
Association between dietary calcium intake and benign prostatic hyperplasia: a population-based result from NHANES 2003 to 2008
Highlight box
Key findings
• Dietary calcium intake was positively associated with increased risk of benign prostatic hyperplasia (BPH).
What is known and what is new?
• Calcium levels are directly associated with BPH, and this relationship may be mediated by regulating androgen receptor and fibroblast growth factor production.
• For every 100 mg increase in dietary calcium intake, the risk of disease increases by 5%. Moreover, there is a non-linear relationship between dietary calcium intake and BPH. When dietary calcium intake exceeds 1,114.11 mg, for every 100 mg increase in intake, the risk of BPH increases by 20%.
What is the implication, and what should change now?
• Monitoring dietary calcium intake and adjusting dietary structure may contribute to the reducing risk of BPH.
Introduction
Benign prostatic hyperplasia (BPH) is a histological diagnosis characterized by the proliferation of smooth muscle and epithelial cells in the transitional zone of the prostate. This condition manifests urodynamically as bladder outlet obstruction (BOO), which can lead to increased bladder pressure and changes in bladder morphology. Lower urinary tract symptoms (LUTS) significantly impact patients’ quality of life (1-3). As the global population ages, the incidence of BPH is both high and increasing. From 1990 to 2019, the prevalence of BPH has risen by 105.7% (4). Research has established a positive correlation between age and the incidence of BPH. The incidence rate of BPH in men exceeds 50%, and this figure can surpass 80% for those aged 80 years and older (5,6). Risk factors for BPH include metabolic syndrome, diabetes, obesity, hypertension, and various dietary nutritional factors. In some men, these factors may coexist (2). Despite extensive investigation, the exact mechanism underlying BPH remains unknown, and clarity on this issue is still lacking (7).
Dietary modifications and nutritional supplementation have demonstrated potential in the prevention and management of BPH and LUTS, while also positively influencing other systemic parameters (8). This includes consuming a diet rich in plant proteins and low in animal proteins, as well as incorporating foods high in zinc and vitamin D. Additionally, supplements such as Saw Palmetto, Cernilton, and Pygeum extracts may aid in controlling prostate growth and alleviating related LUTS (9). Conversely, excessive consumption of specific nutrients may contribute to an elevated risk of BPH (10). Consequently, identifying effective prevention and management strategies, as well as detection indicators, represents a significant focus for BPH research.
Calcium, an essential nutrient for human health, is primarily derived from dietary sources such as dairy products, legumes, leafy green vegetables, and certain supplements (11). While adequate calcium intake is crucial for bone health and has been linked to a reduced risk of osteoporosis (12), excessive calcium consumption has been implicated in the progression of prostate-related diseases (13). However, the specific relationship between dietary calcium intake and the risk of BPH remains insufficiently explored. To address this gap, we utilized data from the National Health and Nutrition Examination Survey (NHANES) collected between 2003 and 2008 to examine the association between dietary calcium intake and BPH risk. This study aims to contribute to the development of evidence-based strategies for the prevention and treatment of BPH. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-43/rc).
Methods
Study design and subjects
The NHANES is a nationwide survey study designed to assess the nutritional and health status of adults and children in the United States. The survey employs a stratified, complex multistage sampling method to record data from interviews, physical examinations, and laboratory tests (14). According to the NHANES database (available from: https://www.cdc.gov/nchs/nhanes/), the study protocol has been reviewed and approved by the National Center for Health Statistics (NCHS) Institutional Review Board, and all participants provided written informed consent upon enrollment. Therefore, further review by the hospital’s ethics committee is unnecessary. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
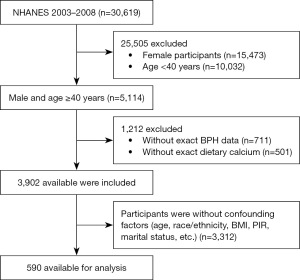
Starting from 1999, NHANES began collecting data on prostate-related diseases through questionnaires, mainly including BPH and prostate cancer (PCa), with data collection ceasing in 2008. Concurrently, since 1999, NHANES has been collecting data related to diet, however, the collection of 2-day dietary data began in 2003. Therefore, we utilized data from three cycles between 2003 and 2008 to explore the relationship between dietary calcium intake and the risk of BPH, where the assessment of 2-day dietary data could enhance its accuracy. According to the study design, a total of 30,619 participants from 2003 to 2008 were initially considered potentially eligible for our study. We performed population exclusion according to the following criteria: (I) females (n=15,473); (II) age less than 40 (n=10,032); (III) missing data on BPH and dietary calcium intake (n=1,212); and (IV) missing data on age, ethnicity, or other covariates (n=3,312). Based on the exclusion criteria, 590 participants were ultimately included in the study (Figure 1).
