
Magnetic resonance imaging for computer-assisted device guidance in transperineal prostate biopsy and cryoablation
Highlight box
Key findings
• Precise and consistent magnetic resonance imaging (MRI)-guided needle placement is feasible using a minimal, easy to use, and readily replicable toolset.
• The developed toolset, with its inexpensive cost and low barriers to entry, supports ready implementation at new sites that may lack prior interventional MRI experience.
What is known and what is new?
• Well-targeted cryoablation can lead to significant and lasting drops in prostate-specific antigen, and precisely targeted biopsies yield more reliable diagnostic results; however, methods for achieving this accuracy are largely developed in-house at each clinical site that offers these services. While advanced robotic systems exist, they are seldom seen outside of the handful of large research centers that focus on robotic development, leading to a gap in available service between healthcare providers with active research departments and those without.
• A minimal toolkit, relying on only a simple rigid plastic targeting grid and a small software package, was able to consistently attain accurate needle placements in 24 procedures. This design is more readily fabricated and utilized compared to existing solutions, and has been made accessible online.
What is the implication, and what should change now?
• With the necessary software and hardware schematics available freely on the corresponding author’s Github (https://github.com/tlilieholm), new centers seeking to implement their own interventional magnetic resonance prostate program may attempt to do so without needing to develop their own solutions or purchase costly commercial products.
Introduction
Background
Magnetic resonance imaging’s (MRI) superior soft tissue contrast, volumetric imaging capabilities, and ability to monitor therapies in real-time make it well-suited for image-guided interventional procedures. Its sensitivity to perfusional and compositional tissue changes and ability to visualize regions of temperature change during thermal ablation make it particularly apt for prostate cancer detection and treatment (1,2). However, its high-strength magnetic fields necessitate specialized equipment, while the narrow bore employed by most scanner designs severely limits accessible space during an intervention (3,4). Advances in magnetic resonance (MR) coils and compatible tools have begun to allow simplified prostate access via a transperineal, rather than transrectal, approach. Concurrently, cryoablation is becoming an increasingly popular treatment for intermediate risk prostate cancers, patients for whom prior therapies limit surgical or radiation-based approaches, or those who wish to avoid surgery or radiation therapy (5,6). Cryoablation has a much faster treatment cycle than radiotherapy and a shorter recovery time than prostatectomy, all with fewer morbidities and greater potential for salvage therapy (5,7-10). The seminal cryoablation service used a unique external visualization platform for device guidance that was unavailable to other institutions and is no longer supported (5,6).
Rationale and knowledge gap
While monitoring procedures with MRI is straightforward through diagnostic gradient-recalled imaging, adoption of MR-guided transperineal procedures has been limited by a lack of complementary hardware and software for assisting clinicians in safe and rapid insertion of needles into the prostate (4). Some sites employ highly skill-dependent freehand needle placement, assisted by real-time imaging and/or cognitive fusion expertise developed through experience (11). For consistent and precise needle placement, some robotic systems have been developed, but these require significant capital investment in acquisition, setup, and maintenance, while being somewhat narrow in usage (12,13). There have been attempts to integrate these robotic systems into standard clinical practice, but, as of yet, none have succeeded in sustained commercial viability (14). Some sites have developed and shared their own guidance solutions as modules of existing research software platforms, but these approaches strictly rely upon specific corresponding hardware, which is not readily available (15,16).
Interventionalists often encounter limitations in implementing new procedures when dedicated on-site engineering support, either from their institution or ablation equipment providers, is not feasible. For adopters’ accessibility, there is a need for tools that are easy to fabricate, simple to learn, usable within the confines of closed bore scanners, and compatible with major MRI vendors. Grid-like device designs have generally been successful in this purpose, across multiple body sites (5,17-19). The transperineal approach simplifies the trajectory to the prostate by avoiding sensitive structures, while also offering a noted reduced risk of infection (20). Utilization of MR allows use of a single imaging modality, eliminating the cognitive and technological complexity of fusing non-contemporaneous multimodal images and reducing the amount of equipment necessary. Simply put, there are 3–5 large research institutions that have made many impressive technological advancements in image-guided prostate interventions over the past decade, yet these transformative clinical innovations are disproportionately underrepresented among virtually all other healthcare providers. Improved accessibility, via a minimal and readily available hardware/software ecosystem, is necessary to see the kind of growth that is warranted by all of the efforts research leaders have put into this clinical domain.
