
Total periurethral reconstruction with preservation of “Hood” structures promotes early recovery of urinary continence in extraperitoneal single-port robot-assisted radical prostatectomy
Highlight box
Surgical highlights
• We proposed a novel parachute-style total periurethral reconstruction technique with preservation of “Hood” structures in extraperitoneal single-port robot-assisted radical prostatectomy.
What is conventional and what is novel/modified?
• Conventional total periurethral reconstruction is challenging due to the need for precise identification of structures and difficulties in achieving tension-free vesicourethral anastomosis, especially in patients with large-volume prostates.
• This procedure is particularly surgeon-friendly, even for beginners, because the structures involved are relatively accessible.
What is the implication, and what should change now?
• Through our investigations, we find the technique creates a tension-free vesicourethral anastomosis which shows promising results in the early recovery of urinary continence.
Introduction
Prostate cancer (PCa) is the second most common malignancy among men worldwide (1). Radical prostatectomy (RP) is the primary treatment for localized cases in patients expected to live >5–10 years. Recently, significant efforts have been made to increase the pentafecta rate [tumor control, urinary continence (UC), preservation of erectile function, negative surgical margins, and absence of perioperative complications] (2). However, the incidence of postprostatectomy incontinence (PPI) remains a major concern for urologists.
Due to its three-dimensional magnified field of view and precise, multi-joint movements, robot-assisted laparoscopic RP (RARP) is increasingly recognized as the preferred treatment for achieving favorable oncologic outcomes and lower complication rates compared to traditional methods (3,4). Various techniques, including Retzius-sparing technique (5,6), Hood technique (7), Vattikuti Institute prostatectomy (VIP) technique (8), total periurethral reconstruction (9), etc., have been proven effective in PPI recovery based on the robotic platform, offering new prospects for achieving early urinary control. Herein, we share our preliminary experience with a novel technique—a total periurethral reconstruction preserving the “Hood” structures in extraperitoneal single-port RARP (sp-RARP)—and explore its impact on the early recovery of UC in selected patients with PCa. We present this article in accordance with the SUPER reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-56/rc).
Preoperative preparations and requirements
Twelve patients with PCa who underwent extraperitoneal sp-RARP performed by a single surgeon (C.W.) from July 2023 to July 2024 at the First Affiliated Hospital of Harbin Medical University participated in this study. All surgeries were conducted using the DaVinci Xi system and a single-port device (100 mm multichannel laparoscopic port) via the extraperitoneal approach. The patients were routinely tested for prostate-specific antigen (PSA), digital rectal examination and prostate magnetic resonance imaging (MRI), patients who were suspected of having PCa were tested for transrectal prostatic biopsy. Patients diagnosed with PCa received whole-body bone scanning to exclude distant metastasis. Patients were selected based on the following inclusion and exclusion criteria. The inclusion criteria included: (I) a pathological diagnosis of PCa confirmed by biopsy, (II) patients with localized or locally progressed PCa (clinical stages cT1–3, cN0–1, cM0), (III) consent to undergo robotic surgery, and (IV) no distant metastasis in cases of locally advanced PCa. The exclusion criteria included: (I) refusal of surgical or robotic surgical treatment, (II) conditions preventing tolerance of surgery and anesthesia, (III) preoperative computed tomography or MRI scans indicating bone or systemic metastases, (IV) preoperative MRI or intraoperative evidence of tumor invasion into the left or right prostate fascia which precludes preservation of the “Hood” structure, and (V) a history of urinary incontinence or urethral stricture.
The study was approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University (No. 2024XJSS74). All procedures performed in this study were in accordance with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patients for publication of this article, accompanying images and video. A copy of the written consent is available for review by the editorial office of this journal.
Step-by-step description
The patient positioning and trocar setup for sp-RARP are described in our previous research (10). Pelvic lymph node dissection (PLND) was routinely performed, and the internal urethral orifice was preserved whenever possible (Figure 1A) before resecting the prostate along with the vas deferens and seminal vesicles using the modified “Hood” technique (Figure 1B). The bladder neck (BN) was reconstructed in cases with a prominent middle prostate lobe.