Assessment of BPH
In the survey on prostate conditions, participants who answered “Yes” to both “Have you ever been told by a doctor or health professional that you had an enlarged prostate gland?” and “Was it a benign enlargement—that is, not cancerous, also called benign prostatic hypertrophy?” were considered to have BPH. Participants who answered “No” to the first question and “No” to the second question after answering “Yes” to the first were identified as non-BPH. We excluded participants who did not know the answer, refused to answer the questions, or had missing data (15,16).
Assessment of dietary calcium intake
The data on dietary calcium intake comes from “What We Eat in America (WWEIA)”, which is conducted as a partnership between the U.S. Department of Agriculture (USDA) and the U.S. Department of Health and Human Services (DHHS). The Food and Nutrient Database for Dietary Studies (FNDDS) of the USDA’s provides the nutritional value of all items in the diet, detailing the nutritional profile of each food reported in the NHANES. Staff convert the reported food items into nutritional values based on the measured tool-assisted food intake reported by participants, and these have been validated for use in nutritional research.
Eligible participants underwent two dietary recall interviews. The first dietary recall interview was conducted at the mobile examination center, and the subsequent second dietary recall interview was completed within 3 to 10 days after the first. The data collection on dietary intake was monitored and reviewed to ensure the feasibility of the data. More information on dietary intake data can be accessed on the NHANES official website: https://wwwn.cdc.gov/Nchs/Nhanes. In this study, we focused only on dietary calcium intake and BPH, not dietary supplement calcium intake.
Potential confounders
Based on a previous study (17), we included age, race, education level, marital status, poverty income ratio (PIR), body mass index (BMI), alcohol consumption, smoking, diabetes, and hypertension in our analysis. Overall, demographic characteristics include age, race, education level, marital status, and PIR. Race is categorized as Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other; education level is divided into below high school, high school, and above high school; marital status is categorized as married or living with partners and living alone; PIR is divided into PIR ≤1.5, 1.5< PIR ≤3.5, and PIR >3.5. Smoking status is classified based on the survey question “Smoke at least 100 cigarettes in a lifetime”; those who smoked less than 100 cigarettes are considered non-smokers, and those who smoked more than 100 are considered smokers. Alcohol consumption is classified based on “Drink at least 12 drinks/year”; hypertension is classified based on systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or self-reported hypertension. Diabetes is classified based on blood glucose levels greater than 126 mg/dL or self-reported diabetes.
