
Three-dimensional measurement and analysis of the compressor urethrae and urethra in postpartum women
Highlight box
Key findings
• This study provides the reference criteria for the volume, thickness, and surface area of compressor urethrae and urethra. The volumes of the upper, middle, and lower regions of the urethra in the control group were 2.58±0.59, 2.10±0.32, and 0.84±0.37 cm3, respectively.
• The volume of compressor urethrae and urethra was negatively correlated with age. Age was associated with the morphological characterization of the compressor urethrae and urethra.
What is known and what is new?
• The middle part of the urethra is the critical point of the stress in surgery for urinary incontinence. The majority of research related to this procedure has focused on the compressor urethrae and its morphology. No studies have examined the volume, thickness, or surface area of different portions of the urethra, and no reports exist regarding the detailed anatomical parameters of the compressor urethrae.
• In this study, novel models of the compressor urethrae and urethra were constructed. Various measurements and analyses, including three-dimensional methods, were performed to clarify the anatomical characteristics of the compressor urethrae and urethra.
What is the implication, and what should change now?
• Our study provides a reference for the volume, thickness, and surface area of upper, middle, and lower urethra, which may aid in urethral sling placement.
• The three-dimensional measurement provides a precise means to quantify the morphological parameters of urological diseases. The compressor urethrae may be used as an entry point in the treatment of stress urinary incontinence.
Introduction
Stress urinary incontinence (SUI) symptoms present as involuntary urine leakage due to a rise in intrabdominal pressure, with childbirth uniquely affecting the parameters of urinary continence. According to a meta-analysis conducted by Dai et al. (1), the incidence of urinary incontinence in postpartum women is 26%. Postpartum-stress incontinence refers to a condition in which women experience symptoms of SUI during the postpartum period, but its cause remains controversial (2). Structural abnormalities of the pelvic floor are key causes of SUI (3), and at the level of anatomy, structural abnormalities of the muscle, organ, and ligament changes are also critically involved in SUI. It has been reported that the anatomical structure index of female pelvis can be used as diagnostic criteria for SUI (4). However, research in this area has mainly focused on major viscera and muscles. The compressor urethrae is positioned with its anterior to the urethra and may be involved in urinary control. The location of the compressor urethrae has been confirmed in a number of studies and has been characterized via imaging and histology (5). However, most of these studies have addressed the morphology of compressor urethrae, but there are no detailed reports regarding its anatomical parameters. The urethra has also garnered attention as it relates to its role in SUI. The majority of studies on this subject indicate that the middle part of the urethra is the critical point in the surgery for SUI (6-8). However, no studies have examined the volume, thickness, or surface area of the different segments of the urethra.
In recent years, three-dimensional (3D) technology, which overcomes the shortcomings of traditional two-dimensional measurement, has been widely applied in the medical field (9,10), including in the measurement and analysis of the human anatomy. For instance, 3D measurement and analysis can be used to construct stereoscopic 3D models of the pelvic floor, and various anatomical indicators can be accurately and automatically analyzed through this approach (11). Although 3D measurement and analysis have been used to investigate the muscles, organs, and ligaments related to the pelvic floor, they have not been applied to study the detailed parameters of the compressor urethrae and urethra. Our previous studies confirmed that 3D analysis can provide measurements of the various metrics of the prostate and uterine body in men with benign prostatic hyperplasia and infertile women, respectively (12,13). This technology provides reference standards for diagnosis and treatment of these two diseases.
This study consisted of four main parts. First, a model of the compressor urethrae was constructed based on imaging examination results. Various measurements and analyses were performed to clarify the anatomical characteristics of the compressor urethrae. Second, the anatomical characteristics of the different urethral segments were clarified via 3D measurement and analysis. Third, the association of age with the parameters of the compressor urethrae and urethra was determined. Fourth, the quantitative reference ranges for the compressor urethrae and urethra were identified. Finally, from these procedures, we could generate a reference standard for SUI sling placement during surgery, the diagnosis of postpartum SUI, and surgical treatments for urinary incontinence. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-2024-695/rc).
Methods
Study population
This retrospective study enrolled 156 patients who underwent T2-weighted imaging examinations 6 weeks after primary vaginal delivery at Jinhua People’s Hospital between February 2019 and January 2021. Patients had completely voided their bladders and drank 200–300 cc of water 1 hour before the scan to ensure a moderately full bladder. During the scanning session, the patients were scanned in the supine position with the median sagittal plane perpendicular to the bed surface. Patients lie down with their head buried in their hands and their legs straight and close together. The scanning range was up to the level of the second sacral vertebra and down to 1.0 cm below the perineum. The scanning parameters were as follows: repetition time/echo time, 4,110/102 ms; matrix, 320×2,256; visual field, 280×280; layer thickness, 2.5 mm; and layer spacing, 0. The demographic information of patients [e.g., age, height, and Incontinence Quality of Life Instrument (I-QOL) score] was extracted from the patients’ records. Among the patients, 58 were diagnosed with postpartum SUI. The remaining patients had no clinical symptoms of postpartum SUI. The T2-weighted imaging data of these patients were collected. The inclusion criteria were (I) primiparous women; (II) a vaginal delivery; and (II) a term birth. Meanwhile, the exclusion criteria were as follows: (I) a history of pelvic floor surgery; (II) pelvic organ prolapse; (III) pelvic tumors; (IV) urinary tract infection; and (V) unclear T2-weighted imaging. After the inclusion and exclusion criteria were applied, 90 patients were ultimately enrolled in this study. Among them, 30 were assigned to the postpartum SUI group, and 60 without any clinical symptoms of SUI were assigned to the control group. The mean age of the study participants was 30.43±3.74 years (range, 23–40 years). This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Institutional Review Board (IRB) of the Jinhua People’s Hospital (No. IRB-2021016-R). The requirement for individual consent was waived due to the retrospective nature of the analysis.