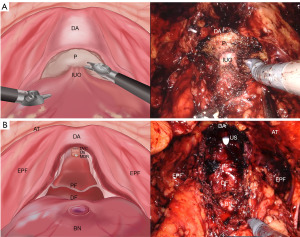
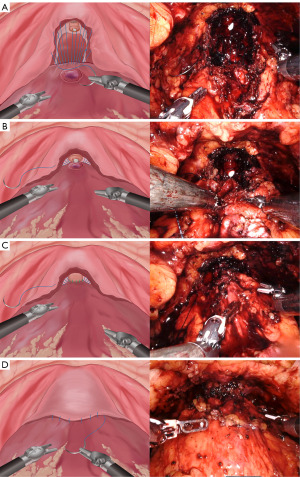
The technique can be divided into two parts. Initially, using a 3/0 V-loc absorbable monofilament barbed suture, the right part of the “Hood” structures was sutured, extending deep into the medial levator ani muscle bundle while avoiding injury to the neurovascular bundle (NVB). The suture was then directed to the detrusor apron (DA) at the posterior layer of the bladder trigone, and subsequently angled towards the median dorsal raphe (MDR). Then, focus shifted to the left part of the “Hood” structure, stitching it to the posterior portion of the BN. To summarize, the lateral levator ani muscle and MDR at the base of the “Hood” structures were joined anticlockwise to their corresponding posterior detrusor muscle of the BN using a parachute-style running suture (PSRS) method (Figure 2A), thus establishing posterior support for the vesicourethral anastomosis (VUA). To ensure a tension-free closure, the suture was tightened sequentially and gradually as the pneumoperitoneum pressure decreased and with the assistance of an aspirator (Figure 2B). Upon completion of the VUA with another suture (Figure 2C), the latter part of the technique involved anastomosing the anterior detrusor muscle of the BN to the rim of the “Hood” structures (prostate fascia and DA above the urethral stump) with the remainder of the aforementioned 3/0 V-loc suture, thereby creating the anterior fixation of the VUA (Figure 2D). The full procedure video is available in the Video 1.
Postoperative considerations and tasks
We collected the patients’ baseline characteristics and perioperative functional and oncologic parameters. Complications were categorized using the Clavien-Dindo classification. To assess postoperative UC, the International Consultation on Incontinence Questionnaire-short form (ICIQ-SF) surveys were conducted via telephone follow-ups at 24 h, 1 week, 4 weeks, and 3 months after catheter removal. Additionally, 24-h pad counts were performed. UC was defined as zero pads used per 24 h and an ICIQ-SF score of ≤6 (11). The American Society of Anesthesiologists (ASA) score assess preoperative patient physical status, and the visual analogue scale (VAS) was used for postoperative pain evaluation. Descriptive statistics summarized the data, with absolute and relative frequencies for categorical variables, and median and interquartile range (IQR) for continuous non-normally distributed variables. The mean was used as an additional measure for daily pad usage. Data analysis was performed using SPSS Statistics for Windows, version 27.0 (IBMCorp., Armonk, NY, USA).
Twelve patients from our medical center participated in this study from July 2023 to July 2024 and the baseline data of the patients are listed in Table 1. The median age of the patients was 70 (IQR, 66.5–76.75) years, and the median body mass index (BMI) was 23.95 (IQR, 22.35–26.15) kg/m2. At surgery, the median prostate volume was 31.13 (IQR, 25.22–58.39) mL, with median total PSA levels at 17.53 (IQR, 8.28–27.98) ng/mL and free PSA levels at 1.57 (IQR, 0.65–2.57) ng/mL.