Statistical analysis
In accordance with the statistical guidelines of the NHANES, we acknowledged the complexity and multiplicity inherent in NHANES’s multistage sampling design. To enhance the precision of our data analysis, we selected appropriate sample weights. The final weights for the weighted analysis were calculated by multiplying the mobile examination center weights by a factor of 1/3. Categorical variables were delineated as percentages, whereas continuous variables are articulated in terms of mean values accompanied by their respective standard deviations. The comparative analysis of inter-group disparities was executed utilizing one-way analysis of variance for data adhering to a normal distribution, the Kruskal-Wallis test for data exhibiting asymmetrical distributional characteristics, and Chi-squared tests for categorical variables. Furthermore, logistic regression models were employed to ascertain the odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) pertaining to the relationship between BPH and dietary calcium intake. We constructed three distinct logistic regression models to explore the risk relationship between dietary calcium intake and BPH. Model 1 is the unadjusted model. In Model 2, we adjusted for certain demographic data, including age, race, education level, marital status, and PIR. Model 3 is the fully adjusted model, where we further adjusted for smoking, alcohol consumption, hypertension, and diabetes on top of the demographic data. We performed two transformations on the dietary calcium intake data to investigate the increase in BPH risk for every 100 mg of dietary calcium intake and to analyze dietary calcium intake as a categorical variable divided into quartiles. Additionally, we further used restricted cubic spline with three knots located at the 5th, 50th, and 95th percentiles of the exposure distribution to assess the dose-response relationship in the logistic regression Model 3. To explore potential differential relationships between dietary calcium intake and BPH risk, we stratified by age (<60 and ≥60 years), BMI (BMI <25, 25≤ BMI <30, BMI ≥30 kg/m2), PIR, smoking, alcohol consumption, hypertension, and diabetes, and investigated the correlation between every 100 mg of dietary calcium intake and BPH under different subgroup analysis conditions. To evaluate the robustness of the constructed model, we conducted two sensitivity analyses. First, we incorporated prostate-specific antigen (PSA) levels (Table S1), and second, we included serum calcium data, examining the association between dietary calcium intake and BPH in each analysis (Table S2).
Analysis was performed using R 4.2.1 (http://www.Rproject.org; The R Foundation, Vienna, Austria) and the Free Statistics software (version 2.0; Beijing Free Clinical Medical Technology Co., Ltd., Beijing, China). A two-sided P value <0.05 was regarded as statistically significant.
Results
Baseline characteristics of study population
We enrolled a total of 590 patients with a mean age of 62.4±13.2 years. The baseline characteristics of the participants, stratified by dietary calcium intake, are presented in Table 1. Significant differences were observed among the groups in terms of age, race, educational level, and smoking status (P<0.05). Additionally, the group with the highest dietary calcium intake had a lower mean age, while the group with the lowest intake exhibited higher BMI levels.
Table 1
| Variables | Total (n=590) | Dietary calcium intake (mg) | P | |||
|---|---|---|---|---|---|---|
| Q1 <508.25 (n=148) | 508.25≤ Q2 <758 (n=147) | 758≤ Q3 <1,135.25 (n=147) | Q4 ≥1,135.25 (n=148) | |||
| Age (years) | 62.4±13.2 | 61.7±13.1 | 63.5±13.1 | 64.6±13.5 | 59.8±12.8 | 0.01 |
| Race | 0.005 | |||||
| Mexican American | 61 (10.3) | 13 (8.8) | 19 (12.9) | 11 (7.5) | 18 (12.2) | |