Objective
Providers of MR-compatible cryoablation tools currently place the responsibility of intraprocedural placement/guidance on the interventionalist. Without guidance, some needles are likely to require repositioning, causing unnecessary percutaneous punctures per reinsertion. The goal of this methodology is to maximize efficiency and accuracy to assist with timely and safe completion of these procedures.
While the treatment space for minimally-invasive prostate interventions is well-developed, new advancements have trended towards increasing procedural and technological complexity to achieve clinical gains in an area where mortality and significant morbidities are already low. There is space for iteration on existing techniques such that complexity is instead reduced while still maintaining clinical efficacy. This is an investigation into the feasibility of a low-investment, easy-to-use toolset for transperineal interventions. The accuracy of this needle-guidance system is demonstrated in 10 phantom trials, and its efficacy in 14 cryoablation procedures, 9 biopsies, and 1 fluid aspiration. We present this article in accordance with the SUPER reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-2024-635/rc).
Methods
Device and software implementation
This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. All data collection involving human subjects was performed with approval from UW Health’s Internal Review Board, under IRB protocol number 2016-1346. All patients consented to the collection and use of their data for research purposes. A plastic targeting grid patterned on a prefabricated design was used to localize, orient, and support needles as they were inserted into soft tissue, following the workflow outlined in Figure 1A. Figure 1B displays the device’s simple design—an array of 13×13 indexed holes, spaced 5 mm apart, makes the face of the grid, which is placed adjacent to the patient, as shown in Figure 1C. The colored circles in Figure 1 denote a set of 3 saline-filled fiducials for use in registering the device based on geometric MR data, visualized in Figure 1D.

The software package and corresponding specifications of the grid device are available for download on GitHub (https://github.com/tlilieholm), along with usage instructions. The complementary software package was developed in Python, relying only on the Numpy library with additional visualization options through the Matplotlib library. The software package is modest in size and requires only basic support to run on standard computer operating systems.
Phantom trials
All phantom trials were performed using a 3T General Electric (GE) SIGNA Premier scanner (Waukesha, WI, USA) with a GE 48-channel head coil receiver. The bespoke phantoms consisted of an open-faced container filled with a water-based gelatine mixture containing 5–10 randomly distributed inclusions. In simulated procedures, the phantom was oriented such that the open side of the container faced the targeting grid, approximating a patient’s perineum (Figure 2A). Then, a scan (0.47 mm × 0.47 mm × 0.7 mm resolution T2w CUBE/SPACE/RARE) containing the grid’s 3 registration fiducials and the volume of the phantom was acquired. Picking 10 target voxels within inclusions, users applied the developed software to identify appropriate grid holes and depths of insertion to drive needles into the inclusions. After each insertion, an additional validation scan was taken (Figure 2B,2C) and the radial discrepancy between the exact targeted voxel and actual needle placement was recorded, as has been reported in other works (21).

Patient preparation and positioning
Demographics for the 24 patients are consolidated in Table 1. All subjects were men over 50 years, on average aged 71±7 years. Among them, 9 had been subject to previous treatments for prostate cancer. New patients were enrolled with referral from partners in radiation oncology or urology, or via self-referral. Each patient was reviewed by the multidisciplinary focal therapy prostate team consisting of one interventional radiologist (IR) and two urologists and was seen in-clinic or virtually by both urology and IR. Appropriate candidates were scheduled and consented for the procedure under a submitted and approved IRB protocol to allow MRI data to be manually entered into grid software, running on separate hardware from the scanner, for determination and validation of projected trajectories.
