
Efficacy of microsurgical denervation of the spermatic cord in patients with chronic scrotal pain following unsuccessful varicocelectomy
Highlight box
Key findings
• Microsurgical denervation of the spermatic cord (MDSC) significantly reduced chronic scrotal pain (CSP) in 84.4% of patients who remained symptomatic after varicocelectomy, with over half achieving near-complete pain relief [Numeric Rating Scale (NRS) ≤1].
• MDSC demonstrated minimal complications, supporting its favorable safety profile.
• Misdiagnosis of varicocele as the primary cause of diffuse scrotal pain could lead patients to undergo unnecessary procedures.
What is known and what is new?
• Varicocelectomy is commonly performed for CSP, yet a substantial proportion of patients experience persistent or worsening pain. Response to spermatic cord block (SCB), generally assessed using longer-acting anesthetics, typically guides patient selection.
• When longer-acting anesthetics are unavailable, short-acting lidocaine may be considered as an alternative, providing reasonable predictive value for surgical outcomes. A subinguinal surgical approach distal to prior varicocelectomy incisions further enhanced outcomes by minimizing neuroma formation and nerve entrapment.
What is the implication, and what should change now?
• Precise diagnosis is crucial, as diffuse scrotal pain (involving the testicle, epididymis, and spermatic cord) rarely stems solely from varicocele. Comprehensive preoperative evaluation including targeted pain mapping, adjunctive imaging, and clinical algorithms is essential to accurately identify the primary pain source.
• When diagnostic uncertainty exists, MDSC may serve as a suitable first-line surgical option. Early consideration of MDSC could reduce reliance on chronic medication and prevent more invasive procedures such as orchiectomy.
Introduction
Chronic scrotal pain (CSP) is a prevalent urological condition that significantly impacts men’s quality of life by disrupting daily activities and causing considerable discomfort. Defined as intermittent or continuous pain in the scrotal region lasting more than three months, CSP can be unilateral or bilateral. This definition aligns with the general concept of chronic pain and was initially proposed by Davis et al. in 1990 during a retrospective study involving patients with prolonged scrotal discomfort (1).
Despite its clinical significance, the exact prevalence of CSP remains unclear due to limited research. Ciftci et al. reported that CSP accounts for approximately 4.75% of annual urology specialist visits by men (2). In contrast, a Swiss study indicated a lower incidence, estimating 350 to 400 cases per 100,000 men aged 25 to 85 years annually (3). These variations highlight the need for more comprehensive epidemiological studies to understand the true burden of CSP.
The etiology of CSP is often multifactorial and can be challenging to identify. Even with advanced diagnostic tools, at least 25% of CSP cases lack an identifiable cause, complicating treatment efforts (1). Management typically begins with conservative approaches, including medical therapy with nonsteroidal anti-inflammatory drugs (NSAIDs), antidepressants, anticonvulsants, psychotherapy, and physical therapy. Surgical intervention is considered only when these methods fail to provide relief.
Varicocele, characterized by dilated veins within the scrotum, is a common cause of scrotal pain and is prevalent among men of reproductive age. Varicocelectomy, the surgical correction of varicocele, is frequently employed to alleviate CSP, improve sperm count and quality, and enhance fertility. While varicocelectomy can significantly reduce pain and improve quality of life for many patients, not all experience relief; some continue to suffer from CSP or may even report worsening pain postoperatively. In clinical practice, diffuse scrotal pain (involving the testicle, epididymis, and spermatic cord) is less likely to stem solely from varicocele. In such cases, careful preoperative evaluation including pain mapping and adjunctive imaging should confirm that varicocele is indeed the primary cause. When doubt exists, microsurgical denervation of the spermatic cord (MDSC) may be a more suitable first-line surgical approach.