| Other Hispanic | 28 (4.7) | 6 (4.1) | 7 (4.8) | 8 (5.4) | 7 (4.7) | |
| Non-Hispanic White | 323 (54.7) | 62 (41.9) | 79 (53.7) | 93 (63.3) | 89 (60.1) | |
| Non-Hispanic Black | 146 (24.7) | 56 (37.8) | 37 (25.2) | 27 (18.4) | 26 (17.6) | |
| Other | 32 (5.4) | 11 (7.4) | 5 (3.4) | 8 (5.4) | 8 (5.4) | |
| Educational level | 0.044 | |||||
| Below high school | 90 (15.3) | 21 (14.2) | 31 (21.1) | 23 (15.6) | 15 (10.1) | |
| High school | 250 (42.4) | 74 (50.0) | 58 (39.5) | 60 (40.8) | 58 (39.2) | |
| Above high school | 250 (42.4) | 53 (35.8) | 58 (39.5) | 64 (43.5) | 75 (50.7) | |
| Marital status | 0.48 | |||||
| Married or living with partners | 453 (76.8) | 107 (72.3) | 113 (76.9) | 116 (78.9) | 117 (79.1) | |
| Living alone | 137 (23.2) | 41 (27.7) | 34 (23.1) | 31 (21.1) | 31 (20.9) | |
| PIR | 0.17 | |||||
| PIR ≤1.5 | 182 (30.8) | 54 (36.5) | 43 (29.3) | 44 (29.9) | 41 (27.7) | |
| 1.5< PIR ≤3.5 | 228 (38.6) | 57 (38.5) | 65 (44.2) | 55 (37.4) | 51 (34.5) | |
| PIR >3.5 | 180 (30.5) | 37 (25.0) | 39 (26.5) | 48 (32.7) | 56 (37.8) | |
| BMI (kg/m2) | 24.9±7.2 | 25.8±7.3 | 24.9±7.9 | 24.5±7.0 | 24.6±6.6 | 0.42 |
| Smoking | 0.009 | |||||
| No | 313 (53.1) | 70 (47.3) | 67 (45.6) | 83 (56.5) | 93 (62.8) | |
| Yes | 277 (46.9) | 78 (52.7) | 80 (54.4) | 64 (43.5) | 55 (37.2) | |
| Drink | 0.08 | |||||
| No | 202 (34.2) | 44 (29.7) | 42 (28.6) | 57 (38.8) | 59 (39.9) | |
| Yes | 388 (65.8) | 104 (70.3) | 105 (71.4) | 90 (61.2) | 89 (60.1) | |
| Hypertension | 0.16 | |||||
| No | 253 (42.9) | 56 (37.8) | 57 (38.8) | 68 (46.3) | 72 (48.6) | |
| Yes | 337 (57.1) | 92 (62.2) | 90 (61.2) | 79 (53.7) | 76 (51.4) | |
| Diabetes | 0.47 | |||||
| No | 452 (76.6) | 107 (72.3) | 117 (79.6) | 112 (76.2) | 116 (78.4) | |
| Yes | 138 (23.4) | 41 (27.7) | 30 (20.4) | 35 (23.8) | 32 (21.6) | |
Data are presented as mean ± SD or n (%). BMI, body mass index; PIR, poverty income ratio; Q, quartile; SD, standard deviation.
Association between dietary calcium intake and BPH
A multivariable logistic regression analysis was performed to investigate the association between dietary calcium intake and the risk of BPH, as depicted in Table 2. The analysis considered dietary calcium intake both as a continuous and as a categorical variable. The findings revealed that across all models, an increment of 100 mg in dietary calcium intake was associated with an increased risk of BPH. Specifically, within the fully adjusted model, a 100 mg increment in dietary calcium intake corresponded to a 5% increase in the risk of BPH (95% CI: 1.01–1.09; P=0.04). When dietary calcium intake was categorized, with the lowest quartile (Q1) serving as the reference, participants in the highest quartile demonstrated an elevated risk of BPH in Model 1 (HR, 2.18; 95% CI: 1.14–4.18; P=0.02), Model 2 (HR, 2.47; 95% CI: 1.32–4.61; P=0.006), and Model 3 (HR, 2.47; 95% CI: 1.26–5.29; P=0.01).
Table 2
| Variables | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |||
| Dietary calcium intake (100 mg) | 1.03 (1.00–1.07) | 0.07 | 1.04 (1.00–1.08) | 0.04 | 1.05 (1.01–1.09) | 0.04 | ||
| Subgroup | ||||||||
| Q1 <508.25 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||||
| 508.25≤ Q2 <758 | 2.05 (0.90–4.64) | 0.08 | 1.95 (0.73–5.25) | 0.18 | 1.95 (0.73–4.92) | 0.18 | ||
| 758≤ Q3 <1,135.25 | 3.03 (1.43–6.44) | 0.005 | 2.15 (0.97–4.77) | 0.058 | 2.15 (0.93–5.17) | 0.07 | ||
| Q4 ≥1,135.25 | 2.18 (1.14–4.18) | 0.02 | 2.47 (1.32–4.61) | 0.006 | 2.47 (1.26–5.29) | 0.01 | ||
Model 1: unadjusted; Model 2: adjusted for age, race, marital status, education level and PIR; Model 3: adjusted for age, race, marital status, education level and PIR, BMI, smoking, drink, diabetes, and hypertension. BMI, body mass index; BPH, benign prostatic hyperplasia; CI, confidence interval; OR, odds ratio; PIR, poverty income ratio; Q, quartile; ref., reference.
