Direct magnetic resonance imaging-guided biopsy of the prostate: lessons learned in establishing a regional referral center
Introduction
Prostate cancer represents a major healthcare burden in the United States. Overall, the lifetime risk of prostate cancer diagnosis for an American man is about 1 in 6, and about 12% of those diagnosed will die of the disease (1). The current standard of care for prostate cancer diagnosis is systematic transrectal ultrasound guided biopsy, during which a urologist uses an ultrasound probe placed in the rectum to localize the prostate (usually not the tumor) and obtains 12 or more needle core biopsies from standard locations in the gland. Approximately one million men undergo prostate biopsy every year in the United States (2), typically after an abnormal serum prostatic specific antigen (PSA) level or digital rectal examination, and about 20% of these men have a positive result. Prostate cancer is the only solid organ malignancy that is diagnosed by such random systematic biopsies. By way of contrast, it is inconceivable that breast cancer would be diagnosed by placing twelve needles at standard locations in the breast, yet this has been the longstanding “state-of-the-art” for prostate cancer. This standard approach is associated with disturbingly high rates of false negative diagnosis (missed cancer, 15−46%), overdiagnosis (detection of indolent Gleason 6 cancer of questionable clinical significance, 45%) and underdiagnosis (undergrading of the cancer when compared to the final surgical pathology, 38%) (3-5). Over the last several decades, multiparametric MRI of the prostate has steadily evolved and currently, in about 60% of patients with suspected prostate cancer, can accurately demonstrate likely sites of disease for targeted biopsy (6). MRI targeted biopsy has been shown to reduce the rates of false negative diagnosis, overdiagnosis, and underdiagnosis (5,7-10). Improved tumor characterization and treatment stratification resulting from targeted biopsy can offset the initial costs associated with MRI over a 10-year interval (11).
The three methods of transrectal MRI-targeted biopsy are direct, fusion, and cognitive. During direct or “in bore” biopsy, the patient is in the MRI scanner and the needle is placed in the target under MRI visualization. Generally, only the target is sampled. During transrectal ultrasound/MRI fusion biopsy, the patient undergoes a standard transrectal ultrasound guided biopsy, but MRI targets from a preceding MRI scan are digitally “fused” to the ultrasound images so that additional cores can also be taken from those locations under ultrasound visualization. Generally, cores are obtained from the target and standard systematic locations. In cognitive MRI-targeted biopsy, the patient undergoes a standard transrectal ultrasound guided biopsy, but in addition the operator biopsies the MRI-target based on visual anatomic co-registration. As might be expected, several studies (12-14), including a prospective comparative trial, have confirmed the inferiority of the cognitive method, which is only mentioned here for completeness. In 2013, we established the first regional referral center for direct MRI-guided prostate biopsy in the Pacific Northwest. Within 2 years we completed the procedure on our fiftieth patient. We undertook this review to describe how we use multiparametric MRI to select patients for direct MRI-guided biopsy, to explain how we perform direct MRI-guided biopsy, to present the results of the first 50 patients to undergo this procedure, and to discuss these results in the wider context of MRI-targeted biopsy.
Patient selection
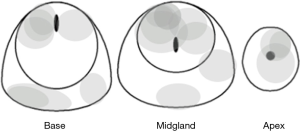
Our preference is to perform a standard diagnostic endorectal coil multiparametric 3T MRI of the prostate, using a previously described technique (15). In brief, we obtain T1-weighted axial images of the whole pelvis and small field of view, high spatial resolution axial, sagittal and coronal T2-weighted images of the prostate. We also obtain axial diffusion-weighted and dynamic contrast enhanced images of the prostate, and these are post-processed to generate apparent diffusion coefficient and colorized perfusion parametric maps. Images are reviewed by one of two attending radiologists (FC and BF), with 20 and 5 years of experience in multiparametric MRI of the prostate, respectively, who identify the presence or absence of biopsy targets. Using an amalgam of published experience, biopsy targets are defined as foci of low T2 signal intensity with an ellipsoid or crescentic subcapsular morphology in the peripheral zone or an infiltrative and non-encapsulated appearance in the central gland accompanied by focal reduction in apparent diffusion coefficient and/or focal early intense enhancement or rapid washout on perfusion imaging (5,16-18). This definition essentially conforms to lesions with scores of 4 or 5 on Pi-RADS version 2 (19). We do not have a strict minimum size, but generally are reluctant to biopsy lesions under 5 mm, given the technical challenges of directing a needle to such very small targets, concern for potentially misleading false negative results, and the questionable clinical significance of such small volume foci. That said, lesions of borderline suitability based on size or Pi-RADS score may be biopsied based on patient or physician preference.
