
Biopsy-free radical prostatectomy for prostate cancer—modern reality or pipe dream?
Current role of prostate biopsy
Treatment for localised prostate cancer generally results in effective local control at the expense of local side effects. A histopathological diagnosis, prior to treatment, has been mandatory according to the World Health Organisation (1), in order to justify radical treatment and potential morbidity (2). Additional information from pathology assists with risk stratification, prognosis estimation and management selection [e.g., use of androgen deprivation therapy (ADT) with radiotherapy, use of pelvic lymph node dissection during surgery] (2). Diagnosis by prostate needle biopsy has changed over time, with use of imaging [e.g., multiparametric magnetic resonance imaging (MRI)] and transperineal prostate biopsy shown to reduce over-biopsying and related side effects (e.g., sepsis) (3,4). Despite these benefits of a biopsy based diagnostic paradigm, some patients and clinicians seek a biopsy free approach (5). Besides avoiding biopsy related risks and side effects, spread of cancer from biopsy-related manipulation as a concept with some supportive data (6,7), is a major concern for some patients.
Biopsy-free radical prostatectomy—data so far
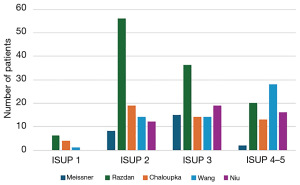
The advent of prostate specific membrane antigen (PSMA) positron emission tomography (PET) has improved staging (8) and intra-prostatic characterization of prostate cancer (9,10). Additionally, PSMA PET serves to improve diagnostic accuracy of prostate biopsy and may translate to a reasonable biopsy avoidance rate in patients with two negative imaging studies (11). Conversely, patients with highly suspicious imaging on both MRI and PSMA PET have a very high likelihood of harbouring a significant prostate cancer (12). Through this unprecedented diagnostic accuracy obtained noninvasively through imaging, a biopsy free treatment approach has gained enthusiasm in recent years (13). This approach has been used for renal, testicular and other tumours for many years. Preliminary, small retrospective studies have reported on patients proceeding to surgery without biopsy on the basis of imaging parameters with encouraging results (Figure 1) (5,14,15). However, in these previous series, several cases of discordant radiology and pathology have been reported—resulting in prostatectomy without significant cancer, thankfully with good functional outcomes (16). A small prospective study using a diagnostic prediction model combining prostate specific antigen (PSA) density with MRI and PSMA PET confirmed insignificant prostate cancer in only 1.8% of patients (1/57) (17).
Potential downfalls of biopsy-free prostatectomy
A pervading concern is whether a biopsy-free paradigm is opening the flood gates without reliable tools and high level evidence to justify safety for patients (18). The study by Tang and colleagues “Benign Prostatic Hyperplasia-Related False-Positive of Prostate-Specific Membrane Antigen-Positron Emission Tomography in the Diagnosis of Prostate Cancer: The Achilles’ Heel of Biopsy-Free Radical Prostatectomy?” (19) provides valuable data to inform this treatment paradigm. They considered patients who had undergone prostate MRI, PSMA PET and prostate biopsy with subsequent negative histology [benign prostatic hyperplasia (BPH) group] or positive histology that preceded to radical prostatectomy (cancer group). Within their study, they reported a 30% false positive rate (PET score 3–5) in the BPH group, with requisite immunohistochemical staining confirming moderate to strong intensity so biopsy sampling error was less likely. Within the cancer cohort, a maximum standardized uptake value (SUVmax) of 9.8 showed a 100% specificity and 60% sensitivity. When considered relative to the BPH cohort, in order to definitely confirm significant cancer (100% specificity), a SUVmax of 15 was required. This confidence was maintained but sensitivity increased by 8% (41% to 49%) if MRI findings were incorporated [Prostate Imaging-Reporting and Data System (PIRADS) ≥4 and PET ≥4].
The findings from this study indicate that a biopsy free treatment pathway is only obtainable for patients with very high SUVmax values on PSMA PET. The available small series have a large proportion of patients below this cut off. While in the PRIMARY trial a SUVmax off of 12 resulted in 100% specificity for significant prostate cancer (12), the study by Tang as well as others (20) necessitate that a very high cut off is required, which means many patients still require biopsy. Furthermore, specific MRI and PSMA quantitative cut-offs are difficult to define due to heterogeneity in MRI and PSMA machines, acquisition parameters tracers used (21). Interobserver variability is also a well-known limitation of MRI (22) and evolving for PSMA PET (23). Such heterogeneity limits reliability and diagnostic accuracy of specific parameters to recommend that biopsy can be safely avoided. Additionally, health system considerations are important, such as cost and access. PSMA PET needs to be used in combination with MRI to guide treatment decisions, which increases cost for patients and health systems of varying degrees depending on the setting (e.g., PSMA PET more expensive and inaccessible in North America compared to Australia, inverse for tissue molecular testing).