Table 1
| Patient ID | Age (years) | Procedure type | Previous prostate treatments |
|---|---|---|---|
| 1 | 74 | Biopsy | None |
| 4 | 65 | Biopsy | None |
| 6 | 51 | Biopsy | None |
| 8 | 56 | Biopsy | None |
| 9 | 81 | Biopsy | Prostatectomy |
| 17 | 69 | Biopsy | None |
| 19 | 69 | Biopsy | None |
| 20 | 67 | Biopsy | Proctectomy |
| 23 | 64 | Biopsy | None |
| 2 | 74 | Cryoablation | External beam radiotherapy |
| 3 | 71 | Cryoablation | Brachytherapy |
| 7 | 82 | Cryoablation | External beam radiotherapy |
| 10 | 76 | Cryoablation | External beam radiotherapy |
| 11 | 71 | Cryoablation | None |
| 12 | 77 | Cryoablation | External beam radiotherapy |
| 13 | 72 | Cryoablation | None |
| 14 | 80 | Cryoablation | None |
| 15 | 72 | Cryoablation | Brachytherapy |
| 16 | 74 | Cryoablation | None |
| 18 | 74 | Cryoablation | None |
| 21 | 72 | Cryoablation | Brachytherapy |
| 22 | 66 | Cryoablation | None |
| 24 | 77 | Cryoablation | None |
| 5 | 77 | Fluid aspiration | None |
All procedures were performed under general anesthesia in a closed-bore 1.5T General Electric 450W scanner with a 70 cm diameter bore (Waukesha, WI, USA). Before entering the bore, patients were placed in a lithotomy-like position, as shown in Figure 1C, with the transperineal targeting grid (Figure 1B) flush to the patient’s perineum and the grid’s base held firm by their body weight. The patients’ legs were elevated, allowing interventionalists to access the rear face of the grid (Figure 1C) and insert needles through its channels. Inserted needles will pass through 6–8 cm of musculature and adipose tissue before reaching the prostate. The patient remains in this position, with their legs elevated, through the entirety of the procedure. For all patients in the cohort, there was sufficient space within the closed bore General Electric 450W scanner to permit this. Other patient positions, such as prone or supine without raised legs, did not offer sufficient access for a transperineal trajectory.
Scanning and targeting
Once the device and patient are positioned correctly, a diagnostic-quality (e.g., high contrast and spatial resolution) T2w fast spin echo (FSE) scan containing both the prostate and the grid’s three, saline-filled fiducials were acquired (Figure 1D) (3). For lesion identification and localization, additional apparent diffusion coefficient (ADC), diffusion, and T1w contrast-enhanced (CE) scans were acquired as well. All of these sequences are standard and will be available on virtually all commercial, diagnostic MR scanners. The standard scanner interface reports spatial coordinates of an acquired image relative to the scan volume isocenter, along the anterior/posterior, right/left, and superior/inferior axes. Suspected malignancies, as in Figure 3, are identified before prescribing an intervention. The spatial coordinates of the grid’s three fiducials and any identified targets within the prostate are entered into the developed, complementary software. Using these data points and prior geometric knowledge of the grid’s design, the software performs a least-squares optimized rigid registration of the coordinates observed in the scan (Magnet Space) to a theoretical coordinate system fully orthogonal to the grid face (Grid Space) (22). This mirrors methodologies commonly employed in stereotactic procedures. Grid Space is orthogonal to the orientation of and centered on the grid- any target point in the prostate may be transformed into Grid Space, backprojected a known distance onto the grid face, and binned into the closest appropriate labeled hole. This 3D registration enables more degrees of freedom in the positioning of the grid and creation of slightly oblique trajectories that may sometimes be appropriate.

Cryoablation
After the interventionalist determined the appropriate grid hole and depth of insertion via scanning and the trajectory-calculating software, 14 G 5.25 inch Angiocaths IV Catheters (BD, Franklin Lakes, NJ, USA) were inserted through the grid holes into the tissue. Then, Boston Scientific VISUAL ICE MRI Cryoprobes (Boston Scientific, Boston, MA, USA) were inserted coaxially through the IV catheters. Needle positioning, post-insertion, was verified with MR imaging. If repositioning was needed, the angiocatheter was removed and reinserted into an adjacent grid hole, or the same hole with a different orientation, such that it would track differently through the tissue. Once the interventionalist determined that all inserted probes were properly positioned, the associated gas compression system cooled the inserted cryoprobes to freezing temperatures via Argon gas in the Joule-Thompson effect.
As iceballs grew around the active region of each cryoprobe, their highly visible signal void was monitored via standard real-time GE sequences to prevent encroachment on sensitive anatomy while sufficiently encompassing the desired treatment area. Figure 4 displays an example of one such sculpted signal void. To further protect sensitive structures, a warming catheter was used to counteract undesired cooling of the urethral urothelium. When necessary, hydrodissection needles, placed using the same targeting techniques, were used to shift local structures to achieve broader margins about the ablation target. Following previous investigations, three cycles of tissue freezing and active thawing were performed over the same ablation volume (5). Active thawing was achieved through the same gas compression system, using Helium gas instead. These cycles took 20–60 minutes, typically 5 minutes freezing and 5 thawing, repeated three times, though durations varied depending on the number of cryoprobes and total volume of ablated tissue. After the final thaw, treatment was completed and the needles were removed. One last round of post-procedural imaging was acquired to verify that the ablated region covered all identified lesions, plus a small margin, and identify any immediate complications (e.g., local bleeding, ablation encroachment on the rectum, etc.). All cryoablation patients were discharged after a planned overnight hospital stay.

