
Machine learning based on automated 3D radiomics features to classify prostate cancer in patients with prostate-specific antigen levels of 4–10 ng/mL
Highlight box
Key findings
• We present a fully automated radiomics model based on prostate magnetic resonance imaging images to predict prostate cancer (PCa) with prostate-specific antigen (PSA) levels between 4 and 10 ng/mL.
What is known and what is new?
• The assessment of patients with PSA levels between 4 and 10 ng/mL for PCa by urologists remains ambiguous, which leads to needless biopsies.
• We provide a new approach combing deep learning-based prostate three-dimensional segmentation and multiple radiomics machine learning methodologies.
What is the implication, and what should change now?
• The automated radiomic model holds the potential to improve diagnostic accuracy of PCa and be an improvement over the PSA and PSA density.
Introduction
Prostate cancer (PCa) is the second most frequent cancer in men and the sixth most common cause of cancer-related death worldwide (1). In 2020, the incidence rate of PCa in Asia was projected to be 11.5/100,000 after age standardization, with a corresponding mortality rate of 4.4/100,000 (2). Prostate-specific antigen (PSA) is a widely used sensitive but non-specific PCa screening method, often leading to overdiagnosis and overtreatment (3-5). According to the American Urological Association (AUA) guidelines and clinical studies, only 17–32% of males with PSA levels of 4–10 ng/mL receive a PCa diagnosis after biopsy, suggesting that most undergo unnecessary biopsies with associated complications (6-8). This intermediate PSA range is termed the “gray zone”, reflecting its limited specificity for PCa detection. Therefore, there is an urgent need to develop a novel non-invasive technique for predicting PCa for males with PSA in the gray zone.
PSA density (PSAD) (9), proPSA isoforms (10), and extracellular RNA molecules in the blood and urine (11) have been found to be helpful in PCa screening. 31% of men could avoid biopsy thanks to magnetic resonance imaging (MRI) scans, and their findings are subsequently confirmed to be non-PCa (e.g., benign prostatic hyperplasia or prostatitis) (12). The most promising technique for PCa screening is the Prostate Imaging Reporting and Data System version 2.1 (PI-RADS v2.1), which standardizes the assessment of prostate MRI images (13). When determining the PI-RADS score, a slow learning curve and high reader subjectivity should be considered (14). From this perspective, the lesion with a score of 3 has the highest level of dispute. For those with a PSA level in the gray zone, a 3-cut-off point had a negative predictive value (NPV) of 97.1% and a lower positive predictive value (PPV) of 44.4% (15).
Radiomics provides an automated approach for objectively and quantitatively evaluating high-throughput tumor imaging data that is non-invasive, affordable, and repeatable. This approach combined with machine learning algorithms has been applied to the diagnosis and prognosis of a variety of cancers. Manually segmenting radiomics might lower the risk of a biopsy and help identify individuals in the PSA gray zone, according to published research (16,17). On the other hand, prostate segmentation usually requires temporal repetition and the participation of two radiologists to pass consistency examinations. It has been shown that deep learning-based three-dimensional (3D) segmentation for prostate MRI image segmentation is a very successful method with results that are independent of human effects (18), and the radiomics features provided by automated segmentation closely match the results obtained by manual segmentation (19). In this study, we developed a fully automated radiomics model based on 3D no-new-Net (nnU-net) to predict male PCa with PSA levels ranging from 4 to 10 ng/mL, achieving non-invasive, simple, and reliable results. We present this article in accordance with the TRIPOD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-2024-731/rc).
Methods
Patients’ data acquisition
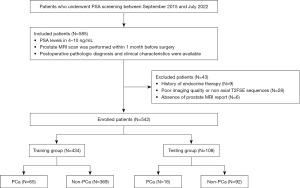
This retrospective study was approved by the Clinical Research Ethic Committee of Renmin Hospital of Wuhan University (No. WDRY2023-K122). Given the retrospective nature of the study, our institutional review board (Renmin Hospital of Wuhan University) waived the need for written informed consent. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. We retrospectively gathered general clinical information and imaging data of 574 patients from the Information Center, Renmin Hospital of Wuhan University between September 2015 and July 2022. The inclusion criteria were: (I) PSA level was in 4–10 ng/mL; (II) prostate MRI scan was performed within 1 month before surgery including prostate biopsy, transurethral resections of the prostate, and radical prostatectomy; and (III) postoperative pathologic diagnosis and clinical characteristics were available. The exclusion criteria included: (I) history of endocrine therapy; (II) poor imaging quality (e.g., motion or metal artifacts) or axial T2-weighted fast spin echo (T2FSE) sequences were unavailable; and (III) absence of prostate MRI report. Finally, 542 patients were enrolled and divided into training group (n=434) and testing group (n=108) at a ratio of 8:2. The study flowchart is shown in Figure 1.