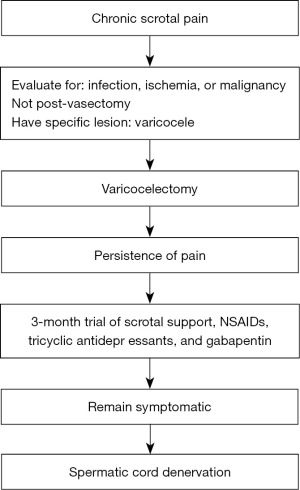
MDSC has emerged as a promising surgical option for patients with CSP unresponsive to prior treatments, including varicocelectomy. As illustrated in Figure 1, patients are first evaluated for infection, ischemia, or malignancy. If varicocele is confirmed, varicocelectomy is performed; in cases of persistent scrotal pain thereafter, MDSC becomes a potential next step in management. MDSC aims to relieve pain by interrupting the sensory nerves within the spermatic cord. At the Urology Department of Binh Dan Hospital, we have observed patients whose scrotal pain persisted despite comprehensive treatment of underlying causes and previous varicocelectomy. Recognizing the need for effective alternatives, we conducted this study to evaluate the effectiveness and safety of MDSC in patients with CSP that persists following varicocelectomy. Our goal is to improve patient outcomes and quality of life by providing evidence for MDSC as a viable treatment option for this challenging condition. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-2024-719/rc).
Methods
We conducted a retrospective study of patients with CSP who failed to respond to varicocelectomy and subsequently underwent MDSC between March 2021 and March 2023 at Binh Dan Hospital, Ho Chi Minh City, Vietnam. The study was approved by the medical ethic council of Pham Ngoc Thach University of Medicine (No. 698/TDHYKPNT-HDDD), and all patients provided informed consent for the procedure and the use of their data for research purposes. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Patient selection
Inclusion criteria were male patients aged 18 years or older who experienced persistent scrotal pain for over one year following varicocelectomy. Patients must have shown no significant response to medical treatments including NSAIDs, antidepressants, and anticonvulsants for more than three months. A successful response to a spermatic cord block (SCB), defined as a reduction of more than 50% in pain intensity lasting at least four hours post-procedure, was also required. Exclusion criteria included identifiable causes of scrotal pain amenable to other treatments (such as infections, tumors, or testicular torsion), previous scrotal surgeries other than varicocelectomy, and patients unwilling or unable to consent to MDSC.
Potential sources of bias and data handling
In this retrospective design, we did not identify major confounders requiring statistical adjustment. We did not observe any missing data for key variables; thus, a complete-case analysis was performed. To minimize selection bias, all eligible patients who met the inclusion criteria were included without additional exclusions. Moreover, to reduce information bias, a standardized NRS tool was administered by the same investigator at each follow-up visit. The sample size of 45 patients was determined based on the number of eligible individuals who underwent MDSC at our facility during the study period from March 2021 to March 2023. Due to the retrospective nature of the study, a formal sample size calculation was not performed. We conducted an initial assessment of potential confounders such as age, duration of pain, and pain location. However, none of these factors were significantly associated with the primary outcome, thus negating the need for further statistical adjustment.
Pain assessment
Pain intensity was assessed using the Numeric Rating Scale (NRS), a validated self-reported tool where patients rate their pain on a scale from 0 (no pain) to 10 (worst possible pain). Assessments were conducted during the initial evaluation, after the SCB, and at each postoperative follow-up visit. Patients reported their average pain level over the past week to account for fluctuations in pain intensity.
SCB
All patients underwent an SCB administered by the primary surgeon. Under aseptic conditions, A 5 mL volume of 1% lidocaine without epinephrine was injected at the level of the external inguinal ring. We chose lidocaine because of its rapid onset and favorable cardiovascular safety profile, which our outpatient protocols prioritize. Furthermore, at our institution, longer-acting local anesthetics (such as bupivacaine or ropivacaine) have not yet received formal approval by the medical board for routine use in this specific procedure. The block was considered successful if there was a reduction of more than 50% in the NRS score compared to the pre-block score. Only patients with a successful SCB were considered candidates for MDSC.