Biopsy
After performing initial scans for registration and target selection, one to two 14 G 5.25 inch IV catheters were inserted through grid holes selected by interventionalists using the initial scanning and trajectory-calculating software, as noted for cryoablation procedures. Invivo 18G automatic core biopsy guns (Philips Medical Systems, Netherlands) were inserted through the IV catheters and their troughs deployed. An additional scan verifying the location of the trough was acquired (Figure 3, right). Once positioning was found satisfactory, samples were taken at 12, 3, 6, and 9 o’clock positions—four samples per needle. If multiple biopsy sites were identified, this was repeated in batches of 1–2 needles until the desired set of locations was sampled. All biopsies were outpatient procedures.
Needle tracking metrics are relevant in biopsy, aspiration, and cryoablation, with the needle placement dictating ablation coverage in cryoablation. Tracking targeting error, as was done with the phantom trials, is less descriptive for needle insertions in in vivo tissues due to the confounding factor of needle deflection (23). Since, for procedural efficacy, inaccurate needle placement is not permissible, tracking the number of repeat needle insertions/adjustments required to attain satisfactory placement was considered a reasonable metric instead. This work is concerned, predominantly, with the mechanics of achieving technical success during a procedure. Prior work has shown that accurate needle placement begets clinical success through sufficient iceball coverage (5,24). Technical success, achieved in every procedure, was defined by clinicians’ satisfaction with needle placement within pre-identified pathologies. For cryoablations, a determination of technical success required production of an ablating iceball that covered all observed lesions plus a small margin, with appropriate spacing (<1 cm) between needles to ensure uniformity and cohesion in the ice. At the end of every procedure, the IR and urologist confirmed technical success (a visibly ablated lesion) with an additional post-treatment acquisition. Figure 3 displays the various MRI sequences used to guide target selection and assess needle placement.
Results
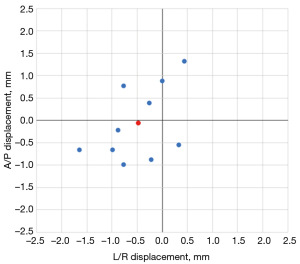
In phantoms, inserted needles achieved a mean radial positioning error of 1.05 mm (max 1.78 mm, min 0.47 mm) with a standard deviation of 0.38 mm. These measurements were derived from high-resolution (0.47 mm × 0.47 mm × 0.7 mm) post-insertion validation scans comparing the distance between targeted voxels and the centers of inserted needles. These displacements are represented in Figure 5.
Implementing the proposed methods clinically, 24 procedures have been completed to date. These included 3 different types of interventions in the prostate region: 14 cryoablations, 9 biopsies, and 1 fluid aspiration. A median procedure saw 5 needle (IV catheter) insertions, with exact numbers varying on the number and extent of identified lesions. Figure 4 displays, for example, how a 3-needle cryoablation procedure, displayed at multiple timepoints, can create a contoured iceball to overlay an ellipsoidal lesion. A per-patient summary of this data is available in Table 2, which shows that needle guidance resulted in a satisfactory placement on the first attempt in 81.8% of insertions in an average (median) procedure. Unsatisfactory first attempt, needles were removed and reinserted until satisfactory, such that all procedures were completed successfully. Needle deflection at tissue interfaces was a common cause of unsatisfactory placement due to deviated trajectories. Three patients reported three mild post-procedural adverse events which did not require additional intervention: mild decrease in sexual function, mild urinary leakage which did not require wearing an incontinence pad, and mild frostbite from the cryoneedle hub resting on the patient’s inner thigh due to the patient’s body habitus (25).
