
Correlation between lifestyle choices, dietary factors, and the risk of adult urolithiasis: insights from a systematic review and meta-analysis
Highlight box
Key findings
• This meta-analysis (a statistical method combining results from multiple studies) identifies key risk factors for urinary stone disease (USD), including advanced age, family history of urolithiasis, high intake of animal protein/fat, low water consumption, and reduced vinegar/legume intake.
• Heterogeneity (variability across studies) was observed in family history and water intake analyses, while age, vinegar, and legume intake showed consistent correlations.
What is known and what is new?
• USD is linked to genetic, dietary, and lifestyle factors, with high recurrence rates globally. Prior studies suggest roles of obesity, diabetes, and dietary habits in stone formation.
• This study systematically quantifies the strength of associations between specific dietary/lifestyle factors (e.g., vinegar, legumes, water intake) and USD risk, highlighting underreported correlations. It also underscores methodological gaps in existing literature.
What is the implication, and what should change now?
• Clinicians and public health strategies should emphasize dietary modifications (e.g., increased water, vinegar, and legume intake) and lifestyle adjustments to reduce USD risk.
• Future research should prioritize longer intervention periods, standardized methodologies, and larger cohorts to validate these findings and refine prevention guidelines.
Introduction
Urinary stone disease (USD) refers to the formation of stones in various parts of the urinary system, encompassing kidney stones, ureteral stones, bladder stones, and urethral calculi. The former two types are classified as upper urinary tract stones, while the latter two are categorized as lower urinary tract stones. The incidence of USD within the Chinese population ranges from 1% to 10%, and notably, the 10-year recurrence rate after treatment can be as high as 50% (1). In Europe and the United States, the lifetime prevalence of kidney stones is approximately 81%, with the incidence continuing to rise (2,3). Given the increasing prevalence and recurrence rates, urolithiasis has emerged as a significant public health concern worldwide. Despite this, the etiology and underlying mechanisms of urolithiasis remain poorly understood. It is widely accepted that genetic, gender, and environmental factors contribute to the development of this condition. For instance, studies have shown that individuals with diabetes and obesity are 3.5 times more likely to develop uric acid stones compared with the general population (4). To effectively manage and reduce the incidence of USD, it is essential to address these underlying factors.
Dietary habits play a critical role in the pathogenesis of adult urolithiasis (5). Various dietary patterns can elevate the concentration of solutes in urine, thereby increasing the risk of stone formation. Diets high in salt, protein, and phosphorus may lead to higher levels of calcium, uric acid, oxalic acid, and other stone-forming components in urine (6,7). Additionally, unhealthy eating habits can contribute to weight gain, exacerbating the supersaturation of solutes in urine and serving as a potential risk factor in the development of urolithiasis.
Lifestyle choices are also significantly linked to the occurrence of adult urolithiasis (8). Poor lifestyle practices, such as inadequate water intake, irregular daily routines, and lack of physical exercise, can adversely affect the dilution and clearance functions of urine. This accumulation of harmful components in urine increases the likelihood of urinary stone formation (9). Therefore, a comprehensive analysis of the interplay between dietary factors, lifestyle, and adult urolithiasis is essential. Such an analysis is expected to provide a scientific foundation for disease prevention and treatment, aiding in the development of effective health management strategies.
Currently, there is a dearth of observational and case-control studies investigating the factors influencing the risk of adult urolithiasis. Existing studies often yield varying conclusions and exhibit substantial differences in study design and evaluation metrics. Relying solely on the findings from individual studies to establish a correlation between specific influencing factors and the risk of adult urolithiasis is insufficient and may lack persuasive power (10). Therefore, robust support from high-quality research evidence is necessary to substantiate these correlations. More comprehensive research is required in this field, and there is an increasing need for credible scientific studies to elucidate the relevant factors affecting the risk of adult urolithiasis.