MRI images acquisition
All axial T2FSE sequences of MRI images were collected from a 3.0 T scanner (GE Discovery MR750W 3.0 T MR, Waukesha, WI, USA) at Renmin Hospital of Wuhan University, with a 3 mm slice thickness, 512×512 image matrix, 0.4279 mm × 0.4279 mm pixel spacing, and recorded in DICOM format.
Development of 3D prostate segmentation model
We use multi-site prostate T2 MRI datasets with segmentation to develop the 3D segmentation model of prostate (n=255), in which samples were collected from Liu’s paper (20), the Medical Segmentation Decathlon (MSD) (21) dataset, and the Prostate158 (22) dataset. We pre-processed all MRI images using center cropping, N4 bias field correction (23), resizing, and normalizing. Prostate axial diameters seldom surpass 100 mm, and the prostate’s surrounding picture size grows throughout regional segmentation, adding to the computational burden. So, center cropping was done to every image by taking away 20% of the image borders from the three dimensions. After N4 bias field correction, images were converted volume of different sizes to same (256×256×40) through the nearest neighbor interpolation method. Excluding extreme values, we applied a z-score normalization to match the image intensities to a conventional normal distribution. The 3D full-resolution U-net architecture, generated by the nnU-net framework (24), was trained in a five-fold cross-validation. The development datasets of each fold were randomly split into training and validating sets at a ratio of 8:2. The nnU-net was trained for 200 epochs and an initial learning rate of 0.01 was used for weight learning. The loss function was the sum of cross-entropy and dice loss. A Linux operating system was employed to train nnU-net model with the following specifications: Intel Core i9-12900K, NVIDIA GeForce RTX 3060 ×2, RAM 64 GB. Dice, relative volume error (RVE), and 95% Hausdorff distance (HD95) were calculated as a measure of consistency and accuracy in prostate segmentation.
Radiomics features extraction and selection
The prostate segmentation prediction for the training and testing groups was performed with all five folds from the cross-validation as an ensemble using the conventional sliding window approach in the nnU-net framework. We utilized the Python package of PyRadiomics (25) to extract the radiomics features from the automatically segmented masks generated by the nnU-net model. A total of 1,595 reproducible radiomic features were conducted based on according to guidelines proposed by the Image Biomarker Standardisation Initiative. We randomly kept one of the characteristics for feature pairs in the training group (n=434) if the correlation was higher than 0.9. Subsequently, Student’s t-test or Welch’s t-test was performed, and features with P values below 0.01 were selected for retention and normalized using z-score. Random forest (RF), least absolute shrinkage and selection operator regression (Lasso), gradient boosting with component-wise linear models (glmBoost), and step-wise glm (Stepglm) possessed the ability of feature selection.
Machine learning model construction
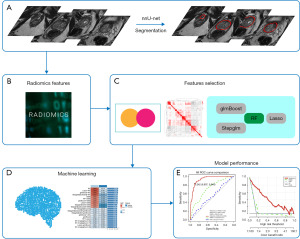
To establish a consistent classification model with high accuracy and good robustness, we adopted 12 machine learning algorithms, including RF, Lasso, glmBoost, Stepglm, support vector machine (SVM), ridge regression (Ridge), elastic net regression (Enet), partial least squares regression (plsRglm), gradient boosting machine (GBM), linear discriminant analysis (LDA), extreme gradient boosting (XGBoost), NaiveBayes. Because of above-mentioned four algorithms with the ability of feature selection, a total of 113 models were built using algorithm combinations and parameter adjustments. Finally, the area under the curve (AUC) for each model was calculated across the training and testing groups to assess the model’s predictive performance. Figure 2 displays the study’s design and procedural steps.
Statistics analysis
R (version 4.2.0) and Python (version 3.10.14) were used for statistical analysis and visualization. Spearman’s correlation coefficients were used to evaluate the correlations between two continuous variables. Categorical variables were compared using the Chi-squared test, while continuous variables were compared using the t-test or the Wilcoxon rank sum test. The AUC differences between the two classifiers were compared using the DeLong test. Benjamini-Hochberg (BH) correction was performed to reduce the bias caused by multiple tests with a false discovery rate (FDR) threshold of α=0.05. Decision curve analysis (DCA) was employed to assess the model’s clinical utility. For all analyses, a P value <0.05 was considered significant.