Table 2
| Patient ID | Procedure type | Needles inserted | Needles readjusted | Single insertion, % |
|---|---|---|---|---|
| 1 | Biopsy | 11 | 4 | 63.64 |
| 4 | Biopsy | 2 | 0 | 100 |
| 6 | Biopsy | 10 | 2 | 80 |
| 8 | Biopsy | 11 | 2 | 81.82 |
| 9 | Biopsy | 1 | 0 | 100 |
| 17 | Biopsy | 11 | 2 | 81.82 |
| 19 | Biopsy | 13 | 1 | 92.31 |
| 20 | Biopsy | 6 | 1 | 83.33 |
| 23 | Biopsy | 4 | 0 | 100 |
| 2 | Cryoablation | 8 | 4 | 50 |
| 3 | Cryoablation | 7 | 1 | 85.71 |
| 7 | Cryoablation | 4 | 2 | 50 |
| 10 | Cryoablation | 7 | 1 | 85.71 |
| 11 | Cryoablation | 5 | 3 | 40 |
| 12 | Cryoablation | 5 | 0 | 100 |
| 13 | Cryoablation | 4 | 0 | 100 |
| 14 | Cryoablation | 4 | 0 | 100 |
| 15 | Cryoablation | 4 | 1 | 75 |
| 16 | Cryoablation | 4 | 4 | 0 |
| 18 | Cryoablation | 5 | 2 | 60 |
| 21 | Cryoablation | 3 | 2 | 33.33 |
| 22 | Cryoablation | 4 | 1 | 75 |
| 24 | Cryoablation | 5 | 1 | 80 |
| 5 | Fluid aspiration | 1 | 0 | 100 |
| Median | – | 5 | 1 | 81.82 |
Discussion
Key findings
The proposed tools and methods were validated in phantoms and then utilized effectively in 24 minimally invasive transperineal prostate interventions using image guidance acquired through a standard, closed-bore, MR scanner. The utilized tools are easy to fabricate, simple to learn, usable within the confines of closed bore scanners, cost-effective, and designed for the most common MRI-guided procedures in the prostate.
Strengths and limitations
This minimal system has seen successful application throughout the first 2 years of our institution’s nascent interventional MRI program. It is inexpensive to fabricate, easy to use, and convenient to store when not in use. The associated targeting software requires no direct communication with the scanner, allowing for simpler integration into clinical practice and fewer administrative hurdles associated with its use. That said, the system has some limitations shared with other trajectory guidance solutions.
The physical design of the grid only permits transperineal access. Its channels are separated with 5 mm spacing along both axes, meaning that, for certain targets, the closest possible trajectories can be as far as 3.5 mm from a targeted location. In cryoablations, this is generally acceptable given the way the ice expands around the cryoprobe. For particularly inauspicious biopsy cases, it may be necessary to adjust the position of the grid relative to the patient.
This study lacks long-term clinical outcomes. Prostate specific antigen (PSA) is the primary biomarker in the diagnosis of prostate cancer. Elevated levels in the blood warrant diagnostic imaging and/or biopsy, while a reduction in levels after treatment indicates therapeutic efficacy. Biochemical and imaging surveillance following treatment is imperative and generally performed around 3, 6, and 12 months post-procedure. Given the recency of the reported program’s establishment, not enough time has yet passed to support a dataset of long-term clinical outcomes of meaningful size.
Another limitation is needle deflection, which is common in these types of minimally invasive interventions, and particularly problematic for the prostate due to heterogeneous tissues and the high potential for scarring caused by prior interventions (23). Subjects for cryoablation often have recurrent cancers, which have previously been treated by radiotherapy or prostatectomy (Table 1). Both treatments result in local tissue changes, which nonuniformly alter the stiffness of the tissue. When asymmetric forces are experienced at tissue interfaces or other heterogeneities, deflection can occur. The detail provided by MR imaging clearly depicts device deflection, as in Figure 6, allowing clinicians to easily identify when deflection has occurred in a planned insertion and correct needle positioning as indicated. While alternative, sturdier needle designs may reduce deflection, few such options exist within the interventional MRI domain. All intraoperative tools for MRI-based interventions must, for patient safety, be MR-compatible, leading to a relatively narrow selection of viable tools, all designed subject to the same major constraints.

Comparison with similar research
Previous work has been done to support interventions within closed bore scanners, though many efforts have faced slow adoption due, in part, to complexity—either too many steps and supplemental tools, or too many degrees of freedom offered by a freehand approach (26,27). The methods proposed here are sufficiently rigid that they offer consistency for the decision-making processes of clinicians and assist with more consistent needle placement while being flexible enough to support a variety of pelvic and prostate interventions and transperineal trajectories. Though all of the procedures reported here concern the prostate and needle insertions near-collinear to the central axis of the bore, the software and hardware are general enough to function with non-collinear trajectories, offering potential application in other body sites.