Three-dimensional reconstruction
The patient imaging data with a layer thickness of 2.5 mm were imported into the Mimics 19.0 software (Materialise, Leuven, Belgium) in Digital Imaging and Communications in Medicine (DICOM) format. Mimics software is the most authoritative software for 3D measurement and analysis and has been widely applied in various medical fields. For example, it was used in one study to measure the condylar morphology in patients with class II malocclusion (14) and in another to measure the volume expansion of orbital soft tissue (15). The 3D models of the compressor urethrae and urethra were constructed based on the corresponding anatomic structures in the images and were then smoothed. Subsequently, the models were imported into 3-matic 11.0 software (Materialise) for measurement and analysis.
Measurement and analysis of parameters
Compressor urethrae thickness (CUT), urethral thickness (UT), compressor urethrae volume (CUV), urethral volume (UV), compressor urethrae surface area (CUS), and urethral surface area (US) were automatically calculated via 3-matic 11.0 software. Compressor urethrae length (CUL) and urethral length (UL) were analyzed automatically by this software in the z axis. The compressor urethrae width (CUW) was measured as follows: first, the left- and right-most point of the compressor urethrae was determined in the x-axis via the 3-matic software. Subsequently, the surface distance of compressor urethrae was measured from the left-most to the right-most point, which was defined as the CUW. The UIA was measured as follows: first, the centerline of urethra was automatically determined by the software based on the 3D model. The sagittal plane was then created by the software, and the angle between the centerline and sagittal plane was defined as the UIA. Finally, the angle tool in the software was used to measure the UIA. The upper, middle, and lower portions of compressor urethrae and urethra were identified as follows: first, the CUL and UL were analyzed automatically by the software in the z-axis, respectively. Second, three equal division points were created by the software according to the CUL and UL, respectively. Subsequently, three corresponding horizontal planes were created by the software based on the three equal division points, respectively. Finally, the compressor urethrae and urethra were divided into the upper, middle, and lower sections by the software. The volume of the upper compressor urethrae (UCUV), thickness of the upper compressor urethrae (UCUT), surface area of the upper compressor urethrae (UCUS), volume of the middle compressor urethrae (MCUV), thickness of the middle compressor urethrae (MCUT), surface area of the middle compressor urethrae (MCUS), volume of the lower compressor urethrae (LCUV), thickness of the lower compressor urethrae (LCUT), surface area of the lower compressor urethrae (LCUS), volume of the upper urethra (UUV), thickness of the upper urethra (UUT), surface area of the upper urethra (UUS), volume of the middle urethra (MUV), thickness of the middle urethra (MUT), surface area of the middle urethra (MUS), volume of the lower urethra (LUV), thickness of the lower urethra (LUT), and surface area of the lower urethra (LUS) were analyzed automatically by the software. The distance between the pubic symphysis and compressor urethrae (L) was considered to span from the upper edge of pubic symphysis to the upper edge of the compressor urethrae and was measured via software. The ratio of the CUL to the UL (CUL/UL) was also determined.
Statistical analysis
All data are expressed as the mean ± SD. The independent-samples t test was used for comparisons between the postpartum SUI group and control group. Different segments of the compressor urethrae and urethra were also compared via the independent-samples t test. Correlation analysis was performed with the Pearson correlation coefficient.
Results
The 3D models of compressor urethrae and urethra were constructed successfully based on magnetic resonance image (Figure 1). The measurement and analysis were conducted via Mimics software. Various anatomical indicators of the compressor urethrae and urethra were carried out using 3D measurement and analysis methods (Figures 2,3). The CUS, CUV, and CUT in the control group were significantly larger than those in the postpartum SUI group (P<0.001, P=0.002, and P=0.02, respectively). The CUL, UCUV, MCUV, LCUV, UCUS, MCUS, LCUS, UCUT, MCUT, and LCUT in the control group were significantly greater than those in the postpartum SUI group (P=0.01, P=0.006, P=0.002, P=0.01, P=0.008, P<0.001, P=0.007, P=0.02, P=0.03, and P=0.004, respectively). The UL, UUV, MUV, UUT, MUT, UUS, and MUS in the control groups were significantly greater than those in the postpartum SUI group (P=0.01, P=0.003, P<0.001, P=0.04, P=0.01, P=0.009, and P=0.002, respectively). The UIA in the control group was smaller than that in postpartum SUI group (P=0.04). L, CUL/UL, CUW, LUV, LUS, LUT, and age did not differ significantly between the two groups (P=0.33, P=0.22, P=0.52, P=0.69, P=0.88, P=0.63, and P=0.98, respectively) (Table 1). After pelvic floor muscle exercises, the I-QOL scores of patients with postpartum SUI was significantly increased (P<0.001) (Table S1).