Results
Patient characteristics
Between September 2015 and July 2022, 542 participants with PSA in the gray zone were included in this study. The training group (n=434/542, 80%) and testing group (n=108/542, 20%) were allocated to the patients at random. Between these groups, there was no significant distinction in clinical characteristics (P>0.05) (Table 1).
Table 1
| Characteristics | Overall (n=542) | Training group (n=434) | Testing group (n=108) | P value† |
|---|---|---|---|---|
| Pathologic diagnosis | 0.97 | |||
| PCa | 81 [15] | 65 [15] | 16 [15] | |
| Non-PCa | 461 [85] | 369 [85] | 92 [85] | |
| Age (years) | 69 [64, 75] | 69 [64, 75] | 68 [64, 73] | 0.20 |
| PSA (ng/mL) | 6.71 [5.30, 8.26] | 6.73 [5.33, 8.26] | 6.55 [4.96, 8.27] | 0.64 |
| PV (mL) | 59 [42, 82] | 59 [43, 81] | 60 [42, 84] | 0.61 |
| PSAD (ng/mL2) | 0.11 [0.08, 0.16] | 0.11 [0.08, 0.16] | 0.11 [0.08, 0.16] | 0.53 |
| Pre-admission PSA screening | 0.64 | |||
| Yes | 140 [26] | 114 [26] | 26 [24] | |
| No | 402 [74] | 320 [74] | 82 [76] | |
| History of prostate surgery | 0.12 | |||
| Yes | 45 [8.3] | 32 [7.4] | 13 [12] | |
| No | 497 [92] | 402 [93] | 95 [88] |
Data are presented as n [%] or median [IQR]. †, Pearson’s Chi-squared test or Wilcoxon rank sum test. IQR, interquartile range; PCa, prostate cancer; PSA, prostate-specific antigen; PSAD, PSA density; PV, prostate volume.
Training effect in nnU-Net model
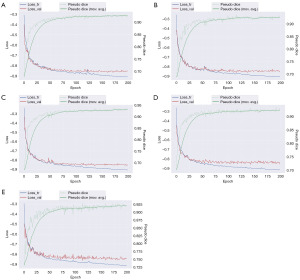
Figure 3 shows the training process of the nnU-Net segmentation model through a five-fold cross-validation process, spanning 200 epochs. Throughout this period, the model exhibited a progressive reduction in loss and a concurrent enhancement in pseudo-dice scores. A convergence trend was observed post 100 epochs, with the exponential moving average of the pseudo dice for the five folds surpassing the threshold of 0.9. Additionally, the model’s performance metrics, included an average dice of 95.33%, an RVE of 1.57%, and an HD95 of 2.73 mm (Table S1). Collectively, these findings underscore the nnU-Net’s precision in segmenting the prostate region.
Radiomics model construction and performance
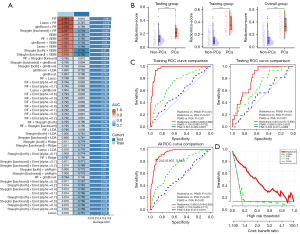
After nnU-Net segmentation, 1,594 radiomic features from MRI images of PSA gray zone patients were reduced to 41 key features using t-tests and correlation analysis, as listed in Table S2 and table available at https://cdn.amegroups.cn/static/public/tau-2024-731-1.xlsx. In the training group, we fitted 113 kinds of prediction models and further calculated the AUC of each model across training and testing groups (Figure 4A, table available at https://cdn.amegroups.cn/static/public/tau-2024-731-2.xlsx). The RF model was selected for its superior performance, with the highest average AUC (Figure 4A). Radiomics-score for each patient was calculated using selected radiomic features weighted by their regression coefficients in each model, the most important feature of both the RF and Lasso + RF model is wavelet.HLH_glszm_ZoneEntropy (table available at https://cdn.amegroups.cn/static/public/tau-2024-731-3.xlsx). The radiomics-score showed significant differences between PCa and non-PCa in the training group, testing group, and the overall group (Figure 4B, P<0.001). The detailed AUC values of radiomics mode were 0.964 [95% confidence interval (CI): 0.949–0.980] in the training group, 0.810 (95% CI: 0.721–0.900) in the testing group, and 0.938 (95% CI: 0.916–0.960) overall (Figure 4C). The DeLong test and BH test revealed that the radiomic model significantly outperformed PSA and PSAD in diagnosing PCa (Figure 4C, all P<0.05, table available at https://cdn.amegroups.cn/static/public/tau-2024-731-4.xlsx). DCA confirmed the radiomic model’s higher net benefit over PSAD (Figure 4D).