MDSC
MDSC was performed under general anesthesia using an operative microscope providing 4–16× magnification. A 3–4 cm subinguinal incision was made to access the spermatic cord, particularly in patients who had previously undergone varicocelectomy via an inguinal approach. This technique places the operative field distal to the old incision, potentially reducing neuroma formation and chronic pain related to prior scarring. Nonetheless, adhesions and fibrosis around the testicular artery may pose challenges, requiring careful dissection to preserve the artery while achieving thorough denervation. The procedure involved meticulous microsurgical dissection to identify and divide all nerve fibers within the spermatic cord.
Specific steps included:
- Nerve identification and division: all identifiable internal and external spermatic nerves were carefully dissected and severed. This included the ilioinguinal nerve, the genital branch of the genitofemoral nerve, and sympathetic fibers accompanying the vas deferens and blood vessels. We distinguished the somatic nerves (ilioinguinal and the genital branch of the genitofemoral nerves), which primarily serve the scrotal skin, from the autonomic nerves (superior, middle, and inferior spermatic nerves) responsible for sensation in the testicular and epididymal regions. The critical anatomical structures encountered during MDSC are illustrated in Figure 2. All nerve fibers carrying noxious stimuli were carefully isolated and divided. We have also adopted the detailed anatomical delineation described by Oka et al. [2016] to guide our dissection (4).
- Cremaster muscle management: all fibers of the cremaster muscle were divided and excised to eliminate the cremasteric nerves contained within the muscle fibers.
- Vas deferens handling: the vas deferens was preserved, and its perivasal sheath and adventitia were carefully stripped to remove any accompanying nerve fibers, ensuring the vasal blood supply remained intact.
- Vascular structures: the testicular artery was meticulously preserved to maintain testicular perfusion. Likewise, both the deferential and cremasteric arteries were preserved whenever present and identifiable, ensuring comprehensive vascular supply to the testis. The testicular artery recognizing that there may be one or multiple branches was meticulously preserved to maintain testicular perfusion. Identification of the artery was based on its typical anatomical location, its tubular morphology, and the palpable pulsation observed along its course. In cases where the arterial branches were difficult to distinguish, papaverine hydrochloride was applied to facilitate vasodilation, and Micro-Doppler ultrasound was utilized to confirm vascular flow and accurately delineate the vessel. Likewise, both the deferential and cremasteric arteries were preserved whenever present and identifiable, ensuring comprehensive vascular supply to the testis. All veins within the spermatic cord, including internal spermatic veins, cremasteric veins, and deferential veins, were ligated and divided regardless of whether they were varicose, aiming to reduce potential sources of nerve fibers accompanying the veins.
- Lymphatic preservation: lymphatic channels were identified and preserved to prevent postoperative hydrocele formation.

Hemostasis was maintained throughout the procedure. After completing the denervation, the spermatic cord was returned to its anatomical position, and the incision was closed in layers using absorbable sutures. A sterile dressing was applied, and patients were advised to limit physical activity for at least two weeks postoperatively. Most patients were discharged the following day after ensuring stable vital signs and adequate pain control.
Postoperative follow-up
Patients were scheduled for follow-up visits at one week, one month, three months, six months, and then every three months, with an average follow-up period of 14 months. During each visit, pain intensity was reassessed using the NRS. Physical examinations and scrotal ultrasonography were performed to monitor for any complications such as hematoma, infection, hydrocele formation, or testicular atrophy. Surgical success was defined as a reduction of more than 50% in the NRS score compared to the preoperative score. Patient satisfaction was evaluated at the final follow-up visit using a simple satisfaction questionnaire categorizing responses as satisfied, neutral, or dissatisfied.
Statistical analysis
Data were collected and managed using Microsoft Excel. Statistical analyses were performed using IBM SPSS Statistics version 26.0 (IBM Corp, Armonk, NY, USA). Continuous variables were presented as means with standard deviations, while categorical variables were presented as frequencies and percentages. Pain score reductions were calculated by subtracting postoperative NRS scores from preoperative scores. Statistical significance was determined using appropriate tests, with a P value of less than 0.05 considered significant. We conducted a predefined subgroup analysis based on the primary location of pain (testicle, epididymis, spermatic cord, or their combinations). No additional interaction or sensitivity analyses were performed due to the relatively small sample size.