Table 1
| Variables | Values (n=12) |
|---|---|
| Age, years | 70 (66.5–76.75) |
| BMI, kg/m2 | 23.95 (22.35–26.15) |
| Prostate volume median, mL | 31.13 (25.22–58.39) |
| Total PSA, ng/mL | 17.53 (8.28–27.98) |
| Prior abdominal surgery | 1 (8.33) |
| Preoperative neoadjuvant therapy | 2 (16.67) |
| Clinical tumor stage | |
| cT2 | 8 (66.7) |
| cT3 | 4 (33.33) |
| Biopsy Gleason score | |
| 3+3 | 3 (25.0) |
| 3+4 | 3 (25.0) |
| 4+3 | 1 (8.33) |
| 4+4 | 1 (8.33) |
| 4+5 | 2 (16.67) |
| 5+4 | 2 (16.67) |
| Clinical risk category | |
| Low | 1 (8.33) |
| Intermediate | 3 (25.0) |
| High | 8 (66.67) |
Data are presented as median (IQR) or n (%). BMI, body mass index; IQR, interquartile range; PSA, prostate-specific antigen.
The patients initial MRI-based stages showed that 8 patients were diagnosed with stage T2N0M0 and 4 patients with T3N0M0. Preoperative Gleason scores were as follows: 3 patients (25%) scored 3+3, 3 (25%) scored 3+4, 1 (8.33%) scored 4+3, 1 (8.33%) scored 4+4, 2 (16.67%) scored 4+5, and 2 (16.67%) scored 5+4. One patient had a history of abdominal surgery, and two patients received a three-month neoadjuvant treatment of treprostinil acetate 3.75 mg subcutaneously once every 4 weeks and bicalutamide 50 mg orally daily.
Table 2 highlights the perioperative and pathologic findings. None of the procedures required conversion to open or standard laparoscopic surgery, nor were additional ports necessary. The median operative time was 152 (IQR, 141.25–180) min, and the median estimated blood loss was 100 (IQR, 50–175) mL. One patient with preoperative mild anemia required a blood transfusion due to vascular anomalies and adhesions during DVC dissection. The median reconstruction time for both posterior and anterior approaches was 13.5 (IQR, 11.0–21.5) min. On the day after surgery, nine patients reported no pain, with VAS scores ranging from 0 to 3.
Table 2
| Variables | Values (n=12) |
|---|---|
| ASA scores | |
| 2 | 2 (16.67) |
| 3 | 10 (83.33) |
| Operative time, min | 152 (141.25–180) |
| EBL, mL | 100 (50–175) |
| Reconstruction time, min | 13.5 (11.0–21.5) |
| Transfusion rate | 1 (8.33) |
| VAS scores | |
| 0–3 | 9 (75.00) |
| 4–6 | 3 (25.00) |
| Postoperative recovery time of gastrointestinal function, days | 2 (1.25–3.00) |
| Postoperative hospitalization time, days | 5.5 (5.0–11.5) |
| Indwelling catheter time, days | 14 (14–14) |
| Drainage tube retention time, days | 5 (3.00–5.75) |
| Complications | |
| Clavien I–II | 3 (25.00) |
| Clavien III–V | 0 |
| PSM | 2 (16.67) |
| Positive lymph nodes | 1 (8.33) |
| Pathological stage | |
| pT2 | 8 (66.67) |
| pT3a | 1 (8.33) |
| pT3b | 3 (25.00) |
| Pathologic Gleason score | |
| 3+3 | 4 (33.33) |
| 3+4 | 1 (8.33) |
| 4+3 | 1 (8.33) |
| 4+4 | 2 (16.67) |
| 4+5 | 2 (16.67) |
| 5+4 | 2 (16.67) |
Data are presented as median (IQR) or n (%). ASA, American Society of Aneshesiologists; EBL, estimated blood loss; IQR, interquartile range; PSM, positive surgical margins; VAS, Visual analogue scale.
