
Analysis of the burden of disease for male infertility globally and in China from 1990 to 2021
Highlight box
Key findings
• China accounts for over one-fifth of the global prevalence and disability-adjusted life years (DALYs) associated with male infertility. The age-standardized rates (ASRs) in China are significantly higher than the global average. However, in contrast to the global trend of increasing burden, China’s rates have stabilized or declined in the past decade.
• Men aged 35–39 years are the most susceptible to male infertility.
• Globally, the burden of male infertility is most severe in Africa and Eastern Europe. Notably, ASRs in Eastern Europe continue to rise.
What is known and what is new?
• Infertility affects millions worldwide, exerting profound negative effects on population health, family and community cohesion, and individual psychological well-being. It is also considered a “poverty trap” in the pursuit of healthcare. Male infertility contributes to approximately 50% of cases among the estimated one in six infertile couples globally.
• Male infertility affects an estimated 55 million individuals globally and accounts for approximately 300,000 DALYs. The burden of disease varies significantly by region and age, and is closely linked to the Socio-Demographic Index (SDI). In recent years, the most rapid increases in burden have been observed in low and lower-middle SDI regions, including Sub-Saharan Africa, South Asia, and Southeast Asia.
Implications and recommendations
• The findings highlight a steadily increasing global burden of male infertility over the past 30 years, indicating a long-standing neglect of this non-fatal condition.
• There is an urgent need for comprehensive interventions, including environmental improvements, prevention and management of sexually transmitted infections, promotion of healthy lifestyles, and expansion of assisted reproductive services. These efforts should be prioritized in low and lower-middle SDI regions and Eastern Europe.
Introduction
The World Health Organization (WHO) defines infertility as the inability to achieve a successful pregnancy after 12 months of unprotected intercourse (1). Infertility often causes significant emotional distress for affected couples, manifesting as depression, guilt, shame, social isolation, and marital stress (2-4). Beyond its psychological toll, infertility poses a substantial and growing disease burden. In the United States, the total expenditure for treating primary male infertility in 2000 was estimated at $17 million, with the cost of assisted reproductive technology (ART) cycles raising the total to approximately $18 billion (5). The WHO highlights infertility as both a critical equity issue and a “medical poverty trap”, as millions of individuals face catastrophic healthcare costs while seeking treatment (6). Recent research has linked male infertility to reduced life expectancy, an increased risk of chronic conditions such as hypertension, and a higher likelihood of certain malignancies (7-10).
Although several epidemiological surveys on male infertility have been conducted, they are often geographically limited, based on cohort studies of high-risk populations or governmental reports (11-15). Male infertility is influenced by numerous factors, with cultural and societal norms significantly impacting diagnosis and treatment in regions such as North Africa and the Middle East. In these patriarchal societies, infertility is frequently attributed to women, while men are reluctant to undergo fertility assessments (14,16). Additionally, male infertility is often diagnosed and treated on an outpatient and self-funded basis, resulting in the absence of large, centralized datasets (11,12). Census-based studies face their own limitations, such as including voluntarily childless couples, who comprised 25–40% of childless couples in the United States during the 1920s and 1930s (12). These challenges collectively hinder the accurate epidemiological assessment of male infertility. Efforts to consolidate existing data have been limited. For instance, the Urologic Diseases Program in the United States attempted to review literature on male infertility in 2007 but found the available data insufficient to draw meaningful conclusions (5). The true prevalence of male infertility remains unclear, and estimates from heterogeneous studies often lack reliability (11). While some studies suggest that male infertility has increased over the past few decades, the extent of this increase and its underlying causes remain contentious (11,17-19).
The Global Burden of Disease (GBD) 2021 dataset provides comprehensive information on 369 diseases and injuries and 88 risk factors across 204 countries and territories from 1990 to 2021 (20). This study aims to utilize the GBD database to conduct a detailed analysis of the global prevalence and disability-adjusted life years (DALYs) associated with male infertility and compare these findings with data from China. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-44/rc).