Results
From March 2021 to March 2023, 45 patients with a mean age of 29.4±6.0 years underwent MDSC surgery for CSP. The average follow-up was 14.0±3.5 months. The pre-surgery pain durations averaged 26.1±6.9 months. Pain was left-sided in 28 patients (62.2%) and right-sided in 17 patients (37.8%). All 45 patients who met the inclusion criteria completed the study without dropouts or exclusions; therefore, a flow diagram was deemed unnecessary. Continuous pain was reported in 31 patients (68.9%), while 14 patients (31.1%) experienced intermittent pain. Eleven patients (24.4%) reported increased pain during ejaculation. All patients had a mean pain reduction of 77.4%±9.6% following an SCB. The demographic data, duration of pain, and pre-/postoperative scores are presented in Table 1.
Table 1
| Variables | Mean and standard deviation |
|---|---|
| Duration of pain prior to surgery, months | 26.1±6.9 |
| NRS score | |
| Preoperative pain | 7.5±0.9 |
| After spermatic cord block | 1.7±0.8 |
| Postoperative pain | 1.8±1.7 |
Data are presented as mean ± standard deviation. NRS, Numeric Rating Scale (a pain screening tool).
In the post-surgery period, 38 patients (84.4%) experienced a reduction in scrotal pain by more than 50%. Due to the limited sample size, 95% confidence intervals (CIs) were not calculated for these proportions. Seven patients (15.6%) achieved complete pain relief, while 31 patients (68.9%) continued to report scrotal pain, albeit with significant improvement compared to pre-surgery levels. Nevertheless, we recognize that many patients aim for a ‘near-pain-free’ state (NRS ≤1) rather than merely a ≥50% reduction in pain. Therefore, in addition to evaluating the ≥50% criterion, we analyzed the subgroup that achieved NRS ≤1 postoperatively to underscore the clinical relevance of near-complete relief. Notably, 24 out of 45 patients (53.3%) in our cohort reached this threshold. This proportion is broadly consistent with prior MDSC series reporting 50% or more patients achieving near or total resolution of pain (5-9). A minority, 15.5%, reported no change in their pain levels. Patient satisfaction with the surgical outcome was high, with 33 patients (73.3%) expressing satisfaction with the results.
Among common pain locations, 14 patients (31.1%) experienced pain in the testicle, making it the most frequent site. The distribution of pain locations and the corresponding total reduction in NRS are detailed in Table 2. Pain in two different locations simultaneously was less common, ranging from 6.7% to 11.1%. Regarding overall pain reduction, the most significant improvement was observed in patients with pain in the testicle, epididymis, and spermatic cord, with a decrease of 89.6±6.0%. Patients with pain solely in the testicle also experienced significant relief, with a reduction of 83.8%±11.8%. Conversely, the lowest pain reduction was noted in patients with combined testicle and spermatic cord pain, with a decrease of 36.9%±10.3%.
Table 2
| Location of pain | Number of patients (%) | Total reduction in pain score, % |
|---|---|---|
| Testicle | 14 (31.1) | 83.8±11.8 |
| Epididymis | 6 (13.3) | 68.6±28.6 |
| Spermatic cord | 5 (11.1) | 60.2±29.4 |
| Testicle and epididymis | 3 (6.7) | 75.4±6.9 |
| Epididymis and spermatic cord | 5 (11.1) | 79.5±12.6 |
| Testicle and spermatic cord | 3 (6.7) | 36.9±10.3 |
| Testicle, epididymis, spermatic cord | 9 (20.0) | 89.6±6.0 |
Data are presented as mean ± standard deviation if not otherwise specified.