The median time to gastrointestinal function recovery was 2 (IQR, 1.25–3.00) days. The median duration for drainage tube removal was 5 (IQR, 3.00–5.75) days, and the median postoperative hospital stay was 5.5 (IQR, 5.0–11.5) days. Catheters were typically removed by day 14 (IQR, 14–14). Final pathology reports revealed Gleason scores as follows: four patients scored 3+3, one scored 3+4, one scored 4+3, two scored 4+4, two scored 4+5, and two scored 5+4. Pathologic staging changed in 2 patients (16.67%) postoperatively, one elevated from preoperative T2N0M0 to postoperative T2N1M0 and the other from T3N0M0 to T3N1M0. One patient exhibited both positive surgical margins (PSM) and lymph node positivity, whereas another displayed only PSM. There were no Clavien grade ≥ III complications, such as significant bleeding, anastomotic leakage, lymphocysts, or blood clots requiring further intervention.
Postoperative continence recovery was tracked (Table 3). Five patients regained UC within 24 h of catheter removal, eight within 1 week, nine within 4 weeks, and eleven within 3 months. The median serum PSA levels were 0.04 (IQR, <0.01–0.08) ng/mL 1 month post-surgery and remained stable at 0.04 (IQR, <0.01–0.10) ng/mL 3 months post-surgery.
Table 3
| Variables | Values (n=12) |
|---|---|
| ICIQ-SF score, median (IQR) | |
| 24 hours | 5.5 (0.5–10.0) |
| 1 week | 2.5 (0–9.0) |
| 4 weeks | 1.5 (0–8.0) |
| 3 months | 0 (0–2.5) |
| Postoperative follow-up PSA, ng/mL, median (IQR) | |
| 4 weeks | 0.035 (<0.01–0.075) |
| 3 months | 0.04 (<0.01–0.10) |
| Patients achieving continence, n (%) | |
| 24 hours | 5 (41.67) |
| 1 week | 8 (66.67) |
| 4 weeks | 9 (75.00) |
| 3 months | 11 (91.67) |
ICIQ-SF, International Consultation on Incontinence Questionnaire-short form; IQR, interquartile range; PSA, prostate-specific antigen.
Tips and pearls
During the initial suture phase of the posterior reconstruction, the needle should be as parallel to NVB as possible in order to avoid damage to the NVB (12), and the impact on the NVB can be minimized by gradual and symmetrical tightening of sutures after the completion of the post-reconstructive suture step as well.
Following posterior PSRS, the sutures were tightened sequentially and gradually as pneumoperitoneum pressure decreased from 12 to ≤6 mmHg, achieving a tension-free VUA. This procedure is particularly surgeon-friendly, even for beginners, as reflected by the median reconstruction time of 13.5 min (IQR, 11.0–21.5).
Discussion
PPI is one of the most common postoperative complications of RARP, with persistence rates ranging from 4% to 31% >1 year post-surgery (13). PPI significantly impairs the quality of life for patients, with its estimated cost burden in the United States ranging from $19 to $32 billion (14). Despite considerable progress in reducing both the rates and recovery time of PPI, it remains a primary concern and challenge for urologists.
Unlike functional urinary incontinence (FUI) and urgency urinary incontinence (UUI), stress urinary incontinence (SUI) is commonly regarded as the primary cause of PPI (15). Although the underlying mechanisms are not fully understood (16), recent advances, particularly with the advent of dynamic MRI (17), have elucidated these processes. Physically, increased abdominal pressure causes the bladder to compress caudally and pelvic organs to rotate downwards and ventrally, using the connection between the anterior bladder wall and abdominal wall as a “fulcrum”, causing compression of the membranous urethra (MU). SUI post-RP may occur when urethral closing pressure is not fully maintained due to BN enlargement, MU instability, and expansion from extensive innervation damage, compromised attachments, inadequate periurethral structures, and a shortened MU length. To maximize the urethral stability, several reconstruction techniques including anterior fixation [periurethral suspension stitch (18)], posterior reconstruction [“Rocco” stitch (19), modified BN suspension (20), vesicoprostatic muscle reconstruction technique (21)], and total reconstruction [total anatomical reconstruction technique (9), advanced reconstruction of vesicourethral support technique (11)] have been applied and improved the recovery of urinary control effectively since 1998 (18). Since the first report in 1982 (22), the maximal preservation of anatomical structures related to UC has been widely accepted as an effective strategy to reduce PPI. Current mainstay techniques include nerve sparing (23,24), BN preservation (25), VIP (8), Retzius-sparing (26), and Hood (7), etc. In our series, sp-RARP were performed using the modified Hood technique (10) with PLND, and the proximal intrinsic sphincter and MU were preserved whenever possible, supporting favorable oncologic and continence outcomes. Inspired by these concepts and techniques, we present here, for the first time, a novel total periurethral reconstruction based on the Hood method.
