
Regulator of cullins-1 predicts a poor prognosis and regulates epithelial-mesenchymal transition process through GSK-3β/Wnt signaling in renal cell carcinoma
Highlight box
Key findings
• The study indicates that regulator of cullins-1 (ROC1) with a high expression in renal cell carcinoma (RCC) predicts an unfavorable prognosis. ROC1 plays an essential role in RCC progression and epithelial-mesenchymal transition (EMT), and our study demonstrates for the first time that ROC1 regulates the GSK-3β/Wnt/β-catenin signaling.
What is known and what is new?
• ROC1 with a high expression in RCC predicts an unfavorable prognosis.
• ROC1 regulates RCC progression and EMT by regulating the GSK-3β/Wnt/β-catenin signaling.
What is the implication, and what should change now?
• Our work preliminarily illuminated the tumor-promoting role of ROC1 in RCC and the potential molecular mechanism, which may provide some evidence for the treatment of RCC.
Introduction
Renal cell carcinoma (RCC) generally refers to a series of histologically heterogeneous tumors occurring in renal parenchyma and renal pelvis (1). RCC represents the malignancy derived from renal parenchymal epithelial cells, accounting for more than 90% of RCC cases. The incidence rate and mortality rate increase by at 2–3% every 10 years worldwide (2). Although the early diagnosis rate of RCC is increasing, about 20–30% of patients are in the middle and advanced stage at the time of diagnosis. Even after surgical resection, 20% of patients still relapse and metastasize during follow-up. In recent decades, the application of molecular targeted drugs and immune checkpoint inhibitors targeting the signaling pathway of RCC has opened a new page to treat RCC (3). But the drug resistance of targeted therapy and the side effects of immunotherapy have significantly affected the clinical efficacy. Most of these treatment methods inhibit tumor progression by depriving the nutrients of tumor microenvironment, However, they cannot weaken the malignant biological characteristics of RCC cell invasion and metastasis.
Epithelial-mesenchymal transition (EMT) is a biological development process, which contributes to cell proliferation and tissue repair, but can also cause tissue and organ fibrosis (4). It is characterized by the loss of epithelial connexin (such as E-cadherin) and the increase of mesenchymal phenotypic protein (such as vimentin), which plays an important role in tumor invasion and metastasis (5). Many cytokines and small molecular mediators are involved in the initiation of EMT process, including the activation of transcription factors, the high expression of specific cell surface receptors, the reorganization of cytoskeleton proteins and the production of extracellular matrix degrading enzymes. GSK-3β/Wnt signaling has been discovered with an important effect on EMT (6).
Regulator of cullins-1 (ROC1), which is referred to as RING box protein-1 (RBX1) as well, accounts for the critical cullin-RING ubiquitin ligase (CRL) subunit responsible for heterodimerization with additional cullins for forming CRL catalytic core (7). ROC1 possesses one small zinc-binding domain (RING finger) with high evolutionary conservation degree, and it has an important role in embryogenesis. Abnormal ROC1 level results in CRL impairment as well as embryonic fatalness. Besides, ROC1 plays a critical role in maintaining tumor progression and genome integrity. We previously reported that ROC1 promoted bladder cancer malignant development through modulating p-IκBα/NF-κB pathway (8). Wang et al. reported that ROC1 had negative effect on regulating SUFU (the regulator of Gli2) for activating hedgehog pathway within bladder cancer (7).
The present work detected ROC1 level within RCC as well as the clinical significance. The effect of ROC1 on EMT process in renal cancer cells was also detected. In addition, we proposed hypothesis that ROC1 regulated the EMT through GSK-3β/Wnt signaling, which was also verified. By constructing the RCC metastasis model in nude mice, the effect of ROC1 knockdown on tumor metastasis and the underlying mechanism were both investigated. We present this article in accordance with the MDAR and ARRIVE reporting checklists (available at https://tau.amegroups.com/article/view/10.21037/tau-2024-646/rc).
