref] [PubMed]
- Rastinehad AR, Turkbey B, Salami SS, et al. Improving detection of clinically significant prostate cancer: magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy. J Urol 2014;191:1749-54. [Crossref] [PubMed]
- Moore CM, Robertson NL, Arsanious N, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol 2013;63:125-40. [Crossref] [PubMed]
- Rud E, Baco E, Klotz D, et al. Does preoperative magnetic resonance imaging reduce the rate of positive surgical margins at radical prostatectomy in a randomised clinical trial? Eur Urol 2015;68:487-96. [Crossref] [PubMed]
- Baur AD, Daqqaq T, Wagner M, et al. T2- and diffusion-weighted magnetic resonance imaging at 3T for the detection of prostate cancer with and without endorectal coil: An intraindividual comparison of image quality and diagnostic performance. Eur J Radiol 2016;85:1075-84. [Crossref] [PubMed]
- Costa DN, Yuan Q, Xi Y, et al. Comparison of prostate cancer detection at 3-T MRI with and without an endorectal coil: A prospective, paired-patient study. Urol Oncol 2016;34:255.e7-e13. [Crossref] [PubMed]
- Beyersdorff D, Taymoorian K, Knösel T, et al. MRI of prostate cancer at 1.5 and 3.0 T: comparison of image quality in tumor detection and staging. AJR Am J Roentgenol 2005;185:1214-20. [Crossref] [PubMed]
- Billing A, Buchner A, Stief C, et al. Poor standard mp-MRI and routine biopsy fail to precisely predict intraprostatic tumor localization. World J Urol 2016;34:1383-8. [Crossref] [PubMed]
- Leake JL, Hardman R, Ojili V, et al. Prostate MRI: access to and current practice of prostate MRI in the United States. J Am Coll Radiol 2014;11:156-60. [Crossref] [PubMed]
- Magnetic resonance imaging (MRI) units. Available online: https://data.oecd.org/healtheqt/magnetic-resonance-imaging-mri-units.html
- Barrett T, Turkbey B, Choyke PL. PI-RADS version 2: what you need to know. Clin Radiol 2015;70:1165-76. [Crossref] [PubMed]
- Kim JY, Kim SH, Kim YH, et al. Low-risk prostate cancer: the accuracy of multiparametric MR imaging for detection. Radiology 2014;271:435-44. [Crossref] [PubMed]
- Falzarano SM, Ferro M, Bollito E, et al. Novel biomarkers and genomic tests in prostate cancer: a critical analysis. Minerva Urol Nefrol 2015;67:211-31. [PubMed]
- Tomlins SA, Day JR, Lonigro RJ, et al. Urine TMPRSS2:ERG Plus PCA3 for Individualized Prostate Cancer Risk Assessment. Eur Urol 2016;70:45-53. [Crossref] [PubMed]
- PCA3 Test Cost Page. Next D 2016. Available online: http://www.pca3.ca/en/pca3cost.php
- Postema A, Mischi M, de la Rosette J, et al. Multiparametric ultrasound in the detection of prostate cancer: a systematic review. World J Urol 2015;33:1651-9. [Crossref] [PubMed]
- Pokorny MR, de Rooij M, Duncan E, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol 2014;66:22-9. [Crossref] [PubMed]
- Puech P, Ouzzane A, Gaillard V, et al. Multiparametric MRI-targeted TRUS prostate biopsies using visual registration. Biomed Res Int 2014;2014:819360. [Crossref] [PubMed]
- Cool DW, Zhang X, Romagnoli C, et al. Evaluation of MRI-TRUS fusion versus cognitive registration accuracy for MRI-targeted, TRUS-guided prostate biopsy. AJR Am J Roentgenol 2015;204:83-91. [Crossref] [PubMed]
- Wysock JS, Rosenkrantz AB, Huang WC, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol 2014;66:343-51. [Crossref] [PubMed]
- Puech P, Rouvière O, Renard-Penna R, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy--prospective multicenter study. Radiology 2013;268:461-9. [Crossref] [PubMed]
- Tonttila PP, Lantto J, Pääkkö E, et al. Prebiopsy Multiparametric Magnetic Resonance Imaging for Prostate Cancer Diagnosis in Biopsy-naive Men with Suspected Prostate Cancer Based on Elevated Prostate-specific Antigen Values: Results from a Randomized Prospective Blinded Controlled Trial. Eur Urol 2016;69:419-25. [Crossref] [PubMed]
- Acar Ö, Esen T, Çolakoğlu B, et al. Multiparametric MRI guidance in first-time prostate biopsies: what is the real benefit? Diagn Interv Radiol 2015;21:271-6. [Crossref] [PubMed]