MRI-guided biopsy technique
All biopsies are performed by one of the two attending radiologists who review the pre-biopsy MRI studies for target identification. Biopsies are performed on a 1.5T whole body MRI scanner (Ingenia; Philips Healthcare, Netherlands) with the patient prone and utilizing a commercially available prostate biopsy system (DynaTRIM, Invivo, Gainesville, FL, USA) in conjunction with a related software package for device tracking and target localization (DynaCAD, Invivo, Gainesville, FL, USA). In accordance with the American Urological Association guidelines for antimicrobial prophylaxis, patients receive oral ciprofoxacin 500 mg twice daily for 3 days, starting the morning before the biopsy (20). A qualified radiology nurse provides moderate, conscious sedation during the procedure, using intravenous fentanyl and midazolam, with dosage titrated to effect. We typically administer 50 mcg fentanyl and 0.5 mg midazolam intravenously at the start of the procedure, as the patient is set up on the scanner table, followed by a second round of 100 mcg fentanyl and 1 mg of midazolam immediately prior to the first biopsy. This dosing scheme generally results in satisfactory anxiolysis and analgesia.
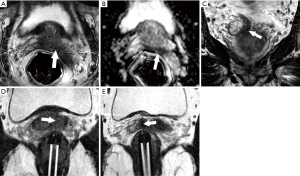
The table-top of the scanner contains an integrated surface coil. A flat plastic holding plate that is part of the biopsy system is then placed on the table top. The patient then lies prone on the table, and is made comfortable with additional pillows or blankets as needed. The needle introducer of the biopsy system, a fingerlike, rigid, hollow-centered device with an outer liquid-filled sleeve that functions both as a guide and as a fiducial marker, is lubricated with topical anesthetic gel and inserted in the rectum. The clamp-stand is then attached to the holding plate under the patient’s legs. The introducer locks into a holder on the clamp-stand, establishing rigid co-registration between the introducer, the patient, and the scanner (Figure 1). Finally, an additional surface coil is placed posteriorly on the patient. The patient is advanced into the scanner and high resolution sagittal and axial T2-weighted images are obtained. The location of the needle sleeve is marked and calibrated to the system and the center of the target lesion(s) is identified. These positional data allows the software to calculate three-dimensional manual adjustments for marked cogs on the device that aim the needle introducer to the target. The system also indicates the correct choice of needle length (the dedicated needles that are part of the system are either 15 or 17.5 cm in length) and whether or not a 1 cm spacer preloaded on the needle introducer should be used. Oblique T2 images are then obtained in a “down the barrel” plane to confirm that the needle introducer is pointing directly at the target. If necessary, minor adjustments and additional confirmation images are obtained. The patient is then removed from the scanner and two or more biopsy cores are obtained from each target using an automatic titanium 18 g MRI-compatible biopsy needle (InVivo, Gainesville, FL, USA). Usually, minor changes of introducer position are made between cores to “step around” the target and optimize sampling.
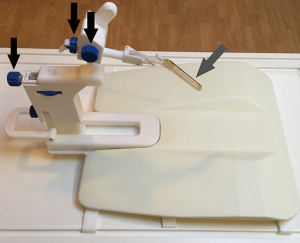
Our method for checking the accuracy of biopsy needle positioning has evolved over time. Initially (n=4), we simply assumed that needle deployment was accurate if the pre-deployment alignment was satisfactory and the post-biopsy images showed no movement of the introducer. Later (n=16), we used the visibility of the biopsy track (linear tissue distortion on post-biopsy T2 images obtained in the plane of the needle deployment) to confirm accurate targeting (21). However, neither of these methods appeared adequately robust, and since then directly image the deployed needle after at least one of the samples using an oblique T2 image obtained in the plane of the needle trajectory. While such post-deployment imaging might raise theoretical concerns that the required several minutes of needle dwell time may increase the risk of bleeding or infection, we are convinced that it is the best method for confirming that the needle has truly traversed the target. This is also the method used at other high volume centers (10,17). Patients are observed for two hours after the procedure, and then discharged if their vital signs are stable and they have urinated satisfactorily without symptoms of clot retention.