Methods
Data acquisition and sources
This study utilized data from the GBD 2021 to assess the global impact of male infertility. The GBD 2021 dataset is available through the Global Health Data Exchange (GHDx) Results Tool (http://ghdx.healthdata.org/) and offers open access to detailed health metrics at global and regional levels. The dataset includes data on 371 diseases and injuries, covering incidence, prevalence, mortality, and DALYs, as well as 88 risk factors spanning behavioral, environmental, occupational, and metabolic domains. This analysis focused on male infertility prevalence and DALYs globally and in China. DALYs, a composite measure of years lived with disability (YLDs) and years of life lost (YLLs) due to premature death, were used to assess both fatal and nonfatal disease impacts. The Socio-Demographic Index (SDI), which combines total fertility rates, mean years of education, and per capita income, was also considered. SDI values range from 0 to 1, classifying countries into five tiers: low, low-middle, middle, high-middle, and high. Data from 204 countries and territories from 1990 to 2021 were analyzed, providing a comprehensive trend analysis and an in-depth evaluation of the global burden of male infertility. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. As the study used publicly available data, ethical approval and informed consent were not required. The research followed guidelines for accurate and transparent reporting in health assessments.
Population and global burden analysis
We examined age-standardized prevalence, DALYs, and their 95% uncertainty intervals (UIs) for male infertility, using data from the GBD 2021 study, which covers 204 countries, 21 GBD regions, and five SDI quintiles. The population was divided into seven age groups (15–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49 years) based on 5-year intervals. High-resolution maps were used to visualize the global burden of male infertility, emphasizing disparities across socio-demographic and geographic contexts. This spatial representation provided valuable insights into global patterns of male infertility.
SDI analysis
The association between the SDI and the burden of male infertility was explored by calculating disease rates specific to each SDI category. The SDI was divided into five levels—low, low-middle, middle, high-middle, and high—to facilitate a comparison of the disease burden at varying stages of socioeconomic development.
Decomposition analysis
Decomposition analysis was used to assess how population growth, aging, and epidemiological changes contributed to trends in total prevalence and DALYs. This method provided a clearer understanding of how demographic shifts and health system factors influence disease burden. The approach is consistent with the analytical framework used in previous GBD studies, which estimate how changes in population structure and risk factors impact disease outcomes.
Health inequality analysis
Health inequality analysis explores disparities in disease burden across different countries and regions, aiding in the formulation of public health policies. We employed the slope index of inequality (SII) and the concentration index (CII) to quantify health inequalities. The SII reflects the relationship between health indicators and socioeconomic status using SDI in a linear regression model. The CII ranges from −1 to 1, indicating variation in health outcomes based on economic status. Values closer to 0 suggest less inequality, positive values favor the wealthy, and negative values favor the impoverished. This study calculated the SII and CII for the prevalence and DALYs of male infertility from 1990 to 2021, emphasizing health inequalities globally, across regions, and among 204 countries.
Prediction analysis
To inform public health policy and medical resource allocation, we used the Bayesian-Aperiodic-People-Cohort (BAPC) model to predict trends in male infertility prevalence and DALYs over the next 15 years. These models, which account for temporal and age-specific variations, provide a reliable and comprehensive outlook on the future disease burden.
Statistical analysis
Statistical analyses were performed using R (version 4.3.3) and Stata 18 (StataCorp, College Station, TX, USA). Custom scripts were created for decomposition analyses. Bayesian analysis was conducted using WinBUGS (version 1.4). Geographic and spatial analyses were carried out with ArcGIS Pro and QGIS (version 3.16), enabling the creation of high-resolution maps to visualize male infertility burden and disparities. Data visualizations, including bi-lateral and two-axis plots, were generated using the ‘ggplot2’ and ‘Benchmarking’ packages in R. P<0.05 was set for all analyses to determine statistical significance, in line with standard practices in epidemiological and public health research, particularly in GBD studies.