Methods
Clinical tissue specimens
Fifty-six paired RCC specimens and adjacent non-tumor tissues were collected from surgical tumor resections performed in The Lishui Hospital of Wenzhou Medical University from October 2014 to September 2017. The pathological morphology had been reviewed by the pathologist of The Lishui Hospital of Wenzhou Medical University and conformed that there was no renal cancer in the adjacent non-tumor tissues. Tissue samples were subject to liquid nitrogen snap-freezing and preservation under −80 ℃ till later analyses. Each tissue was collected from patients with informed consent provided. This work was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Our study protocols gained approval from the Institutional Ethical Review Committee in The Sixth Affiliated Hospital of Wenzhou Medical University (No. 2019-61).
Cell lines and cell culture
We obtained human renal cancer cell lines including 768-0 and A498 in Shanghai Institutes for Biological Sciences Cell Resource Center (Shanghai, China), cultivated them within Dulbecco’s modified Eagle medium (DMEM)/F12 that contained 10% fetal bovine serum (FBS), and incubated them under 37 ℃ and 5% CO2 conditions.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
We utilized TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA) for extracting total RNA from cells in line with specific instructions. To examine microRNA (miRNA) level, we conducted RT-qPCR with TaqMan Universal PCR Master Mix and TaqMan miRNA Reverse Transcription Kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). For ROC1 expression analysis, RT-qPCR was performed by using the TaqMan High-Capacity cDNA Reverse Transcription Kit and TaqMan Fast PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions with corresponding primers: ROC1 forward, 5'-GTTCTATTCGTGGTGCTAGGT-3' and reverse, 5'-AGTGCCTTTCAACTTATCCCT-3'; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward, 5'-TCCTCTGACTTCAACAGCGACAC-3' and reverse, 5'-CACCCTGTTGCTGTAGCCAAATTC-3'. The 2−[(Ct of GENES) − (Ct of GAPDH)] method was applied in determining gene levels relative to GADPH.
Cell transfection
We constructed three small interfering RNA (siRNA) that targeted ROC1 (siROC1#1–3), together with negative control siRNAs within pLKO.1. Afterwards, we transfected cells (70–90% confluence) with those as-constructed plasmid constructs by adopting Lipofectamine 2000 (Invitrogen), followed by additional 24-h transfection. We acquired siRNA/short hairpin RNA (shRNA) targeting GSK-3β (siGSK-3β/shGSK-3β), together with relevant controls in GenePharma Co., Ltd. (Shanghai, China). Lipofectamine 2000 (Invitrogen) was adopted for cell transfection at the eventual 50 nM dose. For over-expressing ROC1, we adopted Lipofectamine 2000 to transfect pcDNA3.1-ROC1 into 768-0 cells. At 24 h after incubation, we harvested cells after transfection for subsequent analysis.
Cell Counting Kit-8 (CCK-8) assay
We inoculated 768-0 cells (1×105/well) into the 96-well plates to culture for a 24-h period before CCK-8 assay (Dojindo Molecular Technologies, Gaithersburg, MD, USA) to analyze cell proliferation. Afterwards, we further incubated cells for 24/48/72/96 h. Later, CCK-8 solution (10 µL) was added to incubate cells for a 4-h period under 37 ℃. To obtain cell growth curves, plates were read at 450 nm using a microplate spectrophotometer (Thermo Fisher Scientific, Inc.). All experiments were performed in triplicate.
5-ethynyl-2′-deoxyuridine (EdU) staining assay
We analyzed cell proliferation using Alexa Fluor 488 (Beyotime, Shanghai, China) and the BeyoClick™ EdU Cell Proliferation Kit (Beyotime) according to specific protocols. Briefly, 786-0 and A498 cells (4×104/well) were cultivated within the 24-well plates for a period of 24 h. Subsequently, 10 µM EdU was adopted to treat cells for 2 h. Then, we utilized 4',6-diamidino-2-phenylindole (DAPI) for 10-min nuclear staining. Later, we observed these cells using the fluorescence microscope (Olympus, Tokyo, Japan). We identified the cell proliferation by EdU-positive cell number.