Preliminary results
Population and procedural results
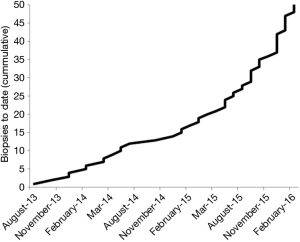
We introduced direct MRI-guided biopsy as a clinical service at our institution in August 2013. To our knowledge, we were the first and remain the only center to offer this service in the Pacific Northwest. Within 19 months of providing the service, we had performed direct MRI-guided prostate biopsy on 50 patients. All procedures were completed successfully, and prostatic tissue was obtained in all patients. With no promotion of the service, beyond informing physicians attending our institutional multidisciplinary genitourinary tumor board and mentioning MRI-guided biopsy of appropriate lesions as an option in our diagnostic prostate MRI reports, the number of referrals grew steadily (Figure 2), suggesting a previously unmet clinical demand. The mean patient age was 66 years (range, 48–78 years). Mean serum PSA level was 12.0 ng/mL (range, 2.8–58.0 ng/mL). In order of frequency, the indications for MRI-guided biopsy were:
- Negative traditional systematic biopsy but with a visible target at multiparametric MRI (30 of 50, 60%);
- Evaluation of patients on active surveillance for previously documented Gleason score 6 cancer and with a visible target at multiparametric MRI (14 of 50, 28%);
- Evaluation of patients with no prior biopsy and a visible target at multiparametric MRI (5 of 50, 10%);
- Evaluation of a patient with a history of prior focal therapy by high intensity focused ultrasound with rising PSA and a visible target concerning for local recurrence at multiparametric MRI (1 of 50, 2%).
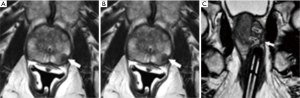
Thirty four of 50 (68%) patients were referred by urologists from our own institution, while the remaining patients were referred by urologists (n=14, 28%) or primary care physicians (n=1, 2%) unaffiliated with our institution. The 50 patients underwent 51 MRI-guided biopsy procedures targeting 60 lesions. Forty patients (80%) had a single target and 10 (20%) had two targets. One patient underwent a second procedure one month after the first direct MRI-guided biopsy because of a suspected false negative result, and the second procedure was positive (Figure 3). The mean procedure time was 49 minutes (range, 25–135 minutes). The mean procedure time was 45 minutes for biopsy sessions with one target and 64 minutes for procedures with two targets (P<0.003). Subgroup analysis showed procedures became shorter with greater operator experience. Specifically, for procedures with one biopsy target, mean procedure time decreased from 49 to 35 minutes over the study period (P<0.0003). There were no immediate or delayed significant post biopsy complications during the study period.
Pathology results
Prostate cancer was detected in 37 of 50 patients (74%), with a Gleason score of 7 or greater in 31 of 37 (84%) of the biopsy positive patients. When considered by indication:
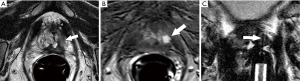
- Prior negative traditional systematic biopsy (n=30): MRI-guided biopsy was positive in 18 (60%) of these patients, with Gleason score 7 or greater in 16. The location and size of tumors missed by prior systematic biopsy and documented by positive direct MRI-guided biopsy are shown in Figure 4. Three of the 12 patients with a negative direct MRI-guided biopsy proceeded to saturation biopsy with two positive results (one for Gleason score 6 cancer and one for Gleason score 7 cancer;
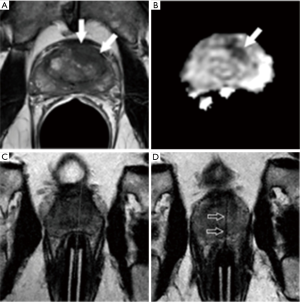
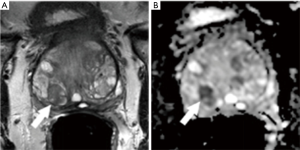
- Prior positive traditional systematic biopsy demonstrating Gleason score 6 cancer and on active surveillance (n=14): MRI-guided biopsy was positive in 13 (93%) of these 14 patients, and positive for an upgraded Gleason score of 7 or above in 10 of 14 (71%). All 10 of the upgraded patients proceeded to definitive therapy by radical prostatectomy (n=9) or radiation therapy (n=1). A representative example is shown in Figure 5;
- Biopsy naïve (n=5): MRI-guided biopsy was positive in all 5 patients (100%), with 4 (80%) having Gleason score 7 or above cancer. The four patients with higher grade disease have since been treated by radical prostatectomy (n=2) or focal therapy by interstitial laser ablation (n=2). The other patient, with Gleason score 6 cancer, has opted for active surveillance. A representative case example is shown in Figure 6;
- Suspected local recurrence after prior focal therapy by high intensity focused ultrasound (n=1): MRI-guided biopsy was positive for Gleason score 7 cancer (Figure 7), and the patient has proceeded to salvage external beam radiotherapy.