Restrictive cubic spline analysis was utilized to assess the dose-response relationship between dietary calcium intake and the risk of BPH, revealing a non-linear association (P for non-linearity <0.05) (Figure 2). Based on these findings, we conducted an inflection point analysis (Table 3). The results indicated that in the two-piece regression model, when dietary calcium intake was less than 1,114.11 mg, each additional 100 mg of dietary calcium was associated with an adjusted OR of 1.20 for BPH (95% CI: 1.05–1.37; P=0.01). In contrast, when dietary calcium intake exceeded 1,114.11 mg, there was no significant association observed between dietary calcium intake and BPH.
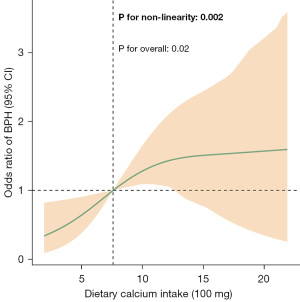
Table 3
| Dietary calcium intake (100 mg) | Crude model | Adjusted model† | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| <1,114.11 mg | 1.24 (1.11, 1.37) | <0.001 | 1.20 (1.05, 1.37) | 0.01 | |
| ≥1,114.11 mg | 1.01 (0.90, 1.13) | 0.90 | 1.05 (0.95, 1.15) | 0.32 | |
†, adjusted for age, race, marital status, education level, PIR, BMI, smoking, drink, diabetes, and hypertension. BMI, body mass index; BPH, benign prostatic hyperplasia; CI, confidence interval; OR, odds ratio; PIR, poverty income ratio.
Subgroup analyses
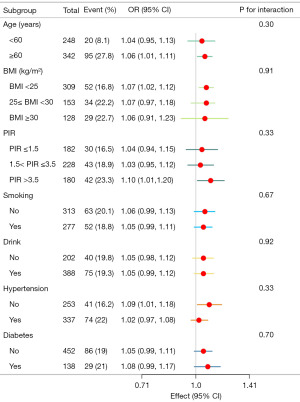
Figure 3 shows the results of subgroup analyses. Each 100 mg increase in dietary calcium intake was associated with an increased BPH risk in individuals over 60 years of age (OR, 1.06; 95% CI: 1.01–1.11), with a BMI <25 kg/m2 (OR, 1.07; 95% CI: 1.02–1.12), the higher PIR (OR, 1.10; 95% CI: 1.01–1.20), and participants without hypertension (OR, 1.09; 95% CI: 1.01–1.18). There was no association between dietary calcium intake and BPH risk in men or participants <60 years of age, with a BMI ≥25 kg/m2, with low or medium PIR, smoking, drinking, diabetes, and participants with hypertension. At the same time, we found no interaction between dietary calcium intake and BPH risk across subgroups (P for interaction >0.05).
Discussion
This study systematically examined the relationship between dietary calcium intake and the risk of BPH utilizing data from the NHANES collected between 2003 and 2008. The findings indicated a significant positive association between dietary calcium intake and BPH risk. After adjusting for confounding variables, each 100 mg increase in dietary calcium intake corresponded to a 5% increase in the risk of BPH. Furthermore, group analyses revealed that the risk of BPH was significantly higher in the highest calcium intake group compared to the lowest. A dose-response relationship, assessed through non-linear model restricted cubic spline analysis, demonstrated a non-linear association between dietary calcium intake and BPH risk. Specifically, when calcium intake was below 1,114.11 mg, the risk of BPH increased significantly with rising calcium intake; however, beyond this critical threshold, the rate of risk increase plateaued. Subgroup analyses indicated that the association between calcium intake and BPH risk was more pronounced among individuals aged ≥60 years, those with PIR >3.5, and those without hypertension. No significant interactions were detected among these subgroups.