To address these gaps, this research employs a rigorous, quantitative, and systematic meta-analysis methodology to examine the findings from related independent investigations. The primary objective is to identify the risk factors associated with adult urolithiasis, thereby providing a scientific foundation for therapeutic applications and informing future research directions in this area. Through this analysis, we aim to contribute to a better understanding of the complex interplay between dietary and lifestyle factors and the risk of urolithiasis, ultimately guiding effective prevention and management strategies for this prevalent condition. We present this article in accordance with the MOOSE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-2024-768/rc).
Methods
Sources and retrieval methods of documents
A comprehensive search was conducted across multiple databases, including the Chinese Biomedical Database (CBM), Wanfang Database, China National Knowledge Infrastructure (CNKI), PubMed, EMBASE, ScienceDirect, Cochrane Library, and the VIP full-text database. Additionally, relevant Chinese and international journals, conference papers, theses, news articles, and manual literature tracking were incorporated into the search strategy. The collected data focused on correlation analyses and multivariate analyses pertaining to the risk factors associated with adult urolithiasis. The literature retrieval utilized key terms such as dietary factors, lifestyle, adult urolithiasis, and USD. The search was conducted for studies published from January 2010 onwards to ensure the inclusion of the most recent and pertinent research regarding the relationship between dietary factors, lifestyle, and the risk of adult urolithiasis.
Criteria for inclusion and exclusion of literature
Criteria for including literature
- Research types: observational studies, including case-control and cohort studies.
- Subjects: (i) there were no restrictions on age, sex, or race; (ii) all cases were diagnosed as urinary calculi via ultrasound, X-ray, or computed tomography (CT) and urography, with relevant literature cited for the diagnostic criteria.
- Exposure factors: factors considered included age, family history of stones, legume intake, vinegar intake, daily drinking water intake, and animal fat protein intake.
- For patients with varying characteristics, the study calculated the relative risk (RR) or odds ratio (OR) adjusted for age, body mass index (BMI), and sex. Additionally, the corresponding 95% confidence interval (95% CI) was determined using complete contingency table data. This adjustment aimed to provide a more accurate and reliable assessment of the correlation between specific factors and the risk of adult urolithiasis, while accounting for potential confounding variables.
Criteria for excluding literature
- In cases where two articles reported the same study or contained duplicate data, the article with the more comprehensive sample size was retained, ensuring the inclusion of the most robust dataset and minimizing redundancy to enhance the reliability of the meta-analysis results.
- Information that was deemed irrelevant or lacking essential components was excluded.
- If research content was repeated, the most recent study was prioritized for inclusion, thereby ensuring that the latest and most relevant findings were used and preventing redundancy in the meta-analysis.
- Studies that provided insignificant evaluations of efficacy were excluded.
- Review articles were not included.
- Clinical case reports were excluded.
Quality evaluation and data extraction
- Bias risk assessment: the bias risk assessment was conducted using the tool recommended in the Cochrane Systematic Review Manual (version 5.3).
- Data extraction and literature review: two independent investigators were responsible for screening the literature, extracting data, and evaluating quality. Any discrepancies or disagreements were resolved through discussion between the two investigators. If consensus could not be reached, a third researcher was consulted to assist in the judgment, ensuring a rigorous and objective review process. Data management and extraction were facilitated using Excel and Note Express document management software. If any data were found to be lacking, the authors of the respective studies would be contacted for clarification. The extracted information included author, publication date, number of cases, method of result evaluation, RR/OR, and 95% CI.
Statistical analysis
RevMan 5.3 software, developed by the Cochrane Collaboration, was utilized for the meta-analysis. Count data were entered into RevMan 5.3 for analysis, using RR as the effect index. Measurement data were similarly analyzed, employing weighted mean difference (WMD) as the effect index, with calculations performed at a 95% CI. The initial analysis involved applying the χ2 test to assess heterogeneity among studies. If P>0.05 and I2<50%, the studies were deemed homogeneous, allowing for the application of a fixed effect model in the meta-analysis. Conversely, when P<0.05 and I2≥50%, the random effects model was implemented. If the source of heterogeneity was indeterminate, a meta-analysis was not conducted, and descriptive analysis was utilized instead. To evaluate publication bias within the literature, an inverted funnel chart (FC) was generated, and Egger’s test was performed to assess the asymmetry of the funnel plot. If the P value from Egger’s test was less than 0.1, the Trim and Fill method was employed to correct for publication bias.