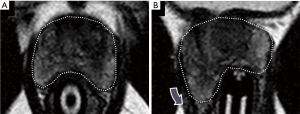
The positive biopsy rate in the study population rose over time, from 68% (17 positive) for the first 25 patients to 84% (21 positive) for the second 25 patients. While this might reflect increasing operator experience and better technique, we suspect the improvement is at least partially due to improved reporting of diagnostic MRI studies resulting in enhanced patient selection. Some of our early targets that yielded negative biopsies were likely nodules of stromal benign prostatic hyperplasia in the central gland that were misinterpreted as concerning for malignancy (Figure 8). While such targets may show reduced diffusion and brisk early enhancement, close attention to the T2 morphology is crucial—nodules of benign prostatic hyperplasia are typically heterogeneous and encapsulated. True central gland cancers are non-encapsulated and have a characteristic homogenous “erased charcoal” appearance (20).
Current status of direct MRI-guided biopsy
The most common indication for direct MRI-guided biopsy in our population was suspected prostate cancer, generally because of an elevated or rising PSA, with one or more prior negative traditional systematic biopsy. Direct MRI-guided biopsy was positive in 60% of this cohort, and most had Gleason score 7 or greater disease. Our positive biopsy rate in this population was slightly higher than published results in the same or similar cohorts, which range from 41% to 52% (10,22). The second commonest indication in our population was patients on active surveillance for previously documented Gleason 6 disease, with 10 of 14 patients (71%) being upgraded to Gleason score 7 or higher cancer. This also compared favorably to the literature, with a published study reporting a tumor upgrading rate of 43% (16 of 37) at targeted biopsy in patients on active surveillance and with high value targets (Pi-RADS 4 or 5) at diagnostic MRI (23). The third commonest indication in our population was biopsy naïve patients, with all 5 such patients (100%) having a positive direct MRI-guided biopsy and with 4 of 5 (80%) having Gleason score 7 or higher cancer. While the number of patients in this group was small, our results are comparable to a published targeted biopsy positive rate of 86% (128 of 149) for a group of biopsy naïve patients with high value targets (scores of 4 or 5), with 80% (103 of 128) having Gleason score 7 or above (9).
To place our results in context, Table 1 compares the positive biopsy rates reported for fusion versus direct MRI-guided biopsy across different published studies (5,17,24-27). Despite the differences between these studies with respect to populations, target definition, and biopsy techniques, it is striking that the higher positive biopsy rates are all from studies utilizing direct MRI guided biopsy, with the highest rate of 84% for direct MRI-guided biopsy as compared to 53% for fusion biopsy. This suggests direct MRI-guided biopsy may be superior to fusion biopsy, particularly in patients with smaller tumors in difficult locations where registration error during fusion biopsy could cause false negative results. These early clinical concerns regarding fusion biopsy are aligned with legitimate conceptual and practical reasons to question the current market rush to adopt fusion biopsy as the preferred approach to MRI-targeted biopsy, which seems to be primarily based on ease of implementation. Conceptually, image-guided biopsy of a target should be performed using the modality that best demonstrates the target, that is: “The absolute requirement for the choice of imaging guidance is that the lesion be visible via the modality chosen” (28). Specifically, the key requirement of fusion biopsy is that the baseline co-registration of the MRI dataset to the transrectal ultrasound images remains fixed during the procedure and is unaffected by gland deformation due to the movement of the ultrasound probe. Current methods of hybrid guidance violate this basic principle. In fact, gland distortion from probe motion is frequently observed during direct MRI-guided biopsy (Figure 9) and may account for an average registration error of approximately 4–5 mm during standard fusion biopsy (29,30). While computer guided techniques including real time elastic fusion and motion compensation are being developed, these are not in widespread use and it is likely residual registration errors are common in practice (31,32). Given that the primary difference between Pi-RADS 4 and 5 lesions is a size threshold of 1.5 cm, it is notable that the cancer detection rate for fusion biopsy is generally significantly lower for Pi-RADS 4 than 5, for example, 62% (94/152) for Pi-RADS 4 lesions versus 89% (99/111) for Pi-RADS 5 lesions in one study (33). One possibility is that registration error is more likely to yield a false negative result for smaller targets (34). In regards to cost containment, given that MRI guided biopsies require a larger initial investment with respect to resource utilization, improved tumor characterization and treatment stratification resulting from targeted biopsy has been shown to offset the initial costs associated with an MRI targeted biopsy approach over a 10-year horizon (11).