Our technique is based on the mainstay theory of PPI to promote early recovery of UC. The Hood structure maximally preserves NVB, proximal intrinsic sphincter, rhabdosphincter DA around the urethral stump, and other periurethral structures, potentially reducing the occurrence of FUI, UUI, and SUI. Additionally, the novel total periurethral reconstruction provides sufficient mechanical support, including dorsal dynamic suspensory support and a musculofascial plate, maintains the junction between the bladder and abdominal wall as a fulcrum, and realizes tension-free VUA to improve urethral healing. All these factors may help maintain proper urethral stability and closing pressure to prevent SUI. Our initial results showed that the continence rates at 24 h, 1 week, 4 weeks, and 3 months post-catheter removal were 41.67% (5/12), 66.67% (8/12), 75% (9/12), and 91.67% (11/12), slightly higher than our previous findings on urinary control rates of 41.67%, 54.17%, 75.0%, and 91.67% at 24 h, 1 week, 4 weeks, and 3 months after urinary catheter removal after the application of the modified Hood technique in sp-RARP, indicating effectiveness in the early recovery of UC.
Posterior reconstruction in RARP is challenging due to the need for precise identification of structures and difficulties in achieving tension-free VUA, especially in patients with large-volume prostates. In this study, the lateral levator ani muscle, MDR, DA of BN, and rim of the Hood structure were relatively accessible. Our initial results showed that estimated blood loss was 100 mL, and no serious complications occurred during the operation (Clavien grade ≥ III). None of the postoperative patients had a VAS score >6, suggesting that this technique is both safe and viable. Since PLND was performed in all cases, for safety reasons and to prevent lymphatic fistulae and urinary leakage, prolonged drainage duration (median 5 days) was implemented despite the fact that most of the patients were able to achieve a drainage of less than 20 mL in 24 hours and recovered well on 1–3 days postoperative. Compared to conventional RARP, the extraperitoneal sp-RARP offers advantages such as smaller incisions (27,28), fewer disturbances to abdominal organs, and potentially faster postoperative recovery. Additionally, the routine use of only one suture during reconstruction may reduce operative time and medical costs to some extent.
Some limitations of our research should be noted as follows. First, PSM remain a critical issue for Retzius-sparing and other preservation surgeries in RARP, with incidence rates ranging from 6% to 47% (29-32), reflecting the clinical challenge of balancing oncologic outcomes with functional preservation. Although only two cases (16.7%) of PSM occurred in our series, this technique may be more suitable for patients with thorough preoperative imaging evaluations. Finally, our findings need further validation through well-designed studies due to inherent shortcomings including a small sample size, retrospective analysis, lack of randomized control trials, and a short follow-up period.
Conclusions
The novel technique demonstrates promising results in the early recovery of UC in extraperitoneal sp-RARP without increasing complications. Therefore, it may be a viable and effective alternative surgical method. However, due to some inherent limitations, well-designed trials should further validate our findings.