Results
Outcomes of literature retrieval and inclusion criteria
Based on the Meta-Analyses of Observational Studies in Epidemiology (MOOSE) guidelines, a total of 872 articles were initially retrieved from the database. After removing duplicates, 651 studies remained. A preliminary review of abstracts and titles led to the retention of 483 articles. Further scrutiny, including the exclusion of irrelevant studies, non-controlled literature, case reports, and reviews, reduced the number to 366 studies. Subsequently, 360 studies were excluded after a thorough examination of the full text due to the absence of primary outcome indicators. Ultimately, 6 studies (11-16) were included in the analysis, with a total sample size of 3,500 cases. Figure 1 displays the flow chart outlining the literature screening process, and Table 1 presents the fundamental characteristics of the included studies.
Table 1
| Literatures | Year of publication | Sample size, n | Male, n | Female, n | Research type | Outcome index |
|---|---|---|---|---|---|---|
| Li YS (11) | 2019 | 1,447 | 658 | 789 | Cohort study | ①②③④ |
| Zhang HL (12) | 2014 | 100 | 52 | 48 | Cohort study | ①②⑤ |
| Chen S (13) | 2017 | 385 | 168 | 217 | Cohort study | ①②③④⑥ |
| Lin FH (14) | 2019 | 1,151 | 723 | 428 | Cohort study | ⑤⑥ |
| Zhang WW (15) | 2017 | 370 | 223 | 147 | Case control | ②⑤ |
| Ryu HY (16) | 2018 | 47 | 30 | 17 | Case control | ⑥ |
① Age; ② family history of calculi; ③ increased intake of vinegar; ④ increased intake of beans; ⑤ an increase in daily drinking water; ⑥ increased intake of animal protein and fat.
Quality assessment of the research methods
All the studies included in the analysis provided detailed descriptions of their research methods and outcome measures. However, none of the studies explicitly reported the quantity or reasons for blinding, lost follow-ups, or participant dropout. According to the Jadad scale, three studies (11-13) were classified as high-quality, scoring ≥3, while another three studies (14-16) were classified as low-quality, scoring ≤2. Figures 2,3 display the results of the bias risk assessment.
Meta-analysis results
Age
Three studies investigated the relationship between age and the risk of adult urolithiasis. The heterogeneity test revealed Chi2=0.70, df=2, P=0.70, I2=0%, indicating no significant heterogeneity among the studies. According to the fixed-effect model analysis, age was identified as a risk factor for adult urolithiasis (combined OR =3.45, 95% CI: 3.43–3.47, P<0.00001) (Figure 4).

Family history of stones
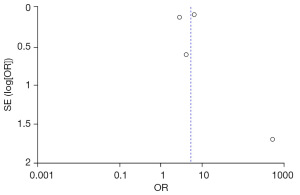
Four studies examined the relationship between a family history of urolithiasis and the risk of developing the condition in adults. The heterogeneity test revealed Chi2=32.66, df=3, P<0.00001, I2=91%, indicating substantial heterogeneity across the studies. Based on the random-effects model analysis (Figure 5), a family history of stones was found to be a significant risk factor for the occurrence of adult urolithiasis (combined OR =5.34, 95% CI: 2.50–11.38, P<0.0001).

Increased intake of vinegar
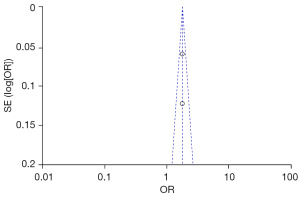
Two studies assessed the relationship between increased vinegar intake and the risk of adult urolithiasis. The heterogeneity test revealed Chi2=0.00, df=1, P=0.95, I2=0%, indicating no notable heterogeneity among the included studies. The fixed-effect model analysis showed that increased vinegar intake acted as a protective factor for the occurrence of adult urolithiasis (combined OR =1.76, 95% CI: 1.58–1.96, P<0.00001) (Figure 6).