While side effects of prostate biopsy can cause harm, they are generally infrequent and minor, particularly using MRI triage to reduce biopsy numbers and a transperineal biopsy approach (3,4). Oncological concerns regarding prostate biopsy have merit, however the majority of low-intermediate risk patients (lowest risk of cancer-mediated metastasis), all of whom undergo biopsy prior to treatment, remain cured at 20-year follow-up (24).
For the small proportion that may be eligible for a biopsy free pathway, they will be deprived of the additional information provided by the biopsy that influence evidence-based management plans. Further to traditional prognostic measures, such as Gleason score and variant morphology (e.g., intraductal carcinoma), molecular classifiers (e.g., DecipherTM) and artificial intelligence image processing (e.g., ArteraAI) serve to improve on prognostication (25) and treatment selection to further personalise treatment. Absence of tissue for these tests would deprive patients of this information for decision-making. Additionally, it is likely that biopsy-free pathway will limit patient choice for local treatment, such as radiotherapy and/or ADT. It would be unlikely that a radiation oncologist would commit to biopsy-free radiotherapy with curative intent without histological diagnosis and grade, much like a medical oncologist is unlikely to give chemotherapy without a tissue diagnosis. So this leaves surgery as the only option, as histopathology is obtained post-operatively, which is inconsistent with shared-decision making principles that underpin prostate cancer management (2).
Future directions
While it is conceivable that clinicians and patients may eventually transition to a biopsy-free pathway for prostate cancer for some patients, improvements in current tools or introduction of new tools are needed before this is a reliable approach that can be widely practised.
Acknowledgments
None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Andrology and Urology. The article has undergone external peer review.
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-8/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-8/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ilbawi A, Varghese C, Loring B, et al. Guide to cancer early diagnosis. World Health Organization; 2017.
- Mottet N, Bellmunt J, Briers E, et al. EAU-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Arnhem: EAU Guidelines Office; 2022.
- Donato P, Morton A, Yaxley J, et al. Improved detection and reduced biopsies: the effect of a multiparametric magnetic resonance imaging-based triage prostate cancer pathway in a public teaching hospital. World J Urol 2020;38:371-9. [Crossref] [PubMed]
- Roberts MJ, Macdonald A, Ranasinghe S, et al. Transrectal versus transperineal prostate biopsy under intravenous anaesthesia: a clinical, microbiological and cost analysis of 2048 cases over 11 years at a tertiary institution. Prostate Cancer Prostatic Dis 2021;24:169-76. [Crossref] [PubMed]
- Meissner VH, Rauscher I, Schwamborn K, et al. Radical Prostatectomy Without Prior Biopsy Following Multiparametric Magnetic Resonance Imaging and Prostate-specific Membrane Antigen Positron Emission Tomography. Eur Urol 2022;82:156-60. [Crossref] [PubMed]
- Joosse SA, Beyer B, Gasch C, et al. Tumor-Associated Release of Prostatic Cells into the Blood after Transrectal Ultrasound-Guided Biopsy in Patients with Histologically Confirmed Prostate Cancer. Clin Chem 2020;66:161-8. [Crossref] [PubMed]
- Volanis D, Neal DE, Warren AY, et al. Incidence of needle-tract seeding following prostate biopsy for suspected cancer: a review of the literature. BJU Int 2015;115:698-704. [Crossref] [PubMed]
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 2020;395:1208-16.