Results
Comparison of global and Chinese epidemiology of male infertility and trend analysis
Prevalence
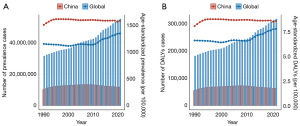
In 2021, the global prevalence of male infertility was 55,000,818 (95% UI: 32,611,257–88,727,953), with an age-standardized rate (ASR) of 1,354.76 per 100,000 (95% UI: 802.12–2,174.77). The number of prevalent cases exhibited a continuous upward trend, with particularly rapid growth observed after 2000. Compared to 1990, the number of cases in 2021 increased by 23,510,436, reflecting a growth of approximately 74.66%. The ASR also demonstrated significant growth after 2011, with an estimated annual percentage change (EAPC) of 0.5 [95% confidence interval (CI): 0.36–0.64] between 1990 and 2021. In China, the prevalence of male infertility similarly showed rapid growth, with an EAPC of 0.5 (95% CI: 0.36–0.64). By 2021, the number of prevalent cases reached 11,845,804 (95% UI: 6,488,726–20,756,171), representing 21.54% of the global total. The ASR in China was 1,591.79 per 100,000 (95% UI: 886.51–2,708.22), significantly exceeding the global average. However, unlike the global trend, the prevalence in China exhibited an initial increase followed by a decline, with the ASR stabilizing but consistently remaining higher than the global average. Between 1990 and 2021, the number of prevalent cases in China grew by only 1,589,177, representing a modest growth rate of approximately 15.5%, with an EAPC of 0.01 (95% CI: −0.05 to 0.06) (Table S1, Figure 1A).
DALYs
In 2021, the global burden of male infertility, as measured by DALYs, reached 317,614 (95% UI: 116,288–752,758), with an ASR of 7.84 per 100,000 (95% UI: 2.85–18.56). Similar to prevalence trends, DALYs displayed a general upward trajectory since 1990, with notable acceleration after 2011. Compared to 1990, DALYs increased by 135,745, representing a growth of approximately 74.6%. The EAPC for DALYs during this period was 0.51 (95% CI: 0.38–0.65). In China, DALYs in 2021 totaled 63,931 (95% UI: 21,752–155,614), accounting for 20.1% of the global total. The corresponding ASR was 8.66 per 100,000 (95% UI: 2.97–21.04), also significantly higher than the global average. However, in contrast to the global pattern, DALYs in China demonstrated an initial increase followed by a decline, with the ASR remaining relatively stable but persistently higher than the global average. Between 1990 and 2021, DALYs in China increased by only 8,776, reflecting a growth rate of 15.9%, with an EAPC of 0.03 (95% CI: −0.04 to 0.09) (Table S2, Figure 1B).
Prevalence and DALYs in subgroups (age, SDI, region, and country)
The global and Chinese distributions of male infertility prevalence and ASRs exhibited a unimodal pattern, heavily concentrated in the 20–44 years age group, particularly among individuals aged 35–39 years. This age group accounted for 24.2% of the global prevalence and 31.1% of the prevalence in China. The corresponding ASRs were 3.5 and 4.3 times higher than the global average, respectively. Since 1990, ASRs in China have consistently surpassed the global average across all age groups (Figure 2). The 30–34 and 35–39 years age groups have showed a dramatic increase in cases in recent years, reflected similarly in DALYs trends (Figure 2).
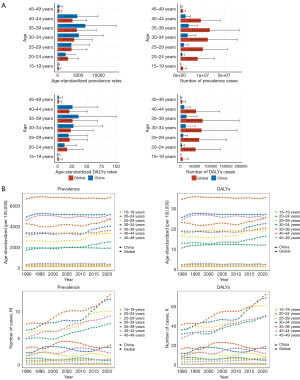
From the perspective of SDI regions, both prevalence and DALYs have steadily increased over time, with a rapid escalation in ASRs observed in high SDI, low-middle SDI, and low SDI regions since 2010 (Figure 3). These shifts have significantly influenced ASRs across various countries and regions (Figure 1). Middle SDI regions have consistently reported the highest number of cases and DALYs, while high-middle SDI regions have maintained the highest ASRs over the long term.

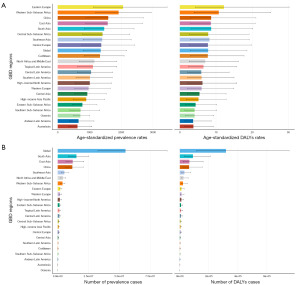
At the regional level, South and East Asia, including China, reported the highest prevalence and DALYs in 2021, contributing 27.7% and 22.1% of global cases and 28.4% and 20.7% of global DALYs, respectively. Southeast Asia closely followed, while Eastern Europe and Western Sub-Saharan Africa recorded the highest ASRs, approximately 1.5 times the global average and three times higher than those in regions such as Australasia or Andean Latin America. The age-standardized prevalence rates (ASPRs) and age-standardized death rates (ASDRs) in Western Sub-Saharan Africa generally exhibited a declining trend from 1990 to 2021, with corresponding EAPCs of −0.62 (95% CI: −0.88 to −0.36) and −0.60 (95% CI: −0.87 to −0.34), respectively. In contrast, Eastern Europe continued to show an upward trend, with EAPCs of 0.26 (95% CI: 0.15 to 0.36) and 0.26 (95% CI: 0.16 to 0.37), respectively. On a global scale, regions with low SDI, including Andean Latin America, Tropical Latin America, Southeast Asia, and South Asia, saw the most rapid increases in ASPRs and ASDRs over time. These regions merit further attention (Tables S1,S2, and Figure 4).