Increased intake of legumes
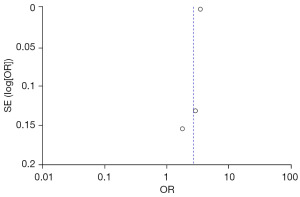
Two studies reported a link between increased legume consumption and the risk of adult urolithiasis. The heterogeneity test showed Chi2=0.00, df=1, P=1.00, I2=0%, indicating no significant variability between the study results. The fixed-effect model analysis indicated that higher legume intake was a protective factor for the development of adult urolithiasis (combined OR =2.40, 95% CI: 1.91–3.01, P<0.00001) (Figure 7).

Increased daily water intake
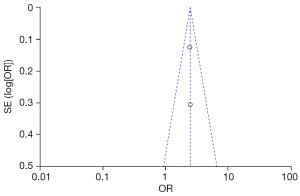
Three studies investigated the impact of increased daily water intake on the risk of adult urolithiasis. The heterogeneity test revealed Chi2=21.80, df=2, P<0.0001, I2=91%, suggesting considerable heterogeneity among the studies. Based on the random-effects model analysis (Figure 8), an increase in daily water intake was associated with a reduced risk of developing adult urolithiasis (combined OR =2.64, 95% CI: 1.81–3.85, P<0.00001).

Increased intake of animal protein and fat
Three studies examined the relationship between increased intake of animal protein and fat and the risk of adult urolithiasis. The heterogeneity test showed Chi2=13.84, df=2, P=0.001, I2=86%, indicating notable heterogeneity across the included studies. According to the random-effects model analysis (Figure 9), higher intake of animal protein and fat was identified as a risk factor for the development of adult urolithiasis (combined OR =4.31, 95% CI: 2.10–8.84, P<0.0001).

Publication bias analysis
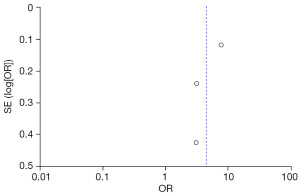
The FC were selected based on age, family history of stones, increased intake of vinegar, legumes, daily water intake, and animal protein and fat intake from the two groups. Publication bias was assessed (Figures 10-15). Most of the funnel plots showed a symmetrical shape, suggesting no significant publication bias. However, a small number of asymmetrical plots were observed, which could indicate some degree of publication bias, likely due to the study’s heterogeneity and the limited number of included studies.
Discussion
Urolithiasis refers to the formation of solid substances in the urinary system, characterized by varying numbers, sizes, shapes, and compositions of stones. These can lead to a range of clinical symptoms, including pain, hematuria, frequent urination, urgency, and discomfort (17). The prevalence and incidence rates of urolithiasis vary significantly across different countries and regions. The overall prevalence ranges from 2% to 20%, with the annual incidence rate approximately 1,500–2,000 cases per 100,000 people (18). Urolithiasis (USD) has been documented since ancient times, with early references in ancient Chinese medical texts, where it was called “Shilin”. Historically, it mainly affected young and middle-aged individuals. In recent years, the incidence of USD in China has risen, particularly in the southern regions, where the incidence is notably high. Considerable advancements have been made in the surgical treatment of urolithiasis, including techniques such as extracorporeal shock wave lithotripsy, ureteroscopy, percutaneous nephrolithotripsy, and other minimally invasive procedures. These methods have been proven successful, allowing over 90% of cases to be treated without the need for traditional surgery. Despite these advancements, the recurrence rate remains high, with approximately 50% of treated patients experiencing a relapse within 5 to 10 years, and around 75% within 20 years (19,20). Recurrent urolithiasis presents a more complex formation mechanism than primary stones, and many aspects of this process are still not fully understood. Consequently, highly effective preventive measures for recurrent stones remain lacking.