Transwell invasion assays
Transwell assays were conducted to detect the invasion capabilities of cells. We later inoculated cells (4×105) into Matrigel-coated upper chamber. We added 10% FBS into bottom chamber to be the chemoattractant. Later, we incubated cells for another 48-h period, while the non-membrane invading cells were eliminated with the cotton swab. Crystal violet was used to stain cells onto bottom chamber, followed by counting under an inverted microscope (Olympus). All the experiments were performed in triplicate.
Western blot (WB) assay
Cells were lysed using radioimmunoprecipitation assay (RIPA) buffer (Sangon, Shanghai, China) containing root mean square fluctuation (RMSF) and the protease inhibitor cocktail. In addition, we utilized bicinchoninic acid (BCA) kit (Takara, Dalian, China) to determine total protein contents. Afterwards, proteins were separated by 80% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transfer onto polyvinylidene fluoride (PVDF) membranes (Takara). Then, we adopted 5% skimmed milk powder contained within TBS buffer that contained 0.1% Tween 20 to block membranes for a 30-min period under 37 ℃. Subsequently, we incubated membranes using primary antibodies under 4 ℃ overnight. Membranes were rinsed thrice, followed by 1-h incubation using secondary antibodies under 37 ℃. An electrochemiluminescence (ECL) detection kit (Takara) was used to determine the blotting. All the experiments were performed in triplicate.
In vivo xenograft experiments
In this research, each animal study gained approval from Animal Ethics Committee in The Sixth Affiliated Hospital of Wenzhou Medical University (No. 2019-61-an09), in compliance with the institutional guidelines for the care and use of animals. A protocol was prepared before the study without registration.
We obtained nude mice (n=18, 6 weeks old, 25–30 g) in SIPPR-BK Laboratory Animal Company, Shanghai, China and kept them under 25 ℃ and 12-h/12-h light-dark cycle conditions and free access to water and food. 786-0 cells transfected with scramble or shROC1 or shROC1 + shGSK-3β were subject to subcutaneous injection in mice through the back center. Each cell sample was injected into six mice with 1×105 cells. On day 56, the tumor weight was weighted after all mice were sacrificed. The pulmonary metastases and pulmonary nodules were observed by hematoxylin and eosin (HE) staining.
Statistical analysis
The results were displayed as mean ± standard deviation (SD) of three separate assays. The statistical analyses were performed by using SPSS 18.0 software (IBM, Armonk, NY, USA). One-way analysis of variance (ANOVA) and Student’s t-test (several/two groups) were adopted to analyze differences across different groups. We deemed overall survival (OS) as duration between surgery to death or final follow-up. We later plotted Kaplan-Meier (K-M) curve to analyze survival, while log-rank test was utilized to compare differences of two groups. P<0.05 stood for statistical significance.
Results
The ROC1 was overexpressed in RCC
For exploring ROC1’s effect on RCC, this work first analyzed ROC1 level within RCC. According to Figure 1A, ROC1 messenger RNA (mRNA) level within cancer tissues markedly increased relative to healthy samples (P=0.007). The relation of ROC1 expression and OS in RCC patients was then examined. It was found that high expression of ROC1 predicted dismal RCC survival (Figure 1B).

ROC1 had a certain effect on RCC cell growth and migration
We further examined how ROC1 silencing affected RCC cells. We later transfected siROC1 or scramble into 768-0 and A498 cells. Cell proliferation, viability and invasion were subsequently tested by CCK-8, EdU, and Transwell, respectively. It was found that ROC1 knockdown inhibited cell proliferation (P=0.006, Figure 2A), cell viability (P=0.006, Figure 2B), and cell invasion (P<0.01, Figure 2C) of 768-0 and A498 cells.