Acknowledgments
Ms. Xilin Shi and Ms. Dan Liao of Heilongjiang University are gratefully acknowledged for their illustrations of the surgical procedures in this study. Moreover, we extend our heartfelt thanks to all the patients who were part of this study.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-56/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-56/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-56/coif). C.W. receives funding from the National Natural Science Foundation of China and the Outstanding Young Medical Talent Training Funding Project of First Affiliated Hospital of Harbin Medical University. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University (No. 2024XJSS74). All procedures performed in this study were in accordance with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patients for publication of this article, accompanying images and video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Patel VR, Sivaraman A, Coelho RF, et al. Pentafecta: a new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur Urol 2011;59:702-7. [Crossref] [PubMed]
- Rassweiler J, Hruza M, Teber D, et al. Laparoscopic and robotic assisted radical prostatectomy--critical analysis of the results. Eur Urol 2006;49:612-24. [Crossref] [PubMed]
- Novara G, Ficarra V, Mocellin S, et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol 2012;62:382-404. [Crossref] [PubMed]
- Galfano A, Ascione A, Grimaldi S, et al. A new anatomic approach for robot-assisted laparoscopic prostatectomy: a feasibility study for completely intrafascial surgery. Eur Urol 2010;58:457-61. [Crossref] [PubMed]
- Nagasubramanian S, Leow JJ, Sooriakumaran P. Retzius-Sparing Robot-Assisted Radical Prostatectomy. J Endourol 2025;39:S27-34. [Crossref] [PubMed]
- Wagaskar VG, Mittal A, Sobotka S, et al. Hood Technique for Robotic Radical Prostatectomy-Preserving Periurethral Anatomical Structures in the Space of Retzius and Sparing the Pouch of Douglas, Enabling Early Return of Continence Without Compromising Surgical Margin Rates. Eur Urol 2021;80:213-21. [Crossref] [PubMed]
- Menon M, Tewari A, Peabody J, et al. Vattikuti Institute prostatectomy: technique. J Urol 2003;169:2289-92. [Crossref] [PubMed]
- Porpiglia F, Bertolo R, Manfredi M, et al. Total Anatomical Reconstruction During Robot-assisted Radical Prostatectomy: Implications on Early Recovery of Urinary Continence. Eur Urol 2016;69:485-95. [Crossref] [PubMed]
- Zhang H, Ning Z, Jia G, et al. Modified hood technique for single-port robot-assisted radical prostatectomy contributes to early recovery of continence. Front Surg 2023;10:1132303. [Crossref] [PubMed]
- Student V Jr, Vidlar A, Grepl M, et al. Advanced Reconstruction of Vesicourethral Support (ARVUS) during Robot-assisted Radical Prostatectomy: One-year Functional Outcomes in a Two-group Randomised Controlled Trial. Eur Urol 2017;71:822-30. [Crossref] [PubMed]
- Tutolo M, Rosiello G, Stabile G, et al. The key role of levator ani thickness for early urinary continence recovery in patients undergoing robot-assisted radical prostatectomy: A multi-institutional study. Neurourol Urodyn 2022;41:1563-72. [Crossref] [PubMed]
- Trinh L, Mingo S, Vanstrum EB, et al. Survival Analysis Using Surgeon Skill Metrics and Patient Factors to Predict Urinary Continence Recovery After Robot-assisted Radical Prostatectomy. Eur Urol Focus 2022;8:623-30. [Crossref] [PubMed]
- Lee R, Te AE, Kaplan SA, et al. Temporal trends in adoption of and indications for the artificial urinary sphincter. J Urol 2009;181:2622-7. [Crossref] [PubMed]
- Kretschmer A, Nitti V. Surgical Treatment of Male Postprostatectomy Incontinence: Current Concepts. Eur Urol Focus 2017;3:364-76. [Crossref] [PubMed]
- John H, Sullivan MP, Bangerter U, et al. Effect of radical prostatectomy on sensory threshold and pressure transmission. J Urol 2000;163:1761-6.