Full table
Conclusions
Our initial experience with establishing a regional direct MRI-guided biopsy service and performing our first 50 biopsies shows, not only high cancer detection rates and high performance metrics when compared to the published literature, but also important treatment pathway alterations among the three major referral indications (suspected prostate cancer with negative prior biopsy, active surveillance for Gleason 6, and biopsy naive). While direct MRI-guided biopsy is currently a niche procedure applied to the diagnostic algorithm in a small subset of men with prostate cancer, the parallel, and more rapid growth of transrectal ultrasound-fusion biopsy as well as growing recognition of a targeted biopsy strategy may play an increasingly important role in the future in a wider population of men. However, the most accurate and cost effective biopsy strategy has yet to be determined and requires future head-to-head prospective trials.
Acknowledgements
Funding: Dr. Chenara Johnson and Dr. Benjamin Addicott supported by NIBIB grant 1R25 EB016671.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Cancer Society. Cancer Facts and Figures 2016. Atlanta: American Cancer So-ciety. 2016:4. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figure-2016.html
- Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol 2011;186:1830-4. [Crossref] [PubMed]
- Dominguez-Escrig JL, McCracken SR, Greene D. Beyond diagnosis: evolving prostate biopsy in the era of focal therapy. Prostate Cancer 2011;2011:386207. [Crossref] [PubMed]
- Kvåle R, Møller B, Wahlqvist R, et al. Concordance between Gleason scores of needle biopsies and radical prostatectomy specimens: a population-based study. BJU Int 2009;103:1647-54. [Crossref] [PubMed]
- Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 2013;64:713-9. [Crossref] [PubMed]
- van de Ven WJ, Barentsz JO. Prostate cancer: MRI/US-guided biopsy--a viable alternative to TRUS-guidance. Nat Rev Urol 2013;10:559-60. [Crossref] [PubMed]
- Klein EA. Prostate cancer: MR-TRUS fusion biopsy--defining a new standard. Nat Rev Clin Oncol 2015;12:253-4. [Crossref] [PubMed]
- Nassiri N, Natarajan S, Margolis DJ, et al. Targeted Prostate Biopsy: Lessons Learned Midst the Evolution of a Disruptive Technology. Urology 2015;86:432-8. [Crossref] [PubMed]
- Mendhiratta N, Rosenkrantz AB, Meng X, et al. Magnetic Resonance Imaging-Ultrasound Fusion Targeted Prostate Biopsy in a Consecutive Cohort of Men with No Previous Biopsy: Reduction of Over Detection through Improved Risk Stratification. J Urol 2015;194:1601-6. [Crossref] [PubMed]
- Hoeks CM, Schouten MG, Bomers JG, et al. Three-Tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol 2012;62:902-9. [Crossref] [PubMed]
- de Rooij M, Crienen S, Witjes JA, et al. Cost-effectiveness of magnetic resonance (MR) imaging and MR-guided targeted biopsy versus systematic transrectal ultrasound-guided biopsy in diagnosing prostate cancer: a modelling study from a health care perspective. Eur Urol 2014;66:430-6. [Crossref] [PubMed]
- Wysock JS, Rosenkrantz AB, Huang WC, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol 2014;66:343-51. [Crossref] [PubMed]
- Valerio M, McCartan N, Freeman A, et al. Visually directed vs. software-based targeted biopsy compared to transperineal template mapping biopsy in the detection of clinically significant prostate cancer. Urol Oncol 2015;33:424.e9-16. [Crossref] [PubMed]
- Cool DW, Zhang X, Romagnoli C, et al. Evaluation of MRI-TRUS fusion versus cognitive registration accuracy for MRI-targeted, TRUS-guided prostate biopsy. AJR Am J Roentgenol 2015;204:83-91. [Crossref] [PubMed]
- Romero G, Foster BR, Pettersson DR, et al. Endorectal multiparametric MRI of the prostate: incremental effect of perfusion imaging on biopsy target identification. Clin Imaging 2016;40:553-7. [Crossref] [PubMed]
- Jung AJ, Westphalen AC, Kurhanewicz J, et al. Clinical utility of endorectal MRI-guided prostate biopsy: preliminary experience. J Magn Reson Imaging 2014;40:314-23. [Crossref] [PubMed]