Urolithiasis, with its high morbidity, incidence, and recurrence rates, continues to pose a significant burden on both public health and the economy (21). The precise mechanisms and factors that trigger and inhibit USD formation are still not fully elucidated. Various risk factors influence the development of urolithiasis, some of which, like dietary habits, can be modified through lifestyle changes, while others, such as climate, family history, and age, remain outside of our control (22). Since metabolic components in urine are closely linked to dietary factors, the increasing incidence and recurrence of USD are directly related to changes in the social and nutritional structure. Understanding these risk factors is crucial in reducing the incidence and recurrence of urolithiasis.
Despite numerous studies examining the causes of urolithiasis, an accurate etiology remains elusive. Extensive research has shown a strong association between the onset of urolithiasis and factors such as dietary habits, lifestyle modifications, heredity, and genetic factors like sex, age, race, hyperoxaluria, and hyperuricemia (23,24). Epidemiological studies indicate that the global prevalence of urolithiasis has been steadily increasing, particularly with age (25). Our findings suggest that age is a significant risk factor for adult urolithiasis, likely due to age-related changes in metabolic status and hormone levels, which affect urine composition.
Studies have also demonstrated that individuals with a family history of kidney stones have a higher prevalence of urolithiasis compared to those without such a history. In our analysis, four of the six included studies reported a correlation between a family history of stones and the risk of adult urolithiasis. The random-effects model analysis confirmed that a family history of stones is indeed a risk factor for developing urolithiasis. Previous research has indicated that urolithiasis is a polygenic disease with genetic polymorphisms (26). Genes that influence the secretion of substances such as oxalate, calcium, and citrate may play a role in stone formation. Notably, alleles of genes like the calcitonin receptor gene, vitamin D receptor gene, and interleukin 1β-51112I locus have been shown to impact bone and calcium metabolism, which may contribute to the development of urolithiasis.
It has also been reported that excessive consumption of animal protein leads to an increase in endogenous acid and purine production, decreases renal tubular calcium reabsorption, and raises the urinary calcium filtration rate. These factors collectively heighten the risk of urolithiasis (27). Studies have shown that individuals on low-protein diets have significantly lower urinary calcium levels, suggesting that a low-protein, low-calcium diet can reduce the incidence of urolithiasis, while excessive consumption of animal protein, beans, and potatoes can increase urinary calcium concentrations (28). Our meta-analysis examined the impact of increased intake of vinegar, beans, and animal protein and fat on the risk of adult urolithiasis. The results indicate that increased intake of beans and vinegar can reduce the risk of urolithiasis, while elevated consumption of animal protein and fat appears to be a risk factor. This may be due to vinegar’s potential benefits in regulating blood sugar, lipid metabolism, and its antioxidant effects. Animal studies have shown that vinegar can inhibit the formation of calcium oxalate crystals in the kidneys, likely by regulating oxalate metabolism and enhancing the antioxidant activity of renal tissue. National epidemiological surveys suggest that the prevalence of urolithiasis in Shanxi, a province with a high consumption of vinegar-based foods, is notably lower than in other regions (1). The mechanism by which increased legume intake reduces the risk of stone formation remains unclear and warrants further investigation.
Numerous studies, both domestically and internationally, have demonstrated that individuals with inadequate daily water intake, delayed hydration, or chronic dehydration are at higher risk for developing urolithiasis compared to those with normal hydration levels. Our study, which included three studies on the relationship between increased daily water intake and urolithiasis risk, found that increasing daily water consumption significantly reduces the risk of adult urolithiasis. This suggests that adequate water intake serves as a protective factor against urolithiasis. Consuming sufficient water helps maintain normal body hydration during sleep and dilutes urine, preventing the supersaturation and precipitation of crystal-forming substances (29). On the other hand, low urine volume can lead to higher concentrations of calcium, oxalate, and uric acid in the urine, increasing the risk of stone formation. Drinking ample water increases urine volume, making it more difficult for stone nuclei to form and persist, thereby reducing the risk of stone formation and recurrence.