Some sites instead employ an ultrasound-based workflow to perform cryoablation, relying on the modality’s significantly lower costs and greater accessibility (7). While this approach is decidedly more convenient, it sacrifices the full volumetric imaging capabilities of MRI, limiting the scope to which clinicians can visualize and target lesions, or monitor and control the tissue-ablating ice front. As more medical centers begin offering cryoablation services, this tradeoff between convenience and precision across imaging modalities warrants further investigation by stakeholders in the treatment space.
Using the grid and software can reduce needle repositioning, limiting complications and improving the efficiency of the procedure. Given the long baseline duration of MRI-guided interventions, procedural efficiency is important for decreasing anesthesia time and usage of a busy diagnostic scanner. While this workflow can improve efficiency and procedure length, it also provides a template for others to use when establishing an MRI intervention program. Given the lack of specific training in this area, sharing current best practices in this space can help develop additional programs and encourage broader adoption of MRI-guided interventions.
Conclusions
The proposed methodology offers accessible tools to simplify device guidance for MRI-guided procedures on the prostate and adjacent pelvic regions. This guidance technique encourages wider adoption of minimally invasive MRI therapies with its minimal capital investment and simple design. The hardware and software are designed to be easily understood and replicated, such that new sites will have low barriers to entry when developing their own interventional MRI programs.
Acknowledgments
We acknowledge David A. Woodrum, MD, PhD for his contributions to this work.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-635/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-635/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-635/prf
Funding: This research was supported by funding from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2024-635/coif). T.L. reports the funding from the University of Wisconsin Madison Biotechnology Training Program via National Institute of General Medical Sciences (No. NIH 5 T32 GM135066), which covered a stipend as a student for 1 year of the time he worked on this project; a travel grant to present this research at one conference in 2024 from the International Society of Magnetic Resonance in Medicine. T.L. and W.F.B. both have financial ties to a company, ImgGyd, that develops tools for image guided neurosurgery, which, at this point in time, has no connection to the prostate body site. W.F.B. also reports the salary support and RA support for setting up software and supporting clinical evaluation described in the manuscript from the University of Wisconsin Madison Radiology; grants for NIH Sub contract for detecting motion during brain scans and consulting fee as a Scientific advisory board from Turing Medical; Travel and hotel support for being on ISMRM Board of Trustees from International Society of Magnetic Resonance in Medicine. W.F.B. was the Ex-officio Board of Trustees of the International Society of Magnetic Resonance in Medicine. E.K.K. reports the Internal University of Wisconsin Radiology support for project software development. E.K.K. also participates in the Boston Scientific Medical Advisory Board. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. All data collection involving human subjects was performed with approval from our institution’s Internal Review Board, under IRB protocol number 2016-1346. All patients consented to the collection and use of their data for research purposes.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Giganti F, Kirkham A, Allen C, et al. Update on Multiparametric Prostate MRI During Active Surveillance: Current and Future Trends and Role of the PRECISE Recommendations. AJR Am J Roentgenol 2021;216:943-51. [Crossref] [PubMed]
- Ozhinsky E, Salgaonkar VA, Diederich CJ, et al. MR thermometry-guided ultrasound hyperthermia of user-defined regions using the ExAblate prostate ablation array. J Ther Ultrasound 2018;6:7. [Crossref] [PubMed]
- Thompson SM, Gorny KR, Koepsel EMK, et al. Body Interventional MRI for Diagnostic and Interventional Radiologists: Current Practice and Future Prospects. Radiographics 2021;41:1785-801. [Crossref] [PubMed]