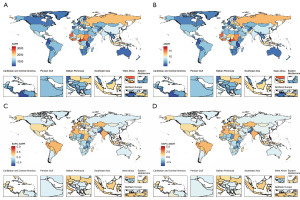
At the country level, India had the highest number of cases of prevalence and DALYs in 2021, followed by China. Together, these two countries accounted for 44% and 43% of the global prevalence and DALYs, respectively. The top 10 countries with the highest ASPRs and ASDRs were all located in Africa, with the majority concentrated in Western Sub-Saharan Africa. These countries include Cameroon, Liberia, Côte d’Ivoire, Sierra Leone, Guinea-Bissau, Ghana, and The Gambia. Among them, Cameroon had the highest ASPR at 3,280.58 per 100,000 (95% UI: 1,939.56 to 5,141.3) and ASDR at 18.96 per 100,000 (95% UI: 7.04 to 44.69), significantly exceeding the global average by 2.4 times and more than 11 times higher than Burundi, the country with the lowest rates. Notably, China ranked 32nd in age-standardized prevalence and 54th in age-standardized DALYs among 204 countries. China’s ASRs were 5.6 and 5.4 times higher than those in the lowest-ranked countries and approximately 2 and 2.2 times lower than those in the highest-ranked countries, respectively. From 1990 to 2021, both ASPRs and ASDRs for male infertility increased in over 40% of countries, including the Philippines, Morocco, Slovenia, Peru, Spain, Ecuador, India, El Salvador, and Brazil. The Philippines exhibited the fastest growth, with EAPCs of 5.33 (95% CI: 3.27 to 7.44) for ASPR and 5.28 (95% CI: 3.29 to 7.3) for ASDR, which was 1.9 and 1.5 times faster than the next fastest-growing country, Morocco, respectively (Tables S1,S2 and Figure 5).
Burden of disease and correlation with SDI
An analysis of the correlation between ASRs and the SDI for male infertility revealed consistent patterns at both national and district levels. ASRs for disease prevalence and DALYs demonstrated an overall declining trend as SDI increased. However, a distinct variation was observed: at the district level, ASR peaks occurred at an SDI of approximately 0.7, while at the national level, the peak was slightly delayed, appearing at an SDI of around 0.8 (Figure 6).
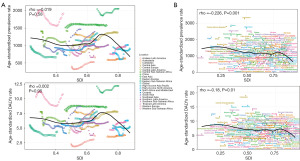
Health inequities
Figure 7 illustrates pronounced absolute and relative income inequalities in the prevalence and DALYs rates of male infertility, predominantly concentrated in low SDI regions. Nevertheless, global health inequalities associated with male infertility had declined over time. The SII in prevalence reduced from −487.7 in 1990 to −345.8 in 2021, alongside a change in the CII from 0.02 to −0.01. Similarly, the SII in DALYs rates decreased from −2.4 to −1.6, with a corresponding CII shift from 0.03 to −0.01. These changes indicate a gradual reduction in health disparities related to male infertility over the past three decades.
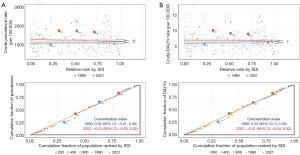
Factors influencing EAPCs
The EAPCs in male infertility prevalence and DALYs rates were evaluated in relation to ASRs and the Human Development Index (HDI) in 2021. EAPCs showed positive correlations with both ASRs and HDI, with HDI exhibiting the strongest association. The Spearman correlation coefficient for prevalence was 0.375 (P<0.01), while for DALYs rate, it was 0.374 (P<0.01). However, the correlation between EAPCs and age-standardized prevalence was weaker and not statistically significant, with a Spearman coefficient of 0.112 (P>0.05) (Figure 8).