Complications of MDSC included one case of surgical site infection (2.2%), one case of postoperative bleeding at the surgical site (2.2%), and two cases of numbness and burning sensation in the corresponding skin area. We did not detect complications such as hydrocele formation or testicular atrophy when comparing testicular size on ultrasound before and after surgery.
Discussion
CSP significantly impacts patients’ quality of life, particularly those who are unresponsive to multiple therapies employing various methods. In numerous instances, patients may consider orchiectomy solely for pain relief. While these surgical interventions can be effective in managing pain, they carry potential psychological consequences. Orchiectomy and epididymectomy, in particular, may lead to sexual dysfunction and infertility as documented in recent studies of testicular surgery survivors (10). These analyses indicate that testicular removal can significantly affect body image, libido, and overall psychosocial well-being, further underscoring the necessity of exploring organ-sparing options like MDSC whenever possible.
In addition to the multifactorial origins of CSP, the phenomenon of Wallerian degeneration has been hypothesized as a critical contributor to persistent neuropathic pain following surgical interventions such as varicocelectomy. When nerve fibers are damaged, this degeneration process can lead to aberrant nerve regeneration or neuroma formation, resulting in ongoing or exacerbated pain. Furthermore, Parekattil et al. have described the “trifecta” nerve distribution within the spermatic cord comprising the sympathetic fibers, the ilioinguinal and genitofemoral nerves, and the autonomic fibers surrounding the vas deferens which illustrates the complexity of innervation in this region and could explain the incomplete relief some patients experience after standard varicocelectomy (11).
MDSC was first introduced in 1978 by Devine and Schellhammer in two cases of CSP (12). Over the years, MDSC has gradually evolved, demonstrating encouraging results and emerging as a promising treatment option with favorable outcomes in some patients. Our study aims to evaluate the effectiveness and safety of MDSC in patients with CSP who have not achieved relief following varicocelectomy. In light of more recent data, Calixte et al. have reported the largest series to date involving 772 patients undergoing MDSC, demonstrating significant improvement in pain outcomes in a majority of cases (7). Similarly, Parekattil et al. have advanced the concept of a multifaceted nerve distribution approach when treating CSP, emphasizing the importance of identifying and addressing all contributing nerve pathways (11). These contemporary studies underline the evolution of current algorithms and surgical strategies aimed at enhancing pain relief for patients with CSP who have not benefited from conventional treatments.
The study showed that the average age of patients was 29.4±6.0 years, indicating that most individuals who failed varicocelectomy were relatively young. These patients experienced scrotal pain for an average duration of 26.1±6.9 months, enduring significant discomfort over a considerable period. Continuous scrotal pain was reported by 68.9% of patients, while sexual activity provoked pain in 31.1%. This underscores the profound impact of CSP on daily activities and quality of life, particularly among younger individuals.
MDSC is a relatively new technique for treating CSP. In our study, the rate of pain reduction post-surgery was up to 84.4%. The rates of complete and partial pain reduction may vary compared to other studies potentially due to differences in pain assessment among patients but the combined rates of pain relief are similar (1,5). Moreover, the failure rate of MDSC surgery was low, at just 15.5%. These findings suggest that MDSC can be an effective treatment option for patients with CSP unresponsive to conservative treatments. However, we acknowledge that approximately 15% of patients in our cohort reported limited or no improvement, a figure aligning with the 15% failure rate commonly described in broader MDSC series (5,7). In response to this, we have refined our surgical protocol by ensuring a minimum 2-cm stripping of nerve fibers down to the serosal surface of both the vas deferens and adjacent vessels to prevent potential residual innervation. We anticipate that this technique refinement may further improve outcomes, particularly for those who did not achieve pain relief with our original approach. Our success rate, encompassing both complete and partial pain reductions, aligns with those reported in previous studies (1,5,13).