- Roberts MJ, Maurer T, Perera M, et al. Using PSMA imaging for prognostication in localized and advanced prostate cancer. Nat Rev Urol 2023;20:23-47. [Crossref] [PubMed]
- Donato P, Morton A, Yaxley J, et al. (68)Ga-PSMA PET/CT better characterises localised prostate cancer after MRI and transperineal prostate biopsy: Is (68)Ga-PSMA PET/CT guided biopsy the future? Eur J Nucl Med Mol Imaging 2020;47:1843-51. [Crossref] [PubMed]
- Emmett L, Buteau J, Papa N, et al. The Additive Diagnostic Value of Prostate-specific Membrane Antigen Positron Emission Tomography Computed Tomography to Multiparametric Magnetic Resonance Imaging Triage in the Diagnosis of Prostate Cancer (PRIMARY): A Prospective Multicentre Study. Eur Urol 2021;80:682-9. [Crossref] [PubMed]
- Ptasznik G, Papa N, Kelly BD, et al. High prostate-specific membrane antigen (PSMA) positron emission tomography (PET) maximum standardized uptake value in men with PI-RADS score 4 or 5 confers a high probability of significant prostate cancer. BJU Int 2022;130:5-7. [Crossref] [PubMed]
- Pomykala KL, Herrmann K, Emmett L, et al. Virtual Prostate Biopsy with Prostate-specific Membrane Antigen and Magnetic Resonance Imaging: Closer to Reality in a Subgroup of Prostate Cancer Patients? Eur Urol Open Sci 2022;44:11-2. [Crossref] [PubMed]
- Chaloupka M, Apfelbeck M, Pyrgidis N, et al. Radical Prostatectomy without Prior Biopsy in Patients with High Suspicion of Prostate Cancer Based on Multiparametric Magnetic Resonance Imaging and Prostate-Specific Membrane Antigen Positron Emission Tomography: A Prospective Cohort Study. Cancers (Basel) 2023;15:1266. [Crossref] [PubMed]
- Niu S, Ding X, Liu B, et al. Radical Prostatectomy Without Prior Biopsy in Selected Patients Evaluated by (18)F-Labeled Prostate-Specific Membrane Antigen-Ligand Positron Emission Tomography/Computed Tomography and Multiparameter Magnetic Resonance Imaging: A Single-Center, Prospective, Single-Arm Trial. J Urol 2024;212:280-9. [Crossref] [PubMed]
- Razdan S, Parekh S, Watts EK, et al. Robot-Assisted Radical Prostatectomy in PIRADS 5 Lesions Without Prior Biopsy: Is Biopsy Really Necessary in This Cohort? J Endourol 2024;38:1062-9. [Crossref] [PubMed]
- Wang C, Xie Q, Yuan L, et al. Radical prostatectomy without prostate biopsy based on a noninvasive diagnostic strategy: a prospective single-center study. Prostate Cancer Prostatic Dis 2025;28:496-502. [Crossref] [PubMed]
- Bahadori A, Woods J, Yuan L, et al. Biopsy-free radical prostatectomy: a narrative review considering rationale, limitations, and current data. Prostate International 2025; [Crossref]
- Tang W, Tang Y, Qi L, et al. Benign Prostatic Hyperplasia-Related False-Positive of Prostate-Specific Membrane Antigen-Positron Emission Tomography in the Diagnosis of Prostate Cancer: The Achilles' Heel of Biopsy-Free Radical Prostatectomy? J Urol 2023;210:845-55. [Crossref] [PubMed]
- Kalapara AA, Ballok ZE, Ramdave S, et al. Combined Utility of (68)Ga-Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography and Multiparametric Magnetic Resonance Imaging in Predicting Prostate Biopsy Pathology. Eur Urol Oncol 2022;5:314-20. [Crossref] [PubMed]
- Ptasznik G, Moon D, Buteau J, et al. A Systematic Review of the Variability in Performing and Reporting Intraprostatic Prostate-specific Membrane Antigen Positron Emission Tomography in Primary Staging Studies. Eur Urol Open Sci 2023;50:91-105. [Crossref] [PubMed]
- Pickersgill NA, Vetter JM, Andriole GL, et al. Accuracy and Variability of Prostate Multiparametric Magnetic Resonance Imaging Interpretation Using the Prostate Imaging Reporting and Data System: A Blinded Comparison of Radiologists. Eur Urol Focus 2020;6:267-72. [Crossref] [PubMed]
- Donswijk ML, Ettema RH, Meijer D, et al. The accuracy and intra- and interobserver variability of PSMA PET/CT for the local staging of primary prostate cancer. Eur J Nucl Med Mol Imaging 2024;51:1741-52. [Crossref] [PubMed]
- Meissner VH, Woll M, Ankerst DP, et al. Long-term and pathological outcomes of low- and intermediate-risk prostate cancer after radical prostatectomy: implications for active surveillance. World J Urol 2021;39:3763-70. [Crossref] [PubMed]
- Tward JD, Huang HC, Esteva A, et al. Prostate Cancer Risk Stratification in NRG Oncology Phase III Randomized Trials Using Multimodal Deep Learning With Digital Histopathology. JCO Precis Oncol 2024;8:e2400145. [Crossref] [PubMed]