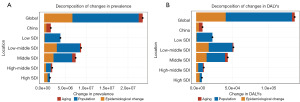
Decomposition analysis
Decomposition analysis identified population growth as the primary factor driving the global prevalence of male infertility across all SDI regions, contributing 62.27% of the total prevalence. Epidemiologic changes were the second most significant contributor, accounting for 28.66%. However, in China, age-related increases played a more significant role in driving prevalence trends compared to the global pattern (Figure 9).
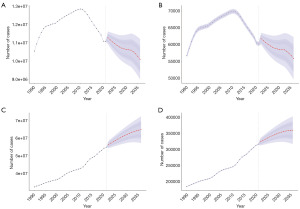
Future projections
Future projections indicate that the global prevalence of male infertility and the number of DALYs will continue to increase over the next decade, while both metrics are expected to decline significantly in China. By 2036, global prevalence is projected to reach approximately 65 million, representing an 18% increase, while DALYs are anticipated to rise to 360,000, an increase of over 40,000. In contrast, China is projected to experience a reduction of 1.8 million cases in prevalence and 0.9 million in DALYs. ASRs are expected to exhibit moderate growth globally and in China, but the pace of this increase is likely to remain limited (Figure 10 and Figure S1).
Discussion
Our study reveals that in 2021, the global prevalence of male infertility surpassed 50 million cases, with over 300,000 associated DALYs. China contributed approximately one-fifth of these figures, with over 10 million cases and 60,000 DALYs. Notably, ASRs in China were significantly higher than the global averages. Since 1990, the global burden of male infertility has steadily increased, with a sharp rise of over 20% recorded in the last decade, primarily driven by regions with low and low-middle SDI. Conversely, China exhibited a relatively stable trend, with prevalence and ASRs gradually declining after peaking in 2008.
According to the United Nations Department of Economic and Social Affairs, the global population in 2019 was estimated at 7.7 billion, with nearly equal numbers of men and women, both continuing to grow (21). Based on extrapolated data, the prevalence of male infertility in 2021 was approximately 14%, consistent with previous reports (6,14). Our findings show that men aged 20–44 years are the most affected, with the highest prevalence and DALYs observed in the 35–39 age group since 1990 (Figure 2). This trend aligns with the reproductive age, a time when many men seek fertility assessments, and is compounded by the natural decline in sperm motility with age. Regions with middle and high-middle SDI accounted for the highest prevalence and DALYs over time (Figure 2B), reflecting global population distribution patterns and the significant role of population growth in the disease burden, as highlighted in our decomposition analysis. High-middle SDI regions also exhibited the highest ASRs, likely influenced by delayed childbearing, industrial environmental pollution, and economic stress. These findings underscore the multifaceted interplay of demographic, socioeconomic, and environmental factors in the global burden of male infertility (Figures 2,9).
Analyses at the regional and national levels showed that ASPRs and DALYs for male infertility were significantly higher in Eastern Europe and Africa compared to other regions, consistent with findings from previous studies (14,22). Another GBD study identified Africa as having the highest global prevalence of sexually transmitted infections (STIs) (23). Reproductive tract infections, which contribute to up to 35% of male infertility cases in some regions, are a major factor. Inflammation and oxidative stress are key mechanisms implicated in these infections, though the exact pathophysiological processes remain unclear (24-27). Common pathogens such as Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma, Ureaplasma, and Spiroplasma syphilis negatively affect sperm quality by reducing sperm viability, inducing apoptosis or necrosis, and altering sperm morphology (28). The stigmatization of male infertility in these regions can hinder patients from receiving timely medical care. While direct evidence on the causes of the high ASPRs in Eastern Europe is limited, several studies suggest that the region’s high rates of smoking and alcohol consumption, along with significant environmental issues—including industrial and agricultural pollution and poor air quality—are key risk factors for male infertility (29-34). Furthermore, a study conducted in Poland indicated that the high prevalence of infertility in Eastern Europe may also be influenced by socio-psychological factors such as high unemployment, elevated daily stress levels, and delayed childbearing (35). Our study also highlights a concerning trend: from 1990 to 2021, the ASPRs and ASDRs of male infertility in low-SDI regions, such as Andean Latin America, Tropical Latin America, Southeast Asia, and South Asia—particularly in the Philippines—have risen sharply. This increase may be associated with rapid urbanization, as well as higher tobacco and drug consumption in these regions (36,37). A recent study by the GBD also suggests that the spread of sexually STIs is accelerating in South and Southeast Asia.