The absorption of dietary calcium primarily occurs in the small intestine, particularly in the duodenum and jejunum. Previous studies have shown that dietary calcium intake is positively correlated with serum calcium levels (18,19). Elevated calcium levels can inhibit the secretion of PTH, which subsequently reduces the synthesis of active vitamin D, namely 1,25-dihydroxyvitamin D [1,25(OH)2D] (20). Evidence indicates that 1,25(OH)2D can inhibit the proliferation of both normal and malignant prostate epithelial cells, suggesting a protective effect against conditions such as BPH and PCa (21,22). Consequently, excessive calcium intake may contribute to the development of BPH by diminishing this protective mechanism, which aligns with our research findings. Additionally, the calcium-sensing receptor (CaSR) plays a significant role in the onset of BPH. A study has demonstrated that CaSR can mediate the recruitment of inflammatory cells, a process that can be triggered by elevated extracellular calcium levels or cytokines such as interleukin-6 (IL-6), thereby promoting the inflammatory response and proliferation of the prostate (23).
Meanwhile, the androgen receptor (AR) mediates the effects of androgens, such as dihydrotestosterone and the increase in AR activity is associated with the proliferation of both prostate epithelial cells and stromal cells (24,25). A study has demonstrated that calcium can activate AR and promote the transcription of androgen-responsive genes critical for prostate growth, potentially contributing to the pathogenesis of BPH (26). Additionally, estrogen can enhance the proliferation of prostate cells and may sensitize the prostate to the effects of androgens, further promoting cell proliferation. Notably, a U-shaped association was observed between serum calcium levels and the concentration of free estradiol, with lower concentrations of free estradiol found in men at both the lowest and highest serum calcium levels, while higher free estradiol concentrations were noted in men within the intermediate range (27). Fibroblast growth factor (FGF) plays a significant role in BPH, FGF7, a potent growth factor for prostatic epithelial cells, is increased by threefold in BPH and is correlated with increased epithelial proliferation in this condition (28). Some studies indicate that calcium can stimulate the production of FGFs independently of PTH and vitamin D (29). Although the subtypes of FGF differ, it can be hypothesized that calcium may influence the progression of prostatic hyperplasia by regulating FGF levels. The findings from the aforementioned studies indirectly support the notion that high calcium intake is associated with an increased risk of BPH, which aligns with our research results.
Nonetheless, some studies have identified an inverse correlation between higher serum calcium levels and prostate volume. A cross-sectional analysis conducted by Haghsheno et al. (30) in the Gothenburg Male Osteoporosis Study suggests that elevated serum calcium levels may play a protective role against prostatic hyperplasia by regulating cell differentiation, inhibiting cell proliferation, or enhancing inflammatory status. This indicates that there may be differences in the effects of dietary calcium intake compared to serum calcium levels. Such complexity underscores the necessity for further research to elucidate the dual effects of calcium intake on prostate health.
Multiple previous studies have directly demonstrated a significant association between high dairy and calcium intake and an increased risk of prostate disease. A systematic review and meta-analysis conducted by Aune et al. (31) integrated data from 32 prospective studies, revealing that high dairy and dietary calcium intake are associated with an elevated risk of PCa. Additionally, Chan et al. (32) analyzed a cohort of 20,885 men and showed that high intakes of dairy products and calcium were significantly associated with an increased risk of PCa.
However, all of these studies focused on PCa, and the relationship between dietary calcium intake and BPH has not been thoroughly investigated. In light of various research findings, we recommend that American men adopt scientifically informed dietary strategies in their daily lives to balance calcium intake and mitigate related health risks. For men who already have BPH or are at high risk, it is advisable to moderate calcium intake and avoid excessive supplementation. Additionally, it is recommended to prioritize a diverse range of dietary sources, such as low-fat dairy products and plant-based calcium sources (e.g., green leafy vegetables), to fulfill calcium requirements while minimizing potential risks. Furthermore, regular monitoring of prostate health is advised, along with discussions with healthcare providers regarding any necessary adjustments to dietary calcium intake.