Discussion
The current researches concentrate on developing reliable predictive models that can accurately assess PCa risk in patients with PSA levels within the gray zone (4–10 ng/mL), thereby potentially reducing unnecessary invasive procedures in cases with low predicted malignancy probability. Preoperative prostate MRI scans, owing to their capacity to detect minute variations in proton signals from different tissues, frequently provide extensive information and are recommended as a promising approach to minimize overdiagnosis and excessive biopsies (3). The PI-RADS v2.1 MRI guidelines (13) often serve as the basis for puncture, however, they heavily rely on the expertise and diagnostic insight of radiologists. How to improve the accuracy and repeatability of PCa detection is an urgent problem to be solved. The combination of radiomics and machine learning methods digitizes high-throughput image results, reducing human factors in the evaluation process and ensuring the robustness of prediction results. However, similar to PI-RADS, the methodology’s reliance on manual segmentation by radiologists for MRI imaging of each patient presents a barrier to its broader dissemination in clinical practice. We employed nnU-net for fully automated 3D segmentation of the prostate MRI images, demonstrating commendable performance. Following this, a stringent feature selection process was undertaken, and an exhaustive comparison of 113 distinct machine learning methodologies was performed to identify the optimal strategy for the development of the radiomic model. The resultant model achieved an AUC of 0.938 when applied to the overall group, markedly surpassing the predictive capabilities of PSA (AUC: 0.542; 95% CI: 0.474–0.611) and PSAD (AUC: 0.718; 95% CI: 0.659–0.777).
Previous studies have evaluated the potential of radiomics models based on MRI or positron emission tomography/computed tomography (PET/CT) in diagnosing PCa in PSA gray zone (15,16,26-28). However, these studies demonstrate the limitations that we aim to address in this study. Wen et al. (15) concluded that PSAD, as a traditional clinical indicator correlating with both PSA levels and prostate volume (PV), has good discriminative performance (AUC: 0.712 in the training group and 0.677 in the testing group), which is similar to our results. Combining PI-RADS can improve the diagnostic performance of the model (0.893 and 0.871). Machine learning based radiological analysis has great potential for effectively distinguishing PCa from non-PCa. Qi et al. (16) and Li et al. (26) first used radiology to analyze the MRI images of the prostate. Qi’s model produced the best AUC of 0.959, but the dataset was small (n=199); Li et al. used a larger dataset (n=381), but only constructed the model using logistic regression without comparing multiple machine learning algorithms. Zhang et al. (17) randomly divided 274 PSA gray zone patients (194 non-PCa patients and 90 PCa patients) into two groups (training set and test set, with a ratio of 7:3). Using a method similar to Li et al., the model showed great potential in distinguishing PCa patients from non-PCa patients. Their model obtained an AUC of 0.941, but similarly, the dataset is small and lacks the use of multiple machine learning algorithms. In addition, Zhong et al. (27) found that radiomics models can help improve the diagnostic ability of primary radiologists compared to PI-RADS scores. Yang et al. (28) included 81 patients based on gallium-68 prostate-specific membrane antigen (68Ga PSMA) PET/CT and multiparametric MRI (mpMRI) to evaluate the performance of using a comprehensive PET/CT, mpMRI, and clinical feature model in diagnosing PCa. The model employed PET/CT maximum standard uptake value (SUVmax) instead of imaging features, resulting in good accuracy (AUC: 0.927). While this model was practical for clinical usage, the dataset was still limited, and using isolated maximum values may result in loss of imaging information. Even yet, the developed model just uses logistic regression.
In our research, we found that wavelet.HLH_glszm_ZoneEntropy, representing the randomness in the distribution of zone sizes and gray levels was the most important feature in our model. The term “ZoneEntropy” is used to describe the texture features of an object, with higher values indicating greater heterogeneity of the texture pattern. In this study, we observed that the ZoneEntropy in PCa cases was higher than that in non-PCa cases based on nnU-net segmentation results. This discovery aligns with clinical practice. The results of MRI imaging indicate that compositional variations within the lesion are a manifestation of PCa’s histological heterogeneity. On T2-weighted imaging (T2WI) images, the tumor in the transition zone shows as “erased charcoal”, an unrestricted homogeneous medium low signal region, whereas the periphery zone appears as focused low signal intensity in the background of high signal intensity glandular tissue. These signal changes are precisely due to the histological features of PCa, which are small and intact tumor-like glands, increased cell density, decreased extracellular fluid, and neovascularization (29,30).