In China, male infertility trends are closely tied to population growth, as reported by the China Population and Development Research Center. China’s population growth can be categorized into three phases: a peak before the 1970s, moderate growth during the 1980s and 1990s, and a decline beginning in the 21st century (38). However, population growth alone does not fully account for these trends. During the 1980s, the reform and opening-up era led to a rapid spread of sexually transmitted diseases (STDs) in China, with an average annual growth rate of 124%. The establishment of a national STD surveillance network in the late 1990s brought these diseases under control (39). Additionally, at the beginning of the 21st century, China’s economy experienced rapid growth, which was accompanied by significant advancements in several fields, including traditional Chinese medicine (TCM), the establishment of assisted reproduction centers and sperm banks, the development of microsurgery, and improvements in the treatment of varicocele (40-43). These developments may have contributed to the decline in male infertility rates in China, particularly after 2008. However, despite these improvements, the ASRs for male infertility in China remain higher than the global average. This discrepancy may be attributed to China’s status as a large industrial and agricultural country, where environmental pollution has increased substantially over the past few decades.
Recent data from the WHO indicate that, despite over three decades of advancements in ART and millions of children born globally through interventions such as in vitro fertilization, access to ART remains severely limited in many countries, particularly in lower-middle-income regions, due to prohibitively high costs (44). Alarmingly, our study highlights a linear increase in ASPRs and ASDRs in these regions since 2010, potentially linked to improved screening efforts (Figure 3C). This trend poses a significant threat to fertility and overall population health, further widening global health inequities. We call on governments in lower-middle-income countries to prioritize the diagnosis and treatment of male infertility in their population and development policies, as well as reproductive health strategies. Key recommendations include increasing public health funding, strengthening the prevention and control of reproductive tract infections, and seeking international assistance to make ART more affordable and accessible. These measures are essential for mitigating the burden of male infertility and addressing the growing disparities in global reproductive healthcare.
In this study, we did not find epidemiological evidence supporting a direct association between regional differences in the burden of male infertility. The interpretations are primarily based on reasoning and speculation derived from limited literature and official data, and thus, these interpretations should be approached with caution. Moreover, this indirectly suggests that the global epidemiology of male infertility remains poorly understood. Furthermore, our study shares several limitations common to other GBD studies. First, part of the GBD data is derived from census records, medical databases, and academic literature, which may inadvertently misclassify voluntarily childless couples as infertile. Additionally, self-paying outpatients, who represent a significant portion of male infertility cases, are often underrepresented. The similarities between our findings and previous studies may also obscure new insights into the evolving trends of male infertility. Lastly, regional differences in diagnostic criteria and variations in data quality further complicate analyses and may mask the true extent of the disease burden.
Conclusions
In conclusion, this study not only aligns with the WHO’s call for global epidemiological surveys on infertility (44), but also reveals a consistent increase in the disease burden of male infertility. This trend is particularly pronounced in regions with low SDI and low-middle SDI over the past decade, where the disease burden is escalating rapidly. In contrast, China has experienced a decline in its disease burden. We urge governments and organizations in lower-middle-income regions, such as Sub-Saharan Africa, South Asia, Southeast Asia (particularly the Philippines), Latin America, and Eastern Europe, to adopt China’s approach. This includes prioritizing male infertility, conducting further etiological investigations, and implementing a comprehensive management strategy that incorporates environmental improvements, STD control, promotion of healthy lifestyles, and expanded access to assisted reproduction services.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-44/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-44/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-44/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. As the study used publicly available data, ethical approval and informed consent were not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thoma ME, McLain AC, Louis JF, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013;99:1324-1331.e1. [Crossref] [PubMed]
- Wright J, Duchesne C, Sabourin S, et al. Psychosocial distress and infertility: men and women respond differently. Fertil Steril 1991;55:100-8.