While the results of this study are compelling, several limitations must be acknowledged. First, this investigation utilized cross-sectional data from NHANES 2003-2008, which limits the establishment of a causal relationship between dietary calcium intake and BPH. Future longitudinal studies are necessary to further validate this association and to elucidate the long-term effects of variations in calcium intake on BPH risk. Second, dietary data were derived from self-reports collected using a standardized 2-day dietary recall method, as officially conducted by NHANES. This approach may introduce recall bias, potentially compromising the accuracy of dietary calcium intake estimates. Given that our study is based on the NHANES research design and publicly available data, we were unable to modify or enhance the original data collection methods. However, future studies could mitigate recall bias by integrating biomarkers, combining multiple dietary assessment methods, or utilizing technology-assisted real-time data collection methods (e.g., smartphone applications). Lastly, despite adjustments for multiple confounders, the influence of unmeasured potential confounders, such as genetic factors, PSA levels, urinary calcium, intake of magnesium and phosphorus in the diet, and vitamin D status may still have impacted the results. Future studies, especially longitudinal designs or randomized controlled trials, are warranted to address these potential unmeasured confounders.
Conclusions
In summary, our study revealed a positive correlation between dietary calcium intake and BPH, accompanied by a significant dose-response trend. Beyond the inflection point, a positive correlation between dietary calcium intake and BPH was observed, suggesting a positive association between higher calcium intake and the occurrence of BPH. Additionally, the risk appears to be greater in older individuals.
Acknowledgments
We sincerely express our gratitude to the participants and investigators of the NHANES study for their invaluable contributions, which have provided significant support and assistance to our research. In addition, we thank Jie Liu, PhD (Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital & Physician-Scientist Center of China) for his constructive review and insightful comments on the manuscript.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-43/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-43/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-43/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Foo KT. What is a disease? What is the disease clinical benign prostatic hyperplasia (BPH)? World J Urol 2019;37:1293-6. [Crossref] [PubMed]
- Chughtai B, Forde JC, Thomas DD, et al. Benign prostatic hyperplasia. Nat Rev Dis Primers 2016;2:16031. [Crossref] [PubMed]
- Sandhu JS, Bixler BR, Dahm P, et al. Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia (BPH): AUA Guideline Amendment 2023. J Urol 2024;211:11-9. [Crossref] [PubMed]
- Zhu C, Wang DQ, Zi H, et al. Epidemiological trends of urinary tract infections, urolithiasis and benign prostatic hyperplasia in 203 countries and territories from 1990 to 2019. Mil Med Res 2021;8:64. [Crossref] [PubMed]
- Berry SJ, Coffey DS, Walsh PC, et al. The development of human benign prostatic hyperplasia with age. J Urol 1984;132:474-9. [Crossref] [PubMed]
- Shao WH, Zheng CF, Ge YC, et al. Age-related changes for the predictors of benign prostatic hyperplasia in Chinese men aged 40 years or older. Asian J Androl 2023;25:132-6. [Crossref] [PubMed]
- Devlin CM, Simms MS, Maitland NJ. Benign prostatic hyperplasia - what do we know? BJU Int 2021;127:389-99. [Crossref] [PubMed]
- Espinosa G. Nutrition and benign prostatic hyperplasia. Curr Opin Urol 2013;23:38-41. [Crossref] [PubMed]
- Das K, Buchholz N. Benign prostate hyperplasia and nutrition. Clin Nutr ESPEN 2019;33:5-11. [Crossref] [PubMed]