Surgical effectiveness varies notably based on the location of the pain. Surgery is highly effective for patients with pain in multiple locations, such as the testicle, epididymis, and spermatic cord, achieving the highest pain reduction rate of 89.6%. Patients with pain localized to the testicle also saw substantial benefits, with an average reduction of 83.8%. However, there was significant variability in pain reduction for certain locations, particularly the epididymis (±28.6%) and spermatic cord (±29.4%), likely due to various influencing factors. Conversely, patients with combined pain in the testicle and spermatic cord experienced the lowest reduction (36.9%), indicating either lower surgical effectiveness or more complex underlying factors. In particular, patients presenting with simultaneous testicle and spermatic cord pain may exhibit more extensive or overlapping nerve pathways and potentially higher central sensitization. Scar tissue or adhesions from prior interventions could also exacerbate nerve involvement, making thorough denervation more challenging and leading to reduced pain relief compared to more localized presentations. Clinically, this information is crucial for physicians to counsel patients on expected pain reduction based on their specific pain location and prioritize surgical interventions for sites showing higher reduction rates.
MDSC appears to have a higher success rate and a longer duration of pain relief than other CSP treatments, such as nerve blocks, medical therapies, and psychotherapy. For instance, the degree of pain reduction following a cord block can be predictive of the pain relief achieved after undergoing MDSC. Patients who respond well to the cord block often experience lasting and complete pain alleviation (14). However, directly comparing different treatment methods is challenging due to variations in patient selection, methodology, and outcome measurements.
In some studies, MDSC has been compared to other CSP treatments. Levine et al. (15) were among the earliest to demonstrate that a positive SCB strongly predicts successful outcomes in microsurgical denervation. A summary of previous MDSC series with etiologies, follow-up, and success rates is shown in Table 3. For instance, a meta-analysis of 14 studies indicated that MDSC has a higher success rate defined as more than 50% pain reduction compared to nerve blocks, medications, and psychotherapy (16). The analysis reported a combined success rate of 75% (95% CI: 65–83%) and an average pain reduction duration of 24 months (95% CI: 12–36 months) (16). However, the authors noted that the quality of evidence could be improved due to the heterogeneity of the studies and the lack of randomized controlled trials.
Table 3
| References | Etiology of orchialgia | No. units | Follow-up (months) | Success, n [%] | ||
|---|---|---|---|---|---|---|
| Complete | Partial | No relief | ||||
| Devine and Schellhammer [1978] (12) | n/a | 2 | n/a | 2 [100] | 0 | 0 |
| Choa and Swami [1992] (16) | Epididymitis | 4 | 18.5 | 4 [100] | 0 | 0 |
| Post-vasectomy | ||||||
| Levine et al. [1996] (15) | Post-vasectomy hernioplasty | 8 | 16.6 | 7 [88] | 1 [13] | 0 |
| Strom et al. [2008] (5) | Post-vasectomy | 95 | 20.3 | 67 [71] | 17 [17] | 11 [12] |
| Herniorrhaphy | ||||||
| Infection | ||||||
| Trauma | ||||||
| Physical straining | ||||||
| Varicocelectomy | ||||||
| Back injury | ||||||
| Back surgery | ||||||
| Previous radiation therapy | ||||||
| Marconi et al. [2015] (6) | Varicocelectomy | 50 | 6 | 40 [80] | 6 [12] | 4 [8] |
| Hernioplasty | ||||||
| Operated epididymal cyst | ||||||
| Epididymectomy | ||||||
| Radical prostatectomy | ||||||
| Chronic epididymitis | ||||||
| Post-vasectomy | ||||||
| Calixte et al. [2018] (7) | Idiopathic & recurrent epididymitis | 860 | 24 | 426 [50] | 292 [34] | 142 [17] |
| Post vasectomy | ||||||
| Sports/trauma | ||||||
| Herniorrhaphy | ||||||
| Others (vas reversal, nephrectomy, penile prosthesis, orchiectomy, etc.) | ||||||
| Varicocele | ||||||
| Post orchiopexy | ||||||
| Chaudhari et al. [2019] (9) | n/a | 38 | 24 | 31 [81.58] | 4 [10.53] | 3 [7.89] |
| Long et al. [2019] (8) | n/a | 30 | 12 | 15 [53.5] | 19 [67.9] | 3 [9.8] |
CSP, chronic scrotal pain; n/a, not available.