Regulation of ROC1 on GSK-3β, and ubiquitination and EMT process
To examine the possible molecular mechanism by which ROC1 mediated cellular function in 768-0 and A498 cells, WB assay was performed to detect the effect of ROC1 on GSK-3 expression and ubiquitination. Thereafter, we transfected overexpression of ROC1 (oeROC1), vector, siROC1, or scramble into 768-0 and A498 cells. It was found that oeROC1 inhibited GSK-3β expression and ubiquitination, while siROC1 promoted the GSK-3β expression and ubiquitination (P=0.04, Figure 3A). We measured the effect of siROC1 or oeROC1 transfection on EMT marker expression (E-cadherin, vimentin, N-cadherin) through WB assay. The results showed that oeROC1 inhibited E-cadherin expression, but increased vimentin and N-cadherin expression within 786-0 and A498 cells, and oeROC1 exhibited the contrary outcome (P=0.02, Figure 3B). To verify that ROC1 mediated EMT by regulating the expression of GSK-3β in 786-0 and A498 cells, 786-0 and A498 cells were co-transfected with oeROC1 and oeGSK-3β, siROC1 and siGSK-3β. E-cadherin, vimentin, N-cadherin, and GSK-3β levels were assessed through WB assay. The results showed that oeGSK-3β and siGSK-3β could counteract the effect of oeROC1 and siROC1, respectively (P=0.01, Figure 3C).

ROC1 regulated the Wnt signaling through GSK-3β
We further explored the effect of ROC1 expression on Wnt signaling pathway. oeROC1 in RCC cells promoted the protein expression of β-catenin and lymphoid enhancing factor (LEF), as well as inhibited GSK-3β expression (Figure 4A). Knockdown of ROC1 inhibited the protein expression of β-catenin and LEF, and stimulated the GSK-3β expression (Figure 4A). However, oeGSK-3β and siGSK-3β could reverse the effect of oeROC1 and siROC1 on the expression of β-catenin and LEF, respectively (P=0.04, Figure 4B).

ROC1 accommodated the EMT process through regulating GSK-3β expression
To further explore the underlying mechanism of ROC1, the 786-0 cells were co-transfected with oeROC1 and oeGSK-3β, siROC1 and siGSK-3β. Cell morphology was observed by light microscope, and the expression of E-cadherin and N-cadherin was examined by immunofluorescence assay. The results showed that oeROC1 promoted cell invasion and EMT process, and siROC1 attenuated cell invasion and EMT process (Figure 5A,5B). Moreover, oeGSK-3β and siGSK-3β could inhibit the effect of oeROC1 and siROC1 on the EMT process in 786-0 cells, respectively (P=0.02, Figure 5A,5B).

ROC1 silencing inhibited tumor metastasis through regulating GSK-3β
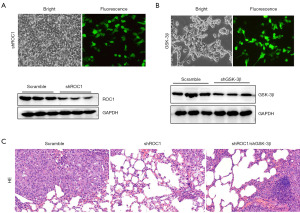
At last, we evaluated the effect of ROC1 knockdown on tumor metastasis. The 786-0 cells were transfected with shROC1 and co-transfected with shGSK-3β. As shown in Figure 6A,6B, the shROC1 and the shGSK-3β were successfully constructed and transfected. The tumor metastasis nude mice model was constructed. As shown in Figure 6C, shROC1 inhibited the tumor metastasis, while shGSK-3β counteracted the shROC1 effect.
Discussion
As we all know, the mechanism of occurrence and development of RCC is not clear, and there is a lack of sensitive and specific targets and early markers, resulting in a very poor prognosis for metastatic RCC cases. The 5-year survival rate is no more than 10%, with median OS being just 13 months. At present, the genetic level of RCC involves many critical pathways, including PI3K/Akt/mTOR, VHL/HIF, Wnt/β-catenin, JAK/STAT, and Raf/ERK (9-14).