- Pokorny MR, de Rooij M, Duncan E, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol 2014;66:22-9. [Crossref] [PubMed]
- Sonn GA, Margolis DJ, Marks LS. Target detection: magnetic resonance imaging-ultrasound fusion-guided prostate biopsy. Urol Oncol 2014;32:903-11. [Crossref] [PubMed]
- Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 2016;69:16-40. [Crossref] [PubMed]
- 20.2012 AUA/SUNA white paper on the incidence, prevention and treatment of complications related to prostate needle biopsy. Avaliable online: http://www.auanet.org/common/pdf/education/clinical-guidance/AUA-SUNA-PNB-White-Paper.pdf
- Nicholson AJ, Pettersson DR, Korngold EK, et al. Direct MRI-guided biopsy of the prostate: use of post-biopsy needle track imaging to confirm targeting. Abdom Imaging 2015;40:2517-22. [Crossref] [PubMed]
- Roethke M, Anastasiadis AG, Lichy M, et al. MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol 2012;30:213-8. [Crossref] [PubMed]
- Da Rosa MR, Milot L, Sugar L, et al. A prospective comparison of MRI-US fused targeted biopsy versus systematic ultrasound-guided biopsy for detecting clinically significant prostate cancer in patients on active surveillance. J Magn Reson Imaging 2015;41:220-5. [Crossref] [PubMed]
- Garmer M, Busch M, Mateiescu S, et al. Accuracy of MRI-Targeted in-Bore Prostate Biopsy According to the Gleason Score with Postprostatectomy Histopathologic Control--a Targeted Biopsy-Only Strategy with Limited Number of Cores. Acad Radiol 2015;22:1409-18. [Crossref] [PubMed]
- Arsov C, Rabenalt R, Blondin D, et al. Prospective randomized trial comparing magnetic resonance imaging (MRI)-guided in-bore biopsy to MRI-ultrasound fusion and transrectal ultrasound-guided prostate biopsy in patients with prior negative biopsies. Eur Urol 2015;68:713-20. [Crossref] [PubMed]
- Schouten MG, Hoeks CM, Bomers JG, et al. Location of Prostate Cancers Determined by Multiparametric and MRI-Guided Biopsy in Patients With Elevated Prostate-Specific Antigen Level and at Least One Negative Transrectal Ultrasound-Guided Biopsy. AJR Am J Roentgenol 2015;205:57-63. [Crossref] [PubMed]
- Filson CP, Natarajan S, Margolis DJ, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer 2016;122:884-92. [Crossref] [PubMed]
- Gervais DA. Percutaneous image-guided biopsies: special approaches. In: Ray CE, Hicks ME, Patel NH. editors. Interventions in Oncology. Philadelphia: Lippincott Williams and Wilkins, 2004:65.
- Martin PR, Cool DW, Romagnoli C, et al. Magnetic resonance imaging-targeted, 3D transrectal ultrasound-guided fusion biopsy for prostate cancer: Quantifying the impact of needle delivery error on diagnosis. Med Phys 2014;41:073504. [Crossref] [PubMed]
- Sparks R, Bloch BN, Feleppa E, et al. Multiattribute probabilistic prostate elastic registration (MAPPER): application to fusion of ultrasound and magnetic resonance imaging. Med Phys 2015;42:1153-63. [Crossref] [PubMed]
- Ukimura O, Desai MM, Palmer S, et al. 3-Dimensional elastic registration system of prostate biopsy location by real-time 3-dimensional transrectal ultrasound guidance with magnetic resonance/transrectal ultrasound image fusion. The Journal of Urology 2012;187:1080-6. [Crossref] [PubMed]
- De Silva T, Fenster A, Cool DW, et al. 2D-3D rigid registration to compensate for prostate motion during 3D TRUS-guided biopsy. Med Phys 2013;40:022904. [Crossref] [PubMed]
- Cash H, Maxeiner A, Stephan C, et al. The detection of significant prostate cancer is correlated with the Prostate Imaging Reporting and Data System (PI-RADS) in MRI/transrectal ultrasound fusion biopsy. World J Urol 2016;34:525-32. [Crossref] [PubMed]
- Cash H, Günzel K, Maxeiner A, et al. Prostate cancer detection on transrectal ultrasonography-guided random biopsy despite negative real-time magnetic resonance imaging/ultrasonography fusion-guided targeted biopsy: reasons for targeted biopsy failure. BJU Int 2016;118:35-43. [Crossref] [PubMed]