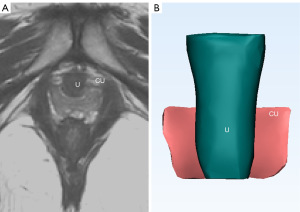


Table 1
| Parameter | Control group | Postpartum SUI group | P value |
|---|---|---|---|
| Age (years) | 30.43±3.74 | 30.47±3.27 | 0.98 |
| CUW (mm) | 24.61±3.79 | 23.22±7.69 | 0.52 |
| CUL (mm) | 23.24±3.29 | 20.45±3.73 | 0.01* |
| CUL/UL | 0.61±0.07 | 0.59±0.1 | 0.22 |
| CUV (cm3) | 1.47±0.49 | 1±0.36 | 0.002** |
| CUS (cm2) | 12.97±2.7 | 9.57±2.85 | <0.001*** |
| CUT (mm) | 6.92±1.86 | 5.82±1.1 | 0.02* |
| UCUV (cm3) | 0.68±0.27 | 0.46±0.14 | 0.006** |
| UCUS (cm2) | 6.39±1.66 | 4.88±1.84 | 0.008** |
| UCUT (mm) | 6.91±1.86 | 5.82±1.1 | 0.02* |
| MCUV (cm3) | 0.44±0.13 | 0.3±0.14 | 0.002** |
| MCUS (cm2) | 3.92±0.69 | 2.73±0.66 | <0.001*** |
| MCUT (mm) | 4.83±1.09 | 4.05±1.15 | 0.03* |
| LCUV (cm3) | 0.34±0.12 | 0.24±0.13 | 0.01* |
| LCUS (cm2) | 2.66±0.79 | 1.97±0.72 | 0.007** |
| LCUT (mm) | 4.42±1.14 | 3.37±0.94 | 0.004** |
| L (mm) | 26.66±3.82 | 27.89±4.03 | 0.33 |
| UV (cm3) | 5.35±1.48 | 3.79±1.17 | <0.001*** |
| US (cm2) | 20.66±5.4 | 17.73±3.95 | 0.005** |
| UT (mm) | 18.55±3.57 | 16.05±3.16 | 0.03* |
| UUV (cm3) | 2.58±0.59 | 1.93±0.74 | 0.003** |
| UUS (cm2) | 11.61±2.64 | 9.2±3.07 | 0.009** |
| UUT (mm) | 18.44±3.7 | 16.02±3.21 | 0.04* |
| MUV (cm3) | 2.10±0.32 | 1.06±0.35 | <0.001*** |
| MUS (cm2) | 5.96±1.13 | 4.81±1.06 | 0.002** |
| MUT (mm) | 13.6±2.08 | 12.03±1.45 | 0.01* |
| LUV (cm3) | 0.84±0.37 | 0.80±0.21 | 0.69 |
| LUS (cm2) | 3.79±1.42 | 3.72±0.73 | 0.88 |
| LUT (mm) | 11.56±2.62 | 11.18±2.11 | 0.63 |
| UL (mm) | 37.93±4.65 | 35.01±2.95 | 0.01* |
| UIA (°) | 3.81±1.55 | 7.34±5.88 | 0.04* |
*, P<0.05; **, P<0.01; ***, P<0.001. CUL, compressor urethrae length; CUL/UL, ratio of the CUL to the UL; CUS, compressor urethrae surface area; CUT, compressor urethrae thickness; CUW, compressor urethrae width; CUV, compressor urethrae volume; LCUS, surface area of the lower compressor urethrae; LCUT, thickness of the lower compressor urethrae; LCUV, volume of the lower compressor urethrae; LUS, surface area of the lower urethra; LUT, thickness of the lower urethral; LUV, lower urethral volume; MCUS, surface area of the middle compressor urethrae; MCUT, thickness of the middle compressor urethrae; MCUV, volume of the middle compressor urethrae; MUS, surface area of the middle urethra; MUT, thickness of the middle urethra; MUV, volume of the middle urethra; SD, standard deviation; SUI, stress urinary incontinence; UCUS, surface area of the upper compressor urethrae; UCUT, thickness of the upper compressor urethrae; UCUV, volume of the upper compressor urethrae; UIA, urethral inclination angle; UL, urethral length; UUS, surface area of the upper urethra; UUT, thickness of the upper urethra; UUV, volume of the upper urethra; US, urethral surface area; UT, urethral thickness; UV, urethral volume.
The UCUV was significantly greater than the MCUV and LCUV, respectively (P<0.001 and P<0.001, respectively); the UCUS was significantly larger than the MCUS and LCUS, respectively (P<0.001 and P<0.001, respectively); the UCUT was significantly larger than the MCUT and LCUT, respectively (P<0.001 and P<0.001, respectively); and the MCUV was significantly larger than the LCUV (P=0.002). There was no significant difference between the MCUT and LCUT (P=0.16) (Table 2).
Table 2
| Parameter | Mean ± SD | P value |
|---|---|---|
| UCUV vs. MCUV (cm3) | 0.68±0.27 vs. 0.44±0.13 | <0.001*** |
| UCUV vs. LCUV (cm3) | 0.68±0.27 vs. 0.34±0.12 | <0.001*** |
| MCUV vs. LCUV (cm3) | 0.44±0.13 vs. 0.34±0.12 | 0.002** |
| UCUS vs. MCUS (cm2) | 6.39±1.66 vs. 3.92±0.69 | <0.001*** |
| UCUS vs. LCUS (cm2) | 6.39±1.66 vs. 2.66±0.79 | <0.001*** |
| MCUS vs. LCUS (cm2) | 3.92±0.69 vs. 2.66±0.79 | <0.001*** |
| UCUT vs. MCUT (mm) | 6.91±1.86 vs. 4.83±1.09 | <0.001*** |
| UCUT vs. LCUT (mm) | 6.91±1.86 vs. 4.42±1.14 | <0.001*** |
| MCUT vs. LCUT (mm) | 4.83±1.09 vs. 4.42±1.14 | 0.16 |
**, P<0.01; ***, P<0.001. LCUS, surface area of the lower compressor urethrae; LCUT, thickness of the lower compressor urethrae; LCUV, volume of the lower compressor urethrae; MCUS, surface area of the middle compressor urethrae; MCUT, thickness of the middle compressor urethrae; MCUV, volume of the middle compressor urethrae; SD, standard deviation; UCUS, surface area of the upper compressor urethrae; UCUT, thickness of the upper compressor urethrae; UCUV, volume of the upper compressor urethrae.