The Wnt/β-catenin is a classical pathway, which plays a crucial role in regulating various cell activities. The sign of Wnt/β-catenin pathway is β-catenin accumulated and transferred to the nucleus. Wnt protein can bind to LRP/6 and Frz onto cell surface for forming the trimer, thus transmitting signal and activating the dishevelled (DSH/DVL) in the cytoplasm. The degradation complex that contains Axin, β-catenin, adenomatous polyposis coli (APC) and GSK-3β has weakened stability by the trimerization. β-catenin phosphorylation decreases while its content elevates within cytoplasm. β-catenin is subsequently transported in the nucleus, followed by T-cell factor (TCF)/LEF interaction for activating Wnt/β-catenin pathway. The expression of target genes downstream is finally activated. TCF/LEF is a kind of transcription factor with bidirectional regulation function. It can inhibit Groucho transcription by combining with Groucho, while promote the transcription of the β-catenin downstream genes by binding to β-catenin. Many articles have detected the participation of Wnt/β-catenin pathway in the regulation of EMT process in cancers (15-18).
In the present study, ROC1 expression remarkably elevated within RCC samples relative to healthy samples. ROC1 expression predicted a poor prognosis in RCC patients. We found that the over-expressing of ROC1 promoted the EMT process, while the interfering of ROC1 inhibited the EMT process. ROC1 regulated the GSK-3β/Wnt signaling in RCC cells. In a previous study, ROC1 level markedly increased within muscle metastasis of bladder cancer, which showed positive relation to EMT (8). As reported by Zhang et al., ROC1 showed over-expression within esophageal squamous cell carcinoma (ESCC), predicted dismal ESCC prognosis (19). ROC1 silencing remarkably suppressed ESCC proliferation in-vivo and in-vitro. From the mechanism perspective, ROC1 knockdown led to arrest of cell cycle at G2 phase and induced apoptosis through NOXA accumulation (a pro-apoptotic protein) (19). According to Wang et al., ROC1 silencing markedly suppressed proliferation of bladder cancer cells, induced G2 phase arrest and caused p53-dependent cell aging (20). In RCC, Wang et al. have reported that ROC1 is overexpressed in RCC, which is positively correlated with poor patient survival (20). Depletion of ROC1 inhibited growth and survival of RCC cells by inducing G2/M arrest, senescence, and apoptosis possibly due to accumulation of WEE1, p21, p27, NOXA, and BIM (20). Wang et al. have investigated that ROC1 expression is correlated with Fuhrman nuclear grade, and which can be considered as an important prognostic factor in RCC (21). Our present work found that downregulation of ROC1 could inhibit cell proliferation, viability, invasion and EMT process.
A large number of studies have shown that EMT plays an important role in malignant epithelial cell tumors (22). Current studies show that EMT leads to the formation of cancer cells in the process of tumor formation and metastasis in the early stage of cancer, accompanied by specific connective tissue damage and release of tumor cells into surrounding tissues and blood vessels (22). Some studies have also confirmed that EMT has a critical effect on renal cancer, lung cancer, bladder cancer, and some malignant epithelial cell tumors (23-26). The mechanism of EMT is as follows: (I) the loss of cell polarity leads to the inhibition of the expression of cell adhesion related molecules, so that the connection between specific cells is destroyed; and (II) the change of cell morphology leads to the acquisition of interstitial cell characteristics, together with a series of changes in molecular composition, resulting in the attack power of cells becoming more aggressive (27-30). Our work showed that downregulation of ROC1 suppressed EMT through modulating GSK-3β expression. Moreover, knockdown of ROC1 also inhibited tumor metastasis in vivo. This study provides important information regarding the role of ROC1 in RCC, but it still has some limitations. Firstly, the clinical sample size is limited, and data from a broader RCC patient population would be more meaningful. Secondly, the paper preliminarily explores the mechanism by which ROC1 regulates the EMT process through the GSK-3β/Wnt signaling pathway, but the specific details of this mechanism and the upstream and downstream molecules involved remain to be further elucidated. Thirdly, the paper proposes the potential of ROC1 as a prognostic marker for RCC, but translating this finding into clinical practice still faces challenges. In summary, this paper has made preliminary progress in exploring the role of ROC1 in RCC, but there are still some limitations. Future research can address and expand upon these limitations to provide a more comprehensive and in-depth understanding.