- Kadono Y, Nohara T, Kawaguchi S, et al. Investigating the mechanism underlying urinary continence using dynamic MRI after Retzius-sparing robot-assisted radical prostatectomy. Sci Rep 2022;12:3975. [Crossref] [PubMed]
- Patel VR, Coelho RF, Palmer KJ, et al. Periurethral suspension stitch during robot-assisted laparoscopic radical prostatectomy: description of the technique and continence outcomes. Eur Urol 2009;56:472-8. [Crossref] [PubMed]
- Rocco B, Gregori A, Stener S, et al. Posterior reconstruction of the rhabdosphincter allows a rapid recovery of continence after transperitoneal videolaparoscopic radical prostatectomy. Eur Urol 2007;51:996-1003. [Crossref] [PubMed]
- Moon HW, Rhew SA, Yoon CE, et al. Impact of modified bladder neck suspension on early recovery of continence after robot-assisted radical prostatectomy (RARP). J Robot Surg 2023;17:2279-85. [Crossref] [PubMed]
- Gao Y, Yang Y, Li X, et al. Vesicoprostatic muscle reconstruction: a step further for immediate and early urinary continence. World J Urol 2023;41:1511-7. [Crossref] [PubMed]
- Walsh PC, Donker PJ. Impotence Following Radical Prostatectomy: Insight into Etiology and Prevention. J Urol 2017;197:S165-70. [Crossref] [PubMed]
- Kadono Y, Ueno S, Kadomoto S, et al. Use of preoperative factors including urodynamic evaluations and nerve-sparing status for predicting urinary continence recovery after robot-assisted radical prostatectomy: Nerve-sparing technique contributes to the reduction of postprostatectomy incontinence. Neurourol Urodyn 2016;35:1034-9. [Crossref] [PubMed]
- Ando S, Sugihara T, Hinotsu S, et al. Early recovery of urinary continence after robot-assisted radical prostatectomy is associated with membranous urethra and neurovascular bundle preservation. Int J Urol 2024;31:492-9. [Crossref] [PubMed]
- Choi J, Yang YJ, Lee CU, et al. Effects of bladder neck sparing on continence outcomes of robotic-assisted radical prostatectomy: a systemic review and metaanalysis. Prostate Int 2024;12:179-85. [Crossref] [PubMed]
- Chierigo F, Caviglia A, Cellini V, et al. Retzius sparing robot-assisted radical prostatectomy: optimizing functional results. World J Urol 2024;42:385. [Crossref] [PubMed]
- Vigneswaran HT, Schwarzman LS, Francavilla S, et al. A Comparison of Perioperative Outcomes Between Single-port and Multiport Robot-assisted Laparoscopic Prostatectomy. Eur Urol 2020;77:671-4. [Crossref] [PubMed]
- Saidian A, Fang AM, Hakim O, et al. Perioperative Outcomes of Single vs Multi-Port Robotic Assisted Radical Prostatectomy: A Single Institutional Experience. J Urol 2020;204:490-5. [Crossref] [PubMed]
- Galfano A, Di Trapani D, Sozzi F, et al. Beyond the learning curve of the Retzius-sparing approach for robot-assisted laparoscopic radical prostatectomy: oncologic and functional results of the first 200 patients with ≥ 1 year of follow-up. Eur Urol 2013;64:974-80. [Crossref] [PubMed]
- Sayyid RK, Simpson WG, Lu C, et al. Retzius-Sparing Robotic-Assisted Laparoscopic Radical Prostatectomy: A Safe Surgical Technique with Superior Continence Outcomes. J Endourol 2017;31:1244-50. [Crossref] [PubMed]
- Dalela D, Jeong W, Prasad MA, et al. A Pragmatic Randomized Controlled Trial Examining the Impact of the Retzius-sparing Approach on Early Urinary Continence Recovery After Robot-assisted Radical Prostatectomy. Eur Urol 2017;72:677-85. [Crossref] [PubMed]
- Lee J, Kim HY, Goh HJ, et al. Retzius Sparing Robot-Assisted Radical Prostatectomy Conveys Early Regain of Continence over Conventional Robot-Assisted Radical Prostatectomy: A Propensity Score Matched Analysis of 1,863 Patients. J Urol 2020;203:137-44. [Crossref] [PubMed]