Compared to previous studies, our research demonstrates several advantages. Firstly, the sample size of our study (n=542) is larger than the above-mentioned studies. Secondly, we utilized the nnU-net model to achieve automatic 3D segmentation of the prostate region. In addition, this study, a stringent feature selection process was undertaken, and an exhaustive comparison of 113 distinct machine learning methodologies was performed to identify the optimal strategy for the development of the radiomic model. It is worth noting that the most significant feature selected by the Top 2 method was consistent. The resultant model achieved an AUC of 0.938 when applied to the overall group, markedly surpassing the predictive capabilities of PSA and PSAD.
There are limitations in this study: the retrospective single-center design inherently carries risks of selection bias and limits generalizability to broader populations. While our use of multiple machine learning algorithms mitigates biases inherent to single-algorithm studies, the top-performing RF model requires validation in prospective cohorts before clinical translation. Not all patients had mpMRI; our model used only T2FSE sequences without PI-RADS scoring. Expanding the model to include prostate dynamic contrast-enhanced-MRI (DCE-MRI) and diffusion-weighted imaging (DWI) is needed. The small sample size limits prostate regional performance analysis; future studies should increase the sample size for broader model validation.
Conclusions
In our research, an automated radiomic model outperforms traditional PSA and PSAD measurements in identifying PCa in patients with PSA levels in the gray zone. This helps reduce unnecessary biopsies and assists urologists in better decision-making.
Acknowledgments
The authors express their sincere gratitude to the Information Center, Renmin Hospital of Wuhan University for their invaluable support.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-731/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-731/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-731/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2024-731/coif). All authors report that they have received funding support from the Primary Health Care Foundation of China (No. 025) and the Natural Science Foundation of Hubei Province (No. 2022CFC036). H.S. reports support from the Teaching and Research Project of Wuhan Municipal Colleges and Universities (No. 2021019). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was approved by the Clinical Research Ethic Committee of Renmin Hospital of Wuhan University (No. WDRY2023-K122). Given the retrospective nature of the study, our institutional review board (Renmin Hospital of Wuhan University) waived the need for written informed consent. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Culp MB, Soerjomataram I, Efstathiou JA, et al. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur Urol 2020;77:38-52. [Crossref] [PubMed]
- Zhu Y, Mo M, Wei Y, et al. Epidemiology and genomics of prostate cancer in Asian men. Nat Rev Urol 2021;18:282-301. [Crossref] [PubMed]
- Schröder FH. Landmarks in prostate cancer screening. BJU Int 2012;110:3-7. [Crossref] [PubMed]
- Vickers AJ, Sjoberg DD, Ulmert D, et al. Empirical estimates of prostate cancer overdiagnosis by age and prostate-specific antigen. BMC Med 2014;12:26. [Crossref] [PubMed]
- Welch HG, Schwartz LM, Woloshin S. Prostate-specific antigen levels in the United States: implications of various definitions for abnormal. J Natl Cancer Inst 2005;97:1132-7. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Gershman B, Van Houten HK, Herrin J, et al. Impact of Prostate-specific Antigen (PSA) Screening Trials and Revised PSA Screening Guidelines on Rates of Prostate Biopsy and Postbiopsy Complications. Eur Urol 2017;71:55-65. [Crossref] [PubMed]
- Greene KL, Albertsen PC, Babaian RJ, et al. Prostate specific antigen best practice statement: 2009 update. J Urol 2009;182:2232-41. [Crossref] [PubMed]
- Aminsharifi A, Howard L, Wu Y, et al. Prostate Specific Antigen Density as a Predictor of Clinically Significant Prostate Cancer When the Prostate Specific Antigen is in the Diagnostic Gray Zone: Defining the Optimum Cutoff Point Stratified by Race and Body Mass Index. J Urol 2018;200:758-66. [Crossref] [PubMed]