MDSC boasts a high patient satisfaction rate of 73.3% and is associated with mild and transient complications, such as wound infection, hematoma, and sensory disturbances. Notably, our study did not record any severe complications, such as atrophy or loss of the testicle. These findings suggest that MDSC is a promising treatment option for CSP, particularly for patients who have not found success with other treatments.
Other studies have reported similar risks associated with MDSC, including testicular atrophy, hydrocele, and recurrence of pain (3,5). These risks highlight the importance of careful patient selection and precise surgical techniques to minimize side effects. This aligns with previous reports indicating that MDSC is a safe procedure with a low recurrence rate and a minimal incidence of post-surgical complications (8.8%). Our study confirms that MDSC is an effective and safe option for treating CSP.
Following varicocelectomy for CSP, some patients may experience disappointment with the surgical outcomes. This also highlights the importance of precise diagnosis: if a varicocele is misattributed as the cause of diffuse scrotal pain, patients may undergo an unnecessary procedure. Comprehensive preoperative workup, including targeted pain mapping, scrotal imaging, and clinical algorithms distinguishing varicocele-related pain from multifactorial scrotal pain, can optimize surgical choices. Otherwise, MDSC might offer more direct relief in cases of multifocal scrotal pain. MDSC serves as an effective adjunctive method for these individuals, offering a promising solution.
For patients who do not achieve pain relief through varicocelectomy, MDSC presents an encouraging option, with many likely to respond well to this procedure. Complete pain relief is not always necessary, as even partial reduction can significantly enhance a patient’s quality of life, enabling them to resume normal activities. Nonetheless, approximately 15% of patients may not experience significant relief, a finding which is consistent with large series in the literature that report MDSC failure rates hovering around 15% across diverse etiologies (17). Although our cohort was restricted to varicocelectomy failures, these parallel findings reinforce the notion that meticulous patient selection and technique remain crucial.
Our study has some limitations, such as a small sample size and a retrospective design, which should be considered when interpreting the results. Additionally, we elected to use lidocaine as our local anesthetic due to institutional restrictions and safety considerations, which may differ from the more conventional practice of administering a larger volume of bupivacaine or ropivacaine. Future research should include larger, more extensive prospective studies with extended follow-up periods and randomized controlled trials to confirm our findings. Additionally, future studies should investigate the mechanisms of action of MDSC, including its effects on central sensitivity and nerve conduction, and explore adjunct therapies, such as concurrent medication, which could also enhance pain relief for patients. Because this study was retrospective and single-center, a certain degree of selection bias may exist if our patient population is not fully representative of broader CSP cases. Measurement bias may also arise due to self-reported pain scores, although we attempted to standardize the assessment using the NRS by the same investigator. The generalizability of our findings may be limited due to the single-center nature of the study and the specific patient population. Further studies in diverse settings are necessary to confirm the applicability of MDSC across different populations and healthcare environments.
Conclusions
CSP remains a challenge for physicians due to its poorly understood pathophysiological mechanisms. Large, well-designed, multi-center trials are necessary to establish level 1 evidence and create a standardized algorithm for approaching this condition. However, the relative rarity of CSP makes conducting randomized controlled trials challenging. When non-surgical treatment methods fail, MDSC becomes a valuable approach, boasting a relatively high success rate. It should be considered for CSP patients unresponsive to medical treatment after varicocelectomy. MDSC can significantly improve the quality of life and help patients with CSP, who experience unsuccessful outcomes post-varicocelectomy, return to their daily activities. Nonetheless, these findings originate from a single tertiary center, which may limit generalizability to other settings or populations. Larger multicenter studies are warranted to validate the efficacy of MDSC in diverse patient groups.