Conclusions
In conclusion, data from the current study indicate that ROC1 with a high expression in RCC predicts an unfavorable prognosis. ROC1 plays an essential role in RCC progression and EMT, and our study demonstrates for the first time that ROC1 regulates the GSK-3β/Wnt/β-catenin signaling. These findings could serve as a novel anticancer target for RCC.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the MDAR and ARRIVE reporting checklists. Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-646/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-646/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-646/prf
Funding: The study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2024-646/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This work was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Our study protocols gained approval from the Institutional Ethical Review Committee in The Sixth Affiliated Hospital of Wenzhou Medical University (No. 2019-61). Each tissue was collected from patients with informed consent provided. Each animal study gained approval from the Animal Ethics Committee in The Sixth Affiliated Hospital of Wenzhou Medical University (No. 2019-61-an09), in compliance with the institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Young M, Jackson-Spence F, Beltran L, et al. Renal cell carcinoma. Lancet 2024;404:476-91. [Crossref] [PubMed]
- Rose TL, Kim WY. Renal Cell Carcinoma: A Review. JAMA 2024;332:1001-10. [Crossref] [PubMed]
- Navani V, Heng DYC. Immunotherapy in renal cell carcinoma. Lancet Oncol 2023;24:1164-6. [Crossref] [PubMed]
- Zhu W, Han L, Wu Y, et al. Keratin 15 protects against cigarette smoke-induced epithelial mesenchymal transformation by MMP-9. Respir Res 2023;24:297. [Crossref] [PubMed]
- Feng J, Zhong H, Mei S, et al. LPS-induced monocarboxylate transporter-1 inhibition facilitates lactate accumulation triggering epithelial-mesenchymal transformation and pulmonary fibrosis. Cell Mol Life Sci 2024;81:206. [Crossref] [PubMed]
- Chen L, Zhu Y, Zhou J, et al. Luteolin Alleviates Epithelial-Mesenchymal Transformation Induced by Oxidative Injury in ARPE-19 Cell via Nrf2 and AKT/GSK-3β Pathway. Oxid Med Cell Longev 2022;2022:2265725. [Crossref] [PubMed]
- Wang W, Qiu J, Qu P, et al. Regulator of cullins-1 (ROC1) negatively regulates the Gli2 regulator SUFU to activate the hedgehog pathway in bladder cancer. Cancer Cell Int 2021;21:75. [Crossref] [PubMed]
- Wu Q, Zhou X, Li P, et al. ROC1 promotes the malignant progression of bladder cancer by regulating p-IκBα/NF-κB signaling. J Exp Clin Cancer Res 2021;40:158. [Crossref] [PubMed]
- Bai Y, You Y, Chen D, et al. Amiloride reduces fructosamine-3-kinase expression to restore sunitinib sensitivity in renal cell carcinoma. iScience 2024;27:109997. [Crossref] [PubMed]
- Ye Z, Fang Z, Li D, et al. Exploring the material basis and mechanism of action of clinacanthus nutans in treating renal cell carcinoma based on metabolomics and network pharmacology. Medicine (Baltimore) 2023;102:e35675. [Crossref] [PubMed]
- Li J, Chen S, Xiao J, et al. FOXC1 transcriptionally suppresses ABHD5 to inhibit the progression of renal cell carcinoma through AMPK/mTOR pathway. Cell Biol Toxicol 2024;40:62. [Crossref] [PubMed]
- Shu G, Chen W, Huang C, et al. Higher concentration of P7C3 than required for neuroprotection suppresses renal cell carcinoma growth and metastasis. J Cancer 2024;15:1191-202. [Crossref] [PubMed]
- Guo T, Zhang J, Wang T, et al. Lactic Acid Metabolism and Transporter Related Three Genes Predict the Prognosis of Patients with Clear Cell Renal Cell Carcinoma. Genes (Basel) 2022;13:620. [Crossref] [PubMed]