- Pecoraro V, Roli L, Plebani M, et al. Clinical utility of the (-2)proPSA and evaluation of the evidence: a systematic review. Clin Chem Lab Med 2016;54:1123-32. [Crossref] [PubMed]
- Mugoni V, Ciani Y, Nardella C, et al. Circulating RNAs in prostate cancer patients. Cancer Lett 2022;524:57-69. [Crossref] [PubMed]
- Siddiqui MR, Ansbro B, Shah PV, et al. Real-world use of MRI for risk stratification prior to prostate biopsy. Prostate Cancer Prostatic Dis 2023;26:353-9. [Crossref] [PubMed]
- Tamada T, Kido A, Takeuchi M, et al. Comparison of PI-RADS version 2 and PI-RADS version 2.1 for the detection of transition zone prostate cancer. Eur J Radiol 2019;121:108704. [Crossref] [PubMed]
- Schieda N, Lim CS, Zabihollahy F, et al. Quantitative Prostate MRI. J Magn Reson Imaging 2021;53:1632-45. [Crossref] [PubMed]
- Wen J, Liu W, Shen X, et al. PI-RADS v2.1 and PSAD for the prediction of clinically significant prostate cancer among patients with PSA levels of 4-10 ng/ml. Sci Rep 2024;14:6570. [Crossref] [PubMed]
- Qi Y, Zhang S, Wei J, et al. Multiparametric MRI-Based Radiomics for Prostate Cancer Screening With PSA in 4-10 ng/mL to Reduce Unnecessary Biopsies. J Magn Reson Imaging 2020;51:1890-9. [Crossref] [PubMed]
- Zhang L, Zhang J, Tang M, et al. MRI-Based Radiomics Nomogram for Predicting Prostate Cancer with Gray-Zone Prostate-Specific Antigen Levels to Reduce Unnecessary Biopsies. Diagnostics (Basel) 2022;12:3005. [Crossref] [PubMed]
- Jin Y, Yang G, Fang Y, et al. 3D PBV-Net: An automated prostate MRI data segmentation method. Comput Biol Med 2021;128:104160. [Crossref] [PubMed]
- Park J, Joo I, Jeon SK, et al. Automated abdominal organ segmentation algorithms for non-enhanced CT for volumetry and 3D radiomics analysis. Abdom Radiol (NY) 2025;50:1448-56. [Crossref] [PubMed]
- Liu Q, Dou Q, Heng PA. Shape-aware meta-learning for generalizing prostate MRI segmentation to unseen domains. In: Medical Image Computing and Computer Assisted Intervention-MICCAI 2020: 23rd International Conference, Lima, Peru, October 4-8, 2020, Proceedings, Part II 23. Cham: Springer International Publishing; 2020:475-85.
- Antonelli M, Reinke A, Bakas S, et al. The Medical Segmentation Decathlon. Nat Commun 2022;13:4128. [Crossref] [PubMed]
- Adams LC, Makowski MR, Engel G, et al. Prostate158 - An expert-annotated 3T MRI dataset and algorithm for prostate cancer detection. Comput Biol Med 2022;148:105817. [Crossref] [PubMed]
- Foltyn-Dumitru M, Schell M, Rastogi A, et al. Impact of signal intensity normalization of MRI on the generalizability of radiomic-based prediction of molecular glioma subtypes. Eur Radiol 2024;34:2782-90. [Crossref] [PubMed]
- Isensee F, Jaeger PF, Kohl SAA, et al. nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation. Nat Methods 2021;18:203-11. [Crossref] [PubMed]
- van Griethuysen JJM, Fedorov A, Parmar C, et al. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res 2017;77:e104-7. [Crossref] [PubMed]
- Li M, Chen T, Zhao W, et al. Radiomics prediction model for the improved diagnosis of clinically significant prostate cancer on biparametric MRI. Quant Imaging Med Surg 2020;10:368-79. [Crossref] [PubMed]
- Zhong JG, Shi L, Liu J, et al. Predicting prostate cancer in men with PSA levels of 4-10 ng/mL: MRI-based radiomics can help junior radiologists improve the diagnostic performance. Sci Rep 2023;13:4846. [Crossref] [PubMed]
- Yang J, Li J, Xiao L, et al. (68)Ga-PSMA PET/CT-based multivariate model for highly accurate and noninvasive diagnosis of clinically significant prostate cancer in the PSA gray zone. Cancer Imaging 2023;23:81. [Crossref] [PubMed]
- Kitzing YX, Prando A, Varol C, et al. Benign Conditions That Mimic Prostate Carcinoma: MR Imaging Features with Histopathologic Correlation. Radiographics 2016;36:162-75. [Crossref] [PubMed]
- Yu X, Liu R, Song L, et al. Differences in the pathogenetic characteristics of prostate cancer in the transitional and peripheral zones and the possible molecular biological mechanisms. Front Oncol 2023;13:1165732. [Crossref] [PubMed]