Our meta-analysis contributes to the existing literature by quantitatively synthesizing and reinforcing evidence regarding lifestyle and dietary factors associated with urolithiasis risk, providing greater clarity on the consistency and strength of these associations across various populations. Notably, our findings emphasize the protective effects of increased vinegar and legume intake against urolithiasis. While vinegar’s protective role has been acknowledged previously, our analysis systematically quantifies this effect, reinforcing its potential clinical significance and applicability for dietary guidelines. Additionally, the protective association identified between increased legume consumption and reduced stone risk highlights an area that remains insufficiently understood and merits focused mechanistic studies. By highlighting these specific dietary associations and explicitly addressing the limitations and gaps in the existing literature, our study adds valuable quantitative support and clear direction for future research in the prevention and management of urolithiasis. There are some limitations in this study. First, the exclusion of some unpublished conference data and studies with incomplete information may introduce publication bias. Second, the small number of included studies and the heterogeneity observed in the dose-effect analysis may affect the reliability of the results. Lastly, the presence of various confounding factors in the studies included in this meta-analysis may contribute to the observed heterogeneity.
Conclusions
In conclusion, the etiology of urolithiasis is multifactorial, involving a complex interaction of individual and environmental risk factors. Key individual factors, such as age and family history, as well as environmental factors like diet and hydration habits, all contribute to the development of urolithiasis. A thorough understanding of these risk factors and the implementation of appropriate preventive measures could help reduce the incidence and recurrence of urolithiasis. Therefore, it is recommended to maintain a balanced diet, avoid excessive consumption of meat and high-fat foods, and consider relevant medications to decrease stone salt saturation and enhance the activity of urinary inhibitors, ultimately mitigating the risk of urolithiasis.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-768/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2024-768/prf
Funding: This study was funded by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2024-768/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Spradling K, Ganesan C, Conti S. Medical Treatment and Prevention of Urinary Stone Disease. Urol Clin North Am 2022;49:335-44. [Crossref] [PubMed]
- Goka SQ, Copelovitch L. Prevention of recurrent urinary stone disease. Curr Opin Pediatr 2020;32:295-9. [Crossref] [PubMed]
- Tekgül S, Stein R, Bogaert G, et al. European Association of Urology and European Society for Paediatric Urology Guidelines on Paediatric Urinary Stone Disease. Eur Urol Focus 2022;8:833-9. [Crossref] [PubMed]
- Jinyi W, Zhang Y, Wang K, et al. Global, regional, and national mortality of tuberculosis attributable to alcohol and tobacco from 1990 to 2019: A modelling study based on the Global Burden of Disease study 2019. J Glob Health 2024;14:04023. [Crossref] [PubMed]
- Wright JF, Craig WY, Lucas FL, et al. Urinary stone disease prevalence and associations in cystic fibrosis. Urolithiasis 2021;49:415-23. [Crossref] [PubMed]
- Wu J, Wang K, Tao F, et al. The association of blood metals with latent tuberculosis infection among adults and adolescents. Front Nutr 2023;10:1259902. [Crossref] [PubMed]
- Lee JA, Abramowitz MK, Kipperman N, et al. Exploring the Association of Asthma with Urinary Stone Disease: Results from the National Health and Nutrition Examination Survey 2007-2014. Eur Urol Focus 2020;6:354-60. [Crossref] [PubMed]
- Wu J, Xiao P, Zhang Y, et al. Evaluation of the Effectiveness of Global Tuberculosis Control Strategies at Different Stages and Analysis of Risk Factors: Findings From the Global Burden of Disease 2021. Arch Bronconeumol 2024;S0300-2896(24)00455-1.
- Bhojani N, Miller LE, Bhattacharyya S, et al. Risk Factors for Urosepsis After Ureteroscopy for Stone Disease: A Systematic Review with Meta-Analysis. J Endourol 2021;35:991-1000. [Crossref] [PubMed]
- Kumar A, Goyal R, Garg K, et al. Insights from a brief study of renal calculi: recent diagnostic and treatment approaches. J Bio-X Res 2024;7:0002.