The UUV was significantly greater than were the MUV and LUV, respectively (P<0.001 and P<0.001, respectively); the UUS was significantly larger than were the MUS and LUS, respectively (P<0.001 and P<0.001, respectively); the UUT was significantly larger than were the MUT and LUT, respectively (P<0.001 and P<0.001, respectively); the MUV was significantly larger than was the LUV (P<0.001); the MUS was significantly larger than was LUS (P<0.001); and the MUT was significantly greater than was the LUT (P=0.001) (Table 3).
Table 3
| Parameter | Mean ± SD | P value |
|---|---|---|
| UUV vs. MUV (cm3) | 2.58±0.59 vs. 2.10±0.32 | <0.001*** |
| UUV vs. LUV (cm3) | 2.58±0.59 vs. 0.84±0.37 | <0.001*** |
| MUV vs. LUV (cm3) | 2.10±0.32 vs. 0.84±0.37 | <0.001*** |
| UUS vs. MUS (cm2) | 11.61±2.64 vs. 5.96±1.13 | <0.001*** |
| UUS vs. LUS (cm2) | 11.61±2.64 vs. 3.79±1.42 | <0.001*** |
| MUS vs. LUS (cm2) | 5.96±1.13 vs. 3.79±1.42 | <0.001*** |
| UUT vs. MUT (mm) | 18.44±3.7 vs. 13.6±2.08 | <0.001*** |
| UUT vs. LUT (mm) | 18.44±3.7 vs. 11.56±2.62 | <0.001*** |
| MUT vs. LUT (mm) | 13.6±2.08 vs. 11.56±2.62 | 0.001** |
**, P<0.01; ***, P<0.001. LUS, surface area of the lower urethra; LUT, thickness of the lower urethra; LUV, lower urethral volume; MUS, surface area of the middle urethra; MUT, thickness of the middle urethra; MUV, volume of the middle urethra; SD, standard deviation; UUS, surface area of the upper urethra; UUT, thickness of the upper urethra; UUV, volume of the upper urethra.
The results of the correlation analysis revealed that the CUL (r=−0.363; P=0.049), CUV (r=−0.506; P=0.004), and CUS (r=−0.523; P=0.003) were negatively correlated with age. Age was negatively correlated with the UV (r=−0.453; P=0.01), UT (r=−0.554; P=0.002), UUV (r=−0.395; P=0.03), MUV (r=−0.403; P=0.03), LUV (r=−0.391; P=0.03), UUT (r=−0.544; P=0.002), MUT (r=−0.629; P<0.001), LUT (r=−0.537; P=0.002), and UL (r=–0.376; P=0.04). Age was not significantly associated with the UIA (r=0.149; P=0.43), CUW (r=−0.314; P=0.09), CUL/UL (r=−0.048; P=0.80), CUT (r=−0.229; P=0.22), UCUT (r=−0.218; P=0.25), MCUT (r=−0.247; P=0.19), LCUT (r=−0.291; P=0.12), US (r=−0.271; P=0.15), L (r=0.115; P=0.55), UUS (r=−0.167; P=0.38), MUS (r=−0.261; P=0.16), or LUS (r=−0.218; P=0.25) (Table 4).
Table 4
| Parameter | r | P |
|---|---|---|
| CUW (mm) | −0.314 | 0.09 |
| CUL (mm) | −0.363 | 0.049* |
| CUL/UL | −0.048 | 0.80 |
| CUV (cm3) | −0.506 | 0.004** |
| CUS (cm2) | −0.523 | 0.003** |
| CUT (mm) | −0.229 | 0.22 |
| UCUV (cm3) | −0.488 | 0.006** |
| UCUS (cm2) | −0.490 | 0.006** |
| UCUT (mm) | −0.218 | 0.25 |
| MCUV (cm3) | −0.448 | 0.01* |
| MCUS (cm2) | −0.437 | 0.02* |
| MCUT (mm) | −0.247 | 0.19 |
| LCUV (cm3) | −0.487 | 0.006** |
| LCUS (cm2) | −0.372 | 0.04* |
| LCUT (mm) | −0.291 | 0.12 |
| L (mm) | 0.115 | 0.55 |
| UV (cm3) | −0.453 | 0.01* |
| US (cm2) | −0.271 | 0.15 |
| UT (mm) | −0.554 | 0.002** |
| UUV (cm3) | −0.395 | 0.03* |
| UUS (cm2) | −0.167 | 0.38 |
| UUT (mm) | −0.544 | 0.002** |
| MUV (cm3) | −0.403 | 0.03* |
| MUS (cm2) | −0.261 | 0.16 |
| MUT (mm) | −0.629 | <0.001*** |
| LUV (cm3) | −0.391 | 0.03* |
| LUS (cm2) | −0.218 | 0.25 |
| LUT (mm) | −0.537 | 0.002** |
| UL (mm) | −0.376 | 0.04* |
| UIA (°) | 0.149 | 0.43 |
*, P<0.05; **, P<0.01; ***, P<0.001. CUL, compressor urethrae length; CUL/UL, ratio of the CUL to the UL; CUS, compressor urethrae surface area; CUT, compressor urethrae thickness; CUW, compressor urethrae width; CUV, compressor urethrae volume; LCUS, surface area of the lower compressor urethrae; LCUT, thickness of the lower compressor urethrae; LCUV, volume of the lower compressor urethrae; LUS, surface area of the lower urethra; LUT, thickness of the lower urethral; LUV, lower urethral volume; MCUS, surface area of the middle compressor urethrae; MCUT, thickness of the middle compressor urethrae; MCUV, volume of the middle compressor urethrae; MUS, surface area of the middle urethra; MUT, thickness of the middle urethra; MUV, volume of the middle urethra; SD, standard deviation; SUI, stress urinary incontinence; UCUS, surface area of the upper compressor urethrae; UCUT, thickness of the upper compressor urethrae; UCUV, volume of the upper compressor urethrae; UIA, urethral inclination angle; UL, urethral length; UUS, surface area of the upper urethra; UUT, thickness of the upper urethra; UUV, volume of the upper urethra; UV, urethral volume; US, urethral surface area; UT, urethral thickness.