- de Gorski A, Rouprêt M, Peyronnet B, et al. Accuracy of Magnetic Resonance Imaging/Ultrasound Fusion Targeted Biopsies to Diagnose Clinically Significant Prostate Cancer in Enlarged Compared to Smaller Prostates. J Urol 2015;194:669-73. [Crossref] [PubMed]
- Radtke JP, Schwab C, Wolf MB, et al. Multiparametric Magnetic Resonance Imaging (MRI) and MRI-Transrectal Ultrasound Fusion Biopsy for Index Tumor Detection: Correlation with Radical Prostatectomy Specimen. Eur Urol 2016;70:846-53. [Crossref] [PubMed]
- Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 2013;64:713-9. [Crossref] [PubMed]
- Gayet M, van der Aa A, Beerlage HP, et al. The value of magnetic resonance imaging and ultrasonography (MRI/US)-fusion biopsy platforms in prostate cancer detection: a systematic review. BJU Int 2016;117:392-400. [Crossref] [PubMed]
- Boegemann M, Stephan C, Cammann H, et al. The percentage of prostate-specific antigen (PSA) isoform [-2]proPSA and the Prostate Health Index improve the diagnostic accuracy for clinically relevant prostate cancer at initial and repeat biopsy compared with total PSA and percentage free PSA in men aged ≤65 years. BJU Int 2016;117:72-9. [Crossref] [PubMed]
- Radtke JP, Boxler S, Kuru TH, et al. Improved detection of anterior fibromuscular stroma and transition zone prostate cancer using biparametric and multiparametric MRI with MRI-targeted biopsy and MRI-US fusion guidance. Prostate Cancer Prostatic Dis 2015;18:288-96. [Crossref] [PubMed]
- Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol 2010;28:126-31. [Crossref] [PubMed]
- Itatani R, Namimoto T, Atsuji S, et al. Negative predictive value of multiparametric MRI for prostate cancer detection: outcome of 5-year follow-up in men with negative findings on initial MRI studies. Eur J Radiol 2014;83:1740-5. [Crossref] [PubMed]
- Taylor AK, Zembower TR, Nadler RB, et al. Targeted antimicrobial prophylaxis using rectal swab cultures in men undergoing transrectal ultrasound guided prostate biopsy is associated with reduced incidence of postoperative infectious complications and cost of care. J Urol 2012;187:1275-9. [Crossref] [PubMed]
- Carignan A, Roussy JF, Lapointe V, et al. Increasing risk of infectious complications after transrectal ultrasound-guided prostate biopsies: time to reassess antimicrobial prophylaxis? Eur Urol 2012;62:453-9. [Crossref] [PubMed]
- Liss MA, Billimek J, Osann K, et al. Consideration of comorbidity in risk stratification prior to prostate biopsy. Cancer 2013;119:2413-8. [Crossref] [PubMed]
- Koerber F, Waidelich R, Stollenwerk B, et al. The cost-utility of open prostatectomy compared with active surveillance in early localised prostate cancer. BMC Health Serv Res 2014;14:163. [Crossref] [PubMed]
- Eldefrawy A, Katkoori D, Abramowitz M, et al. Active surveillance vs. treatment for low-risk prostate cancer: a cost comparison. Urol Oncol 2013;31:576-80. [Crossref] [PubMed]
- Dall'Era MA. The economics of active surveillance for prostate cancer. Curr Opin Urol 2013;23:278-82. [PubMed]
- Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol 2012;61:177-84. [Crossref] [PubMed]
- Turkbey B, Merino MJ, Gallardo EC, et al. Comparison of endorectal coil and nonendorectal coil T2W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: correlation with whole-mount histopathology. J Magn Reson Imaging 2014;39:1443-8. [Crossref] [PubMed]
- Muller BG, Kaushal A, Sankineni S, et al. Multiparametric magnetic resonance imaging-transrectal ultrasound fusion-assisted biopsy for the diagnosis of local recurrence after radical prostatectomy. Urol Oncol 2015;33:425.e1-6. [Crossref] [PubMed]
- Nam RK, Wallis CJ, Stojcic-Bendavid J, et al. A Pilot Study to Evaluate the Role of Magnetic Resonance Imaging for Prostate Cancer Screening in the General Population. J Urol 2016;196:361-6. [Crossref] [PubMed]