- Kim N, Kim S, Lee MW, et al. MITF Promotes Cell Growth, Migration and Invasion in Clear Cell Renal Cell Carcinoma by Activating the RhoA/YAP Signal Pathway. Cancers (Basel) 2021;13:2920. [Crossref] [PubMed]
- Chen Y, Yang Y, Wang N, et al. β-Sitosterol suppresses hepatocellular carcinoma growth and metastasis via FOXM1-regulated Wnt/β-catenin pathway. J Cell Mol Med 2024;28:e18072. [Crossref] [PubMed]
- Xiao G, Lu W, Yuan J, et al. Fbxw7 suppresses carcinogenesis and stemness in triple-negative breast cancer through CHD4 degradation and Wnt/β-catenin pathway inhibition. J Transl Med 2024;22:99. [Crossref] [PubMed]
- Zhang J, Cai H, Sun L, et al. LGR5, a novel functional glioma stem cell marker, promotes EMT by activating the Wnt/β-catenin pathway and predicts poor survival of glioma patients. J Exp Clin Cancer Res 2018;37:225. [Crossref] [PubMed]
- Zhang TT, Yi W, Dong DZ, et al. METTL3-mediated upregulation of FAM135B promotes EMT of esophageal squamous cell carcinoma via regulating the Wnt/β-catenin pathway. Am J Physiol Cell Physiol 2024;327:C329-40. [Crossref] [PubMed]
- Zhang J, Li S, Shang Z, et al. Targeting the overexpressed ROC1 induces G2 cell cycle arrest and apoptosis in esophageal cancer cells. Oncotarget 2017;8:29125-37. [Crossref] [PubMed]
- Wang W, Liu Z, Qu P, et al. Knockdown of regulator of cullins-1 (ROC1) expression induces bladder cancer cell cycle arrest at the G2 phase and senescence. PLoS One 2013;8:e62734. [Crossref] [PubMed]
- Wang Y, Tan M, Li H, et al. Inactivation of SAG or ROC1 E3 Ligase Inhibits Growth and Survival of Renal Cell Carcinoma Cells: Effect of BIM. Transl Oncol 2019;12:810-8. [Crossref] [PubMed]
- Yang S, Zhang D, Sun Q, et al. Single-Cell and Spatial Transcriptome Profiling Identifies the Transcription Factor BHLHE40 as a Driver of EMT in Metastatic Colorectal Cancer. Cancer Res 2024;84:2202-17. [Crossref] [PubMed]
- Kahlert UD, Joseph JV, Kruyt FAE. EMT- and MET-related processes in nonepithelial tumors: importance for disease progression, prognosis, and therapeutic opportunities. Mol Oncol 2017;11:860-77. [Crossref] [PubMed]
- Akrida I, Mulita F, Plachouri KM, et al. Epithelial to mesenchymal transition (EMT) in metaplastic breast cancer and phyllodes breast tumors. Med Oncol 2023;41:20. [Crossref] [PubMed]
- Recouvreux MV, Moldenhauer MR, Galenkamp KMO, et al. Glutamine depletion regulates Slug to promote EMT and metastasis in pancreatic cancer. J Exp Med 2020;217:e20200388. [Crossref] [PubMed]
- Sadrkhanloo M, Entezari M, Orouei S, et al. STAT3-EMT axis in tumors: Modulation of cancer metastasis, stemness and therapy response. Pharmacol Res 2022;182:106311. [Crossref] [PubMed]
- Stashko C, Hayward MK, Northey JJ, et al. A convolutional neural network STIFMap reveals associations between stromal stiffness and EMT in breast cancer. Nat Commun 2023;14:3561. [Crossref] [PubMed]
- Xu Z, Zhang Y, Dai H, et al. Epithelial-Mesenchymal Transition-Mediated Tumor Therapeutic Resistance. Molecules 2022;27:4750. [Crossref] [PubMed]
- Sadeghi M, Karimi MR, Karimi AH, et al. Network-Based and Machine-Learning Approaches Identify Diagnostic and Prognostic Models for EMT-Type Gastric Tumors. Genes (Basel) 2023;14:750. [Crossref] [PubMed]
- Guo D, Sheng K, Zhang Q, et al. Single-cell transcriptomic analysis reveals the landscape of epithelial-mesenchymal transition molecular heterogeneity in esophageal squamous cell carcinoma. Cancer Lett 2024;587:216723. [Crossref] [PubMed]