Discussion
The structure of the female pelvic floor is complex, but studies in this area have mainly employed methods involving subjective judgment of the two-dimensional plane. This plane is an abstract image that cannot provide a clear visualization of the female pelvic floor structure. Pelvic floor muscles have been extensively examined in terms of their relation to SUI (16) and have been indicated to play an important role in female urinary continence.
The compressor urethrae is located anteriorly to the urethra and may be relevant to a urinary effect. Studies on the compressor urethrae, however, have mainly focused on imaging and histological findings (17,18), but interest in the structural features of the compressor urethrae has grown in recent years.
Wang et al. found that patients with overactive bladder disease had a significantly lower CUV than did healthy individuals (19). This indicates that there may be an association between the compressor urethrae and female urinary continence. In our study, we found that compressor urethrae is shaped like a “C”, which is consistent with the study by Wu et al. (20). Moreover, we quantified the location, length, and width of the compressor urethrae and measured and analyzed its morphological characteristics. We found that the lengths of the compressor urethrae and urethra were not associated. In other research, the ratio of the length of a urogenital diaphragm to its compressor urethrae and urethrovaginal sphincter to the UL was 0.54 to 0.76 (21), which is similar to our study, in which the CUL was shorter than the UL.
The associations between the compressor urethrae and SUI in the postpartum period have not been extensively investigated. A study by Shi et al. found that CUV can be used as a diagnostic indicator for SUI (22). In contrast, a study by Franchi et al. found that worsening of SUI symptoms was not associated with the impact of SUI surgery on the compressor urethrae (23). Similar findings were reported by Rostaminia et al., who found that the compressor urethrae is not related to SUI (24). The divergence in these results may be due to methodological differences in their respective studies. Shi et al. created a 3D model of the compressor urethrae, Franchi et al. examined the effect of SUI surgery on the female pelvic floor, and Rostaminia et al. used 3D ultrasound imaging technology to assess the SUI. Although 3D ultrasound can provide improved quality of imaging, it is essentially two-dimensional. In our study, 3D models of compressor urethrae and urethra were constructed according to the two-dimensional images. The measurements and analyses conducted based on the 3D models were reliable and accurate. This method’s automated analysis is more scientifically objective than conventional two-dimensional subjective measures. The morphometric parameters of our study were automatically analyzed via Mimics software and included volume, thickness, lines, and surface area, among others, with subjective judgment being largely avoided. In traditional two-dimensional measurement, the volume of the body structure is estimated according to length, width, and height through the use of the formula for an ellipsoid. This process requires the subjective judgment of researchers regarding the regular shape of body structure. In contrast, Mimics software is able to analyze volume regardless of the shape of the body structure. The parameters included in our study were repeatedly, objectively, and automatically measured. Automated measurements may be affected by the quality of two-dimensional images due to the 3D model being built from these two-dimensional images. However, due to specialist anatomical knowledge and professional training in the use of medical software, we were able to position the compressor urethrae and the urethra and its related structures accurately for two-dimensional magnetic resonance imaging. In addition, compared to ultrasound and computerized tomography images, magnetic resonance imaging is able to clearly identify the anatomical structure. Thus, these are able to minimize subjective effects.
We further found that the CUV, CUS, and CUT in the control group were larger than those in the postpartum SUI group. This indicates that the compressor urethrae is likely related to SUI. In line with this, the CUL, UCUV, UCUS, UCUT, MCUV, MCUS, MCUT, LCUV, LCUS, and LCUT in the control group were larger than those in postpartum SUI group. This suggests that the different segments of the compressor urethrae are involved in maintaining urinary continence. Reid et al. similarly found the resection of the compressor urethrae to be associated with SUI (25). However, the compressor urethrae is not present at the anterosuperior direction of the urethra. The compressor urethrae is located anterior to the middle and lower portion of the urethra and may thus further strengthen the middle urethra in conditions of urinary continence. L did not differ significantly between the control group and postpartum SUI group. Although the position of compressor urethrae was not significantly altered, the morphological characterization was different.