- Barkhausen J, Kahn T, Krombach GA, et al. White Paper: Interventional MRI: Current Status and Potential for Development Considering Economic Perspectives, Part 1: General Application. Rofo 2017;189:611-23. [Crossref] [PubMed]
- Woodrum DA, Kawashima A, Karnes RJ, et al. Magnetic resonance imaging-guided cryoablation of recurrent prostate cancer after radical prostatectomy: initial single institution experience. Urology 2013;82:870-5. [Crossref] [PubMed]
- Woodrum DA, Gorny KR, Mynderse LA. MR-Guided Prostate Interventions. Top Magn Reson Imaging 2018;27:141-51. [Crossref] [PubMed]
- de Marini P, Cazzato RL, Garnon J, et al. Percutaneous MR-guided prostate cancer cryoablation technical updates and literature review. BJR Open 2019;1:20180043. [Crossref] [PubMed]
- Onik G. Percutaneous image-guided prostate cancer treatment: cryoablation as a successful example. Tech Vasc Interv Radiol 2007;10:149-58. [Crossref] [PubMed]
- Lee H, Thakker S, Pineault K, et al. Salvage Cryoablation for Recurrent Prostate Cancer Following Radiation-A Comprehensive Review. Cancers (Basel) 2024;16:2717. [Crossref] [PubMed]
- Pio F, Murdock A, Fuller RE, et al. The Role of Whole-Gland and Focal Cryotherapy in Recurrent Prostate Cancer. Cancers (Basel) 2024;16:3225. [Crossref] [PubMed]
- Gangi A, Tsoumakidou G, Abdelli O, et al. Percutaneous MR-guided cryoablation of prostate cancer: initial experience. Eur Radiol 2012;22:1829-35. [Crossref] [PubMed]
- Cornud F, Bomers J, Futterer JJ, et al. MR imaging-guided prostate interventional imaging: Ready for a clinical use? Diagn Interv Imaging 2018;99:743-53. [Crossref] [PubMed]
- Fischer GS, Iordachita I, Csoma C, et al. MRI-Compatible Pneumatic Robot for Transperineal Prostate Needle Placement. IEEE ASME Trans Mechatron 2008;13:295-305. [Crossref] [PubMed]
- Hata N, Moreira P, Fischer G. Robotics in MRI-Guided Interventions. Top Magn Reson Imaging 2018;27:19-23. [Crossref] [PubMed]
- Herz C, MacNeil K, Behringer PA, et al. Open Source Platform for Transperineal In-Bore MRI-Guided Targeted Prostate Biopsy. IEEE Trans Biomed Eng 2020;67:565-76. [Crossref] [PubMed]
- Tokuda J, Song SE, Tuncali K, et al. Configurable automatic detection and registration of fiducial frames for device-to-image registration in MRI-guided prostate interventions. Med Image Comput Comput Assist Interv 2013;16:355-62.
- Kaye EA, Granlund KL, Morris EA, et al. Closed-Bore Interventional MRI: Percutaneous Biopsies and Ablations. AJR Am J Roentgenol 2015;205:W400-10. [Crossref] [PubMed]
- Tokuda J, Tuncali K, Iordachita I, et al. In-bore setup and software for 3T MRI-guided transperineal prostate biopsy. Phys Med Biol 2012;57:5823-40. [Crossref] [PubMed]
- Taneja S, Jena A, Kumar K, et al. Technical Note: MRI-guided breast biopsy - our preliminary experience. Indian J Radiol Imaging 2010;20:218-20. [Crossref] [PubMed]
- Miller J, Perumalla C, Heap G. Complications of transrectal versus transperineal prostate biopsy. ANZ J Surg 2005;75:48-50. [Crossref] [PubMed]
- Olsen ME, Brodsky EK, Oler JA, et al. Real-time trajectory guide tracking for intraoperative MRI-guided neurosurgery. Magn Reson Med 2023;89:710-20. [Crossref] [PubMed]
- Arun KS, Huang TS, Blostein SD. Least-squares fitting of two 3-d point sets. IEEE Trans Pattern Anal Mach Intell 1987;9:698-700. [Crossref] [PubMed]
- Abolhassani N, Patel RV. Deflection of a flexible needle during insertion into soft tissue. Conf Proc IEEE Eng Med Biol Soc 2006;2006:3858-61. [Crossref] [PubMed]
- Moreira P, Tuncali K, Tempany CM, et al. The Impact of Placement Errors on the Tumor Coverage in MRI-Guided Focal Cryoablation of Prostate Cancer. Acad Radiol 2021;28:841-8. [Crossref] [PubMed]
- Khalilzadeh O, Baerlocher MO, Shyn PB, et al. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol 2017;28:1432-1437.e3. Erratum in: J Vasc Interv Radiol 2018;29:146. [Crossref] [PubMed]
- Busse H, Garnov N, Thörmer G, et al. Flexible add-on solution for MR image-guided interventions in a closed-bore scanner environment. Magn Reson Med 2010;64:922-8. [Crossref] [PubMed]
- Rothgang E, Gilson WD, Wacker F, et al. Rapid freehand MR-guided percutaneous needle interventions: an image-based approach to improve workflow and feasibility. J Magn Reson Imaging 2013;37:1202-12. [Crossref] [PubMed]