- Li YS, Zeng GH, Mai ZL, et al. Analysis on the associated factors of adult urolithiasis in China based on two-level Logistic regression model. Chinese Journal of Disease Control & Prevention 2019;23:866-70.
- Zhang HL. Risk factors of urolithiasis. Inner Mongolia Traditional Chinese Medicine. 2014;33:50-51.
- Chen S, Mai Z, Wu W, et al. Associated factors of urolithiasis for adult residents in rural areas of China. Journal of Clinical Urology 2017;32:429-32.
- Lin FH, Chen XZ, Cai BJ, et al. Investigation on pathogenic factors of 1151 cases of urolithiasis. Chinese Journal of Primary Medicine 2019;26:5.
- Zhang WW, Yu Q, Wu HX, et al. Investigation and analysis of related factors of urolithiasis. Zhejiang Medical Education 2017;16:55-7.
- Ryu HY, Lee YK, Park J, et al. Dietary risk factors for urolithiasis in Korea: A case-control pilot study. Investig Clin Urol 2018;59:106-11. [Crossref] [PubMed]
- Basiri A, Naji M, Houshmand M, et al. CAG repeats and one polymorphism in androgen receptor gene are associated with renal calcium stone disease. Urologia 2022;89:391-6. [Crossref] [PubMed]
- Siener R. Nutrition and Kidney Stone Disease. Nutrients 2021;13:1917. [Crossref] [PubMed]
- Gianella FG, Prado VE, Poindexter JR, et al. Spot urinary citrate-to-creatinine ratio is a marker for acid-base status in chronic kidney disease. Kidney Int 2021;99:208-17. [Crossref] [PubMed]
- Önal B, Kırlı EA, Canpolat N, et al. Different approaches among physicians to treat pediatric stone disease: a survey-based study. Arch Argent Pediatr 2021;119:83-90. [Crossref] [PubMed]
- Premakumar Y, Gadiyar N, Hameed BMZ, et al. Association of Kidney Stone Disease (KSD) with Primary Gastrointestinal Surgery: a Systematic Review over Last 2 Decades. Curr Urol Rep 2021;22:34. [Crossref] [PubMed]
- Miller AW, Penniston KL, Fitzpatrick K, et al. Mechanisms of the intestinal and urinary microbiome in kidney stone disease. Nat Rev Urol 2022;19:695-707. [Crossref] [PubMed]
- Singh P, Harris PC, Sas DJ, et al. The genetics of kidney stone disease and nephrocalcinosis. Nat Rev Nephrol 2022;18:224-40. [Crossref] [PubMed]
- Jones P, Karim Sulaiman S, Gamage KN, et al. Do Lifestyle Factors Including Smoking, Alcohol, and Exercise Impact Your Risk of Developing Kidney Stone Disease? Outcomes of a Systematic Review. J Endourol 2021;35:1-7. [Crossref] [PubMed]
- Xiao P, Li C, Mi J, et al. Evaluating the distinct effects of body mass index at childhood and adulthood on adult major psychiatric disorders. Sci Adv 2024;10:eadq2452. [Crossref] [PubMed]
- Ando R, Nagaya T, Suzuki S, et al. Independent and interactive effects of kidney stone formation and conventional risk factors for chronic kidney disease: a follow-up study of Japanese men. Int Urol Nephrol 2021;53:1081-7. [Crossref] [PubMed]
- Zhang Y, Wang K, Zhu J, et al. A network suspected infectious disease model for the development of syphilis transmission from 2015 to 2021 in Hubei province, China. J Appl Microbiol 2023;134:lxad311. [Crossref] [PubMed]
- Howles SA, Thakker RV. Genetics of kidney stone disease. Nat Rev Urol 2020;17:407-21. [Crossref] [PubMed]
- Jones P, Somani BK. Authors Reply to Editorial Comment: “Do Lifestyle Factors Including Smoking, Alcohol, and Exercise Impact Your Risk of Developing Kidney Stone Disease? Outcomes of a Systematic Review” by Jones, P et al. J Endourol 2021;35:737-8. [Crossref] [PubMed]