The urethra is bounded superiorly by the bladder and posteriorly by the uterus and vagina. The urethra is composed of the internal urethral sphincter and the external urethral sphincter. Female urinary continence has been associated with the muscles, ligaments, and organs of the pelvic floor, with the urethra playing a particularly important role in female urinary continence. The location of urethral kinking has been reported to be associated with SUI (8), and in one study, the highest increase in pressure occurred in the distal urethra due to kinking of the urethra (26), and it was also found that the upper and middle urethral mobility vector was altered dramatically during the Valsalva maneuver. In our study, the volume and surface area of the upper and middle urethrae were significantly larger than those of the lower urethrae, respectively. This may suggest that the urinary control function of the upper and middle urethra is much stronger than that of the lower urethra. Therefore, the upper and middle urethral mobility profile appears to be altered dramatically during the Valsalva maneuver. Our studies provide an explanation for the observation by Venema et al. (26) in that the highest increase in pressure occurred in the distal urethra due to kinking of the urethra.
The UL or UIA have been examined in terms of their relationship to urinary incontinence. A study by Guo et al. found that a short UL is not beneficial for urinary continence (27). The shorter the urethra, the more concentrated the pressure inside the urethra is that the sphincter needs to counteract. Moreover, as intra-abdominal pressure increases, the UL also increases (28). A long urethra may reduce the severity of female urinary continence, as this elongation can expand the periurethral tissue to further augment the pressure transmission to the proximal urethra, and an increased UL can spread out the intraurethral pressure. Generally, the female urethra is short, wide, and straight; the shorter the UL is, the faster the flow rates of the urethra. Mao et al. reported there being significant differences in the UIA between nulliparous and primiparous women (29). Meanwhile, Minardi et al. found that the UIA changes dramatically depending on different intra-abdominal pressure states (30). The inconsistency in the findings from these other studies and our own may be due to differences in the participants included. The participants in our study were women with vaginal delivery. Meanwhile, Mao et al. included women with intrapartum caesarean section, while Minardi et al. did not examine postpartum women.
Most of the reports on this topic indicate that the middle urethra plays a key role in female urinary continence. A study by Ling et al. found that the more tortuous the middle urethra is, the greater the likelihood of SUI (8). Furthermore, several studies support the use of midurethral sling surgery for treating SUI (31,32). Lo et al. found that patients with intrinsic sphincter deficiency had significantly worse clinical SUI outcomes (32). This shows that the urethral muscles play an important role in female urinary continence. However, the urethra is composed of both the internal and external urethral sphincters, yet no study has examined the morphology of these urethrae different parts. In our study, we found that the UUV, UUS, and UUT were significantly larger than the LUV, LUS, and LUT, respectively, while the MUV, MUS, and MUT were significantly larger than were LUV, LUS, and LUT, respectively.
This may suggest that the effect of upper and middle urethral sphincter muscles in female urinary continence is stronger than that of the lower urethral sphincter muscles. Our findings can serve as a reference standard for different segments of the urethra and for urethral sling surgery. We further discovered that L was not significantly associated with age, indicating that age exerts little effect on the position of compressor urethrae.
In a study conducted by Dinh et al., the thickness of the inner layer of the urethra in the control group was significantly larger than that of the SUI group (33). Moreover, participants in the control group were older than those in the SUI group. However, this study did not examine the association between muscle thickness and age. Meanwhile, Komemushi et al. found that the levator ani muscle volume was significantly inversely correlated with age (34). In our study, the CUV, CUS, UCUV, MCUV, LCUV, UCUS, MCUS, LCUS, UV, UUV, MUV, LUV, UUT, MUT, and LUT were significantly and negatively correlated with age. This may be attributable to a reduction in female hormone secretion. Among women, as age increases, estrogen level decreases, and changes in estrogen can elicit changes in the function of pelvic floor muscles (35). Decreased estrogen secretion may exert certain negative effects. For instance, it may lead to a reduction of support provided by the pelvic floor muscles to the pelvic floor, which may result in pelvic floor dysfunction. In such situations, patients may be more prone to SUI. This may explain why SUI increases after menopause. For menopausal women, estrogen therapy can help ameliorate urinary system symptoms (36), and it may also help those women with postpartum SUI. Lifestyle factors are also important in the maintenance of pelvic floor muscle strength. Postnatal pelvic floor exercises can enhance muscle strength (37) and improve the clinical symptoms of patients with SUI (38) through increasing the thickness of the pelvic floor muscle (39). The negative effects of delivery can be lessened to a varing extent via pelvic floor exercises and may be beneficial to postpartum women with SUI as long as adherence to exercise can be maintained.
It has been reported that exercising the levator ani muscle can effectively increase its thickness (40) and may potentially improve the clinical symptoms of patients with postpartum SUI (41). The findings from our study corroborate this speculation. After pelvic floor muscle exercises, the CUV, CUS, CUT, UV, US, UT, and I-QOL score of patients with postpartum SUI were significantly increased. The lower the I-QOL scores were, the more severe the SUI symptoms. This may suggest that the compressor urethrae and urethra play a role in female urinary continence. These parameters are provided in Table S1. Performing functional exercise may be beneficial for pelvic floor function recovery in older women, and it has been found that pelvic floor muscle fiber strength diminishes after delivery (16), which may disrupt normal pelvic floor structures. In our study, we found that the CUV, CUS, CUT, UV, US, and UT decreased significantly with parity while UIA increased significantly with parity (Table S2). This indicates that a higher number of deliveries adversely affects the morphological characteristics of the compressor urethrae and urethra. However, the association between age and UIA was not significant.