- Suzuki S, Platz EA, Kawachi I, et al. Intakes of energy and macronutrients and the risk of benign prostatic hyperplasia. Am J Clin Nutr 2002;75:689-97. [Crossref] [PubMed]
- Huang F, Wang Z, Zhang J, et al. Dietary calcium intake and food sources among Chinese adults in CNTCS. PLoS One 2018;13:e0205045. [Crossref] [PubMed]
- Al-Daghri NM, Hussain SD, Alnaami AM, et al. Dietary Calcium Intake and Osteoporosis Risk in Arab Adults. Nutrients 2023;15:2829. [Crossref] [PubMed]
- Capiod T, Barry Delongchamps N, Pigat N, et al. Do dietary calcium and vitamin D matter in men with prostate cancer? Nat Rev Urol 2018;15:453-61. [Crossref] [PubMed]
- Chen TC, Clark J, Riddles MK, et al. National Health and Nutrition Examination Survey, 2015-2018: Sample Design and Estimation Procedures. Vital Health Stat 2 2020;1-35. [PubMed]
- Chen T, Huang Y. Red blood cell folate and benign prostatic hyperplasia: results from the NHANES 2001-2008. Aging Male 2024;27:2336625. [Crossref] [PubMed]
- Yang L, Liu Z, Peng Z, et al. Exposure to Di-2-ethylhexyl Phthalate and Benign Prostatic Hyperplasia, NHANES 2001-2008. Front Endocrinol (Lausanne) 2021;12:804457. [Crossref] [PubMed]
- Feng X, Chen Y, Xia W, et al. Association between dietary niacin intake and benign prostatic hyperplasia: a population-based results from NHANES 2003-2008. J Health Popul Nutr 2024;43:130. [Crossref] [PubMed]
- Pal R, Bhadada SK, Aggarwal A, et al. Dietary Calcium Intake and Association with Serum Calcium in Healthy Urban North Indian Adults: The Calcium-Chandigarh Urban Bone Epidemiological Study. Indian J Endocrinol Metab 2024;28:596-600. [Crossref] [PubMed]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. Ross AC, Taylor CL, Yaktine AL, et al. editors. Washington: National Academies Press (US); 2011.
- Morris HA, Vitamin D. Status: Current Opinion on Critical Levels for Plasma Calcium and Bone Mineral Homeostasis. EJIFCC 2011;22:57-65. [PubMed]
- Flores O, Burnstein KL. GADD45gamma: a new vitamin D-regulated gene that is antiproliferative in prostate cancer cells. Endocrinology 2010;151:4654-64. [Crossref] [PubMed]
- Manchanda PK, Kibler AJ, Zhang M, et al. Vitamin D receptor as a therapeutic target for benign prostatic hyperplasia. Indian J Urol 2012;28:377-81. [Crossref] [PubMed]
- Anract J, Baures M, Barry Delongchamps N, et al. Microcalcifications, calcium-sensing receptor, and cancer. Cell Calcium 2019;82:102051. [Crossref] [PubMed]
- Izumi K, Li L, Chang C. Androgen receptor and immune inflammation in benign prostatic hyperplasia and prostate cancer. Clin Investig (Lond) 2014;4:935-50. [Crossref] [PubMed]
- Madersbacher S, Sampson N, Culig Z. Pathophysiology of Benign Prostatic Hyperplasia and Benign Prostatic Enlargement: A Mini-Review. Gerontology 2019;65:458-64. [Crossref] [PubMed]
- Sharawi ZW, Khatrawi SM, Wang Q, et al. Calcium Activation of the Androgen Receptor in Prostate Cells. Int J Endocrinol 2023;2023:9907948. [Crossref] [PubMed]
- Van Hemelrijck M, Michaelsson K, Nelson WG, et al. Association of serum calcium with serum sex steroid hormones in men in NHANES III. Aging Male 2013;16:151-8. [Crossref] [PubMed]
- Giri D, Ittmann M. Interleukin-1alpha is a paracrine inducer of FGF7, a key epithelial growth factor in benign prostatic hyperplasia. Am J Pathol 2000;157:249-55. [Crossref] [PubMed]
- Rayego-Mateos S, Doladé N, García-Carrasco A, et al. The Increase in FGF23 Induced by Calcium Is Partially Dependent on Vitamin D Signaling. Nutrients 2022;14:2576. [Crossref] [PubMed]
- Haghsheno MA, Mellström D, Behre CJ, et al. Low 25-OH vitamin D is associated with benign prostatic hyperplasia. J Urol 2013;190:608-14. [Crossref] [PubMed]
- Aune D, Navarro Rosenblatt DA, Chan DS, et al. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr 2015;101:87-117. [Crossref] [PubMed]
- Chan JM, Stampfer MJ, Ma J, et al. Dairy products, calcium, and prostate cancer risk in the Physicians' Health Study. Am J Clin Nutr 2001;74:549-54. [Crossref] [PubMed]