Acknowledgments
The authors are grateful to Pham Ngoc Thach University of Medicine and Andrology Department at Binh Dan Hospital for their invaluable support, guidance, and resources in completing this research project.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-719/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-719/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-719/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2024-719/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the medical ethic council of Pham Ngoc Thach University of Medicine (No. 698/TDHYKPNT-HDDD), and all patients provided informed consent for the procedure and the use of their data for research purposes. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davis BE, Noble MJ, Weigel JW, et al. Analysis and management of chronic testicular pain. J Urol 1990;143:936-9. [Crossref] [PubMed]
- Ciftci H, Savas M, Yeni E, et al. Chronic orchialgia and associated diseases. Current Urology 2010;4:67-70. [Crossref]
- Rottenstreich M, Glick Y, Gofrit ON. Chronic scrotal pain in young adults. BMC Res Notes 2017;10:241. [Crossref] [PubMed]
- Oka S, Shiraishi K, Matsuyama H. Microsurgical Anatomy of the Spermatic Cord and Spermatic Fascia: Distribution of Lymphatics, and Sensory and Autonomic Nerves. J Urol 2016;195:1841-7. [Crossref] [PubMed]
- Strom KH, Levine LA. Microsurgical denervation of the spermatic cord for chronic orchialgia: long-term results from a single center. J Urol 2008;180:949-53. [Crossref] [PubMed]
- Marconi M, Palma C, Troncoso P, et al. Microsurgical Spermatic Cord Denervation as a Treatment for Chronic Scrotal Content Pain: A Multicenter Open Label Trial. J Urol 2015;194:1323-7. [Crossref] [PubMed]
- Calixte N, Tojuola B, Kartal I, et al. Targeted Robotic Assisted Microsurgical Denervation of the Spermatic Cord for the Treatment of Chronic Orchialgia or Groin Pain: A Single Center, Large Series Review. J Urol 2018;199:1015-22. [Crossref] [PubMed]
- Long H, Bai W, Zhang X, et al. A Clinical Study on Microsurgical Denervation of Spermatic Cord for Refractory Chronic Orchialgia. Urol Int 2019;103:62-7. [Crossref] [PubMed]
- Chaudhari R, Sharma S, Khant S, et al. Microsurgical Denervation of Spermatic Cord for Chronic Idiopathic Orchialgia: Long-Term Results from an Institutional Experience. World J Mens Health 2019;37:78-84. [Crossref] [PubMed]
- Raffo M, Di Naro A, Napolitano L, et al. Testicular Cancer Treatments and Sexuality: A Narrative Review. Medicina (Kaunas) 2024;60:586. [Crossref] [PubMed]
- Parekattil SJ, Gudeloglu A, Brahmbhatt JV, et al. Trifecta nerve complex: potential anatomical basis for microsurgical denervation of the spermatic cord for chronic orchialgia. J Urol 2013;190:265-70. [Crossref] [PubMed]
- Devine CJ Jr, Schellhammer PF. The use of microsurgical denervation of the spermatic cord for orchialgia. Trans Am Assoc Genitourin Surg 1978;70:149-51. [PubMed]
- Sun HH, Tay KS, Jesse E, et al. Microsurgical Denervation of the Spermatic Cord: A Historical Perspective and Recent Developments. Sex Med Rev 2022;10:791-9. [Crossref]
- Benson JS, Abern MR, Larsen S, et al. Does a positive response to spermatic cord block predict response to microdenervation of the spermatic cord for chronic scrotal content pain? J Sex Med 2013;10:876-82. [Crossref] [PubMed]
- Levine LA, Matkov TG, Lubenow TR. Microsurgical denervation of the spermatic cord: a surgical alternative in the treatment of chronic orchialgia. J Urol 1996;155:1005-7. [Crossref] [PubMed]
- Choa RG, Swami KS. Testicular denervation. A new surgical procedure for intractable testicular pain. Br J Urol 1992;70:417-9. [Crossref] [PubMed]
- Kavoussi PK, West BT, Machen GL. Preoperative Predictors of Failure of Microsurgical Spermatic Cord Denervation for Men With Chronic Orchialgia. Urology 2021;149:30-3. [Crossref] [PubMed]