The clinical implications of this study are as follows. The urethral sling procedure is the gold-standard treatment for SUI, with the middle urethra being the common position selected for this procedure. First, our study provides a reference for the volume, thickness, and surface area of the upper, middle, and lower urethra in terms of urethral sling placement. Second, the material of the urethral sling can be chosen on the basis of these morphological parameters. We additionally found that the compressor urethrae may play a role in female urinary continence. The volume, thickness, and surface area of compressor urethrae in the control group were significantly larger than those in the postpartum SUI group. Our findings constitute a noninvasive reference for the diagnosis of SUI, offering an alternative to urodynamic examination, which is invasive, costly, and potentially damaging. Finally, we examined the various morphological parameters of the compressor urethrae and found that it can be used as an entry point in the treatment of SUI.
This study involves three principal limitations. First, we employed a retrospective design, which may involve inherent biases. Second, the sample size of the study was not large, but it will be expanded in subsequent studies to clarify the relationship between the compressor urethrae and SUI. Finally, the urethra was studied as a whole based on the characteristics derived through images, and thus, a distinction could not be made between the internal and external urethral sphincters.
Conclusions
This study provides the reference criteria for different volumes, thicknesses, and surface areas of the compressor urethrae and urethra, along with their different segments. The upper and middle urethra may be more relevant to female urinary continence than the lower urethra. Moreover, strengthening the compressor urethrae might help ameliorate female urinary continence. The morphological characteristics of the compressor urethrae and urethra may change with age.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-695/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-695/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-695/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2024-695/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Institutional Review Board (IRB) of the Jinhua People’s Hospital (No. IRB-2021016-R). The requirement for individual consent was waived due to the retrospective nature of the analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dai S, Chen H, Luo T. Prevalence and factors of urinary incontinence among postpartum: systematic review and meta-analysis. BMC Pregnancy Childbirth 2023;23:761. [Crossref] [PubMed]
- Wang C, Wei W, Ma D, et al. Prevalence and Determinants of Stress Urinary Incontinence in Middle-Aged and Older Women: A Systematic Review and Meta-Analysis. Arch Esp Urol 2025;78:46-55. [Crossref] [PubMed]
- Zhang L, Wang X, Hou X, et al. Assessment of lower urinary tract symptoms 6 weeks after delivery and the relationship of pelvic floor muscle function. Front Glob Womens Health 2024;5:1416429. [Crossref] [PubMed]
- Pietrus M, Pityński K, Socha MW, et al. Enhanced Non-Invasive Diagnosis of Female Urinary Incontinence Using Static and Functional Transperineal Ultrasonography. Diagnostics (Basel) 2024;14:2549. [Crossref] [PubMed]
- Stein TA, DeLancey JO. Structure of the perineal membrane in females: gross and microscopic anatomy. Obstet Gynecol 2008;111:686-93. [Crossref] [PubMed]
- Lo TS, Kamarudin M, Sun MJ, et al. Predictors and outcomes of Mid-urethral sling continence surgeries for stress urinary incontinence among Taiwanese women: What works best? Taiwan J Obstet Gynecol 2024;63:826-35. [Crossref] [PubMed]
- Guan X, Wang F, Zhang D, et al. Mid-urethral sling with proper sling tension is an effective treatment for stress urinary incontinence in women after pelvic radiotherapy: a pilot study of case series. Front Surg 2024;11:1475030. [Crossref] [PubMed]
- Ling C, Shek KL, Gillor M, et al. Is location of urethral kinking a confounder of association between urethral closure pressure and stress urinary incontinence? Ultrasound Obstet Gynecol 2021;57:488-92. [Crossref] [PubMed]
- Grosso AA, Di Maida F, Lambertini L, et al. Three-dimensional virtual model for robot-assisted partial nephrectomy: a propensity-score matching analysis with a contemporary control group. World J Urol 2024;42:338. [Crossref] [PubMed]
- Grosso AA, Di Maida F, Tellini R, et al. Robot-assisted partial nephrectomy with 3D preoperative surgical planning: video presentation of the florentine experience. Int Braz J Urol 2021;47:1272-3. [Crossref] [PubMed]
- Ma X, Li Y, Xu S, et al. The analysis of different types of anorectal abscesses was conducted using the MRI 3D reconstruction technique. Sci Rep 2024;14:18473. [Crossref] [PubMed]
- Feng Y, Wu J, Zhu H, et al. Three-dimensional measurement and analysis of benign prostatic hyperplasia. Transl Androl Urol 2021;10:2384-96. [Crossref] [PubMed]
- Feng Y, Zhang S, Zhou Y, et al. Three-dimensional measurement and analysis of morphological parameters of the uterus in infertile women. Quant Imaging Med Surg 2022;12:2224-37. [Crossref] [PubMed]
- Shi Q, Gu Z, Lai D, et al. Three-dimensional evaluation of condylar morphology after orthodontic treatment in adult patients with Class II malocclusion by cone-beam computed tomography. BMC Oral Health 2024;24:48. [Crossref] [PubMed]
- Xiong C, Ren Z, Li X, et al. Orbital computed tomography imaging characteristics of thyroid-associated ophthalmopathy. Sci Rep 2024;14:28960. [Crossref] [PubMed]
- Shao FX, He P, Mao YJ, et al. Association of pre-pregnancy body mass index and gestational weight gain on postpartum pelvic floor muscle morphology and function in Chinese primiparous women: A retrospective cohort study. Int J Gynaecol Obstet 2025;168:680-92. [Crossref] [PubMed]
- DeLancey JO. Structural aspects of the extrinsic continence mechanism. Obstet Gynecol 1988;72:296-301. [PubMed]
- Li JR, Lei L, Luo N, et al. Architecture of female urethral supporting structures based on undeformed high-resolution sectional anatomical images. Anat Sci Int 2021;96:30-41. [Crossref] [PubMed]
- Wang Y, Yao J, Chen N, et al. Study of female pelvic floor muscle in overactive bladder based on MRI 3D reconstruction. BMC Urol 2022;22:132. [Crossref] [PubMed]
- Wu Y, Dabhoiwala NF, Hagoort J, et al. Architectural differences in the anterior and middle compartments of the pelvic floor of young-adult and postmenopausal females. J Anat 2017;230:651-63. [Crossref] [PubMed]
- DeLancey JO. Correlative study of paraurethral anatomy. Obstet Gynecol 1986;68:91-7. [PubMed]
- Shi L, Zhao Y, Li W, et al. Evaluation of pelvic structural abnormalities in primiparous women with stress urinary incontinence. Int Urogynecol J 2024;35:369-80. [Crossref] [PubMed]
- Franchi M, Uccella S, Zorzato PC, et al. Vaginal flap for urethral neomeatus reconstruction after radical surgery for vulvar cancer: a retrospective cohort analysis. Int J Gynecol Cancer 2019;29:1098-104. [Crossref] [PubMed]
- Rostaminia G, White DE, Quiroz LH, et al. Visualization of periurethral structures by 3D endovaginal ultrasonography in midsagittal plane is not associated with stress urinary incontinence status. Int Urogynecol J 2013;24:1145-50. [Crossref] [PubMed]
- Reid GC, DeLancey JO, Hopkins MP, et al. Urinary incontinence following radical vulvectomy. Obstet Gynecol 1990;75:852-8. [PubMed]
- Venema PL, Heesakkers JP, de Vries AM, et al. The female urethral closure mechanism during physical stress. Neurourol Urodyn 2024;43:1647-54. [Crossref] [PubMed]
- Guo X, Ding C, Zhang S. 4D Transperineal Ultrasound for the Diagnosis and Classification of Stress Urinary Incontinence in Postmenopausal Women. J Coll Physicians Surg Pak 2023;33:438-42. [Crossref] [PubMed]
- Martin LC, Routzong MR, Abramowitch SD, et al. Effect of Squeeze, Cough, and Strain on Dynamic Urethral Function in Nulligravid Asymptomatic Women: A Cross-Sectional Cohort Study. Urogynecology (Phila) 2023;29:740-7. [Crossref] [PubMed]
- Mao YJ, Zheng ZJ, Xu JH, et al. Pelvic floor biometry in asymptomatic primiparous women compared with nulliparous women: a single-center study in Southern China. J Int Med Res 2020;48:300060520920393. [Crossref] [PubMed]
- Minardi D, Piloni V, Amadi A, et al. Correlation between urodynamics and perineal ultrasound in female patients with urinary incontinence. Neurourol Urodyn 2007;26:176-82; discussion 183-4. [Crossref] [PubMed]
- Mansy I, Elsayed D, Saafan A, et al. Transobturator hybrid tape versus synthetic tape in treatment of female stress urinary incontinence: A prospective randomized clinical study. Urologia 2025;92:154-60. [Crossref] [PubMed]
- Lo TS, Ng KL, Lin YH, et al. Impact of intrinsic sphincter deficiency on mid-urethral sling outcomes. Int Urogynecol J 2022;33:887-96. [Crossref] [PubMed]
- Dinh Au H, Dung VT, Hien MM, et al. Evaluation of anatomical factors affecting stress urinary incontinence in female patients via dynamic pelvic floor magnetic resonance imaging. Clin Ter 2023;174:491-7. [PubMed]
- Komemushi Y, Komemushi A, Morimoto K, et al. Quantitative evaluation of age-related changes to pelvic floor muscles in magnetic resonance images from 369 patients. Geriatr Gerontol Int 2019;19:834-7. [Crossref] [PubMed]
- Castelán F, Cuevas-Romero E, Martínez-Gómez M. The Expression of Hormone Receptors as a Gateway toward Understanding Endocrine Actions in Female Pelvic Floor Muscles. Endocr Metab Immune Disord Drug Targets 2020;20:305-20. [Crossref] [PubMed]
- Christmas MM, Iyer S, Daisy C, et al. Menopause hormone therapy and urinary symptoms: a systematic review. Menopause 2023;30:672-85. [Crossref] [PubMed]
- Chen Y, Zhang F, Zheng P, et al. Postpartum Stress Urinary Incontinence: Current Advances in Non-Pharmacological Therapies. Arch Esp Urol 2025;78:1-9. [Crossref] [PubMed]
- Lai F, Liu H, Wang H. Effect of a Nurse-Led Remote Guided Pelvic Floor Exercise Program on Stress Urinary Incontinence, Pelvic Floor Function and Sexual Function in Patients after Total Hysterectomy: A Retrospective Study. Arch Esp Urol 2024;77:992-8. [Crossref] [PubMed]
- Zhang L, Zhao S, Wu S, et al. Application of Four-Dimensional Pelvic Floor Ultrasound in the Diagnosis of Postpartum Pelvic Floor Dysfunction and Evaluation of Curative Effect. Altern Ther Health Med 2024;30:294-9. [PubMed]
- Al-Dossari R, Kalra M, Adkison J, et al. Non-Surgical Management of Urinary Incontinence. J Am Board Fam Med 2024;37:909-18. [Crossref] [PubMed]
- Yu H, Zheng H, Zhang X, et al. Association between elastography findings of the levator ani and stress urinary incontinence. J Gynecol Obstet Hum Reprod 2021;50:101906. [Crossref] [PubMed]
(English Language Editor: J. Gray)