- Smith JF, Walsh TJ, Shindel AW, et al. Sexual, marital, and social impact of a man’s perceived infertility diagnosis. J Sex Med 2009;6:2505-15. [Crossref] [PubMed]
- Nelson CJ, Shindel AW, Naughton CK, et al. Prevalence and predictors of sexual problems, relationship stress, and depression in female partners of infertile couples. J Sex Med 2008;5:1907-14. [Crossref] [PubMed]
- Meacham RB, Joyce GF, Wise M, et al. Male infertility. J Urol 2007;177:2058-66. [Crossref] [PubMed]
- Harris E. Infertility Affects 1 in 6 People Globally. JAMA 2023;329:1443. [Crossref] [PubMed]
- Chen T, Belladelli F, Del Giudice F, et al. Male fertility as a marker for health. Reprod Biomed Online 2022;44:131-44. [Crossref] [PubMed]
- Jensen TK, Jacobsen R, Christensen K, et al. Good semen quality and life expectancy: a cohort study of 43,277 men. Am J Epidemiol 2009;170:559-65. [Crossref] [PubMed]
- Walsh TJ, Croughan MS, Schembri M, et al. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med 2009;169:351-6. [Crossref] [PubMed]
- Eisenberg ML, Li S, Cullen MR, et al. Increased risk of incident chronic medical conditions in infertile men: analysis of United States claims data. Fertil Steril 2016;105:629-36. [Crossref] [PubMed]
- Winters BR, Walsh TJ. The epidemiology of male infertility. Urol Clin North Am 2014;41:195-204. [Crossref] [PubMed]
- Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod 1998;13:33-44. [Crossref] [PubMed]
- Ikechebelu JI, Adinma JI, Orie EF, et al. High prevalence of male infertility in southeastern Nigeria. J Obstet Gynaecol 2003;23:657-9. [Crossref] [PubMed]
- Agarwal A, Mulgund A, Hamada A, et al. A unique view on male infertility around the globe. Reprod Biol Endocrinol 2015;13:37. [Crossref] [PubMed]
- Philippov OS, Radionchenko AA, Bolotova VP, et al. Estimation of the prevalence and causes of infertility in western Siberia. Bull World Health Organ 1998;76:183-7.
- Matetakufa SN. Infertility: Our Own Gift. New Internationalist. 1998. Available online: http://newint.org/features/1998/07/05/infertility/. Accessed 17 December 2014.
- Skakkebaek NE, Jørgensen N, Main KM, et al. Is human fecundity declining? Int J Androl 2006;29:2-11. [Crossref] [PubMed]
- Auger J, Kunstmann JM, Czyglik F, et al. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med 1995;332:281-5. [Crossref] [PubMed]
- Carlsen E, Giwercman A, Keiding N, et al. Evidence for decreasing quality of semen during past 50 years. BMJ 1992;305:609-13. [Crossref] [PubMed]
- Global age-sex-specific mortality, life expectancy, and population estimates in 204 countries and territories and 811 subnational locations, 1950-2021, and the impact of the COVID-19 pandemic: a comprehensive demographic analysis for the Global Burden of Disease Study 2021. Lancet 2024;403:1989-2056. [Crossref] [PubMed]
World Population Prospects 2019 . Available online: https://population.un.org/wpp/Publications/?utm_source- Huang B, Wang Z, Kong Y, et al. Global, regional and national burden of male infertility in 204 countries and territories between 1990 and 2019: an analysis of global burden of disease study. BMC Public Health 2023;23:2195. [Crossref] [PubMed]
- Zheng Y, Yu Q, Lin Y, et al. Global burden and trends of sexually transmitted infections from 1990 to 2019: an observational trend study. Lancet Infect Dis 2022;22:541-51. [Crossref] [PubMed]
- Guo Y, Zhou G, Feng Y, et al. The Association Between Male Viral Infections and Infertility: A Systematic Review and Meta-Analysis. Rev Med Virol 2024;34:e70002. [Crossref] [PubMed]
- Henkel R, Maass G, Jung A, et al. Age-related changes in seminal polymorphonuclear elastase in men with asymptomatic inflammation of the genital tract. Asian J Androl 2007;9:299-304. [Crossref] [PubMed]
- Tjagur S, Mändar R, Poolamets O, et al. Mycoplasma genitalium Provokes Seminal Inflammation among Infertile Males. Int J Mol Sci 2021;22:13467. [Crossref] [PubMed]
- Weidner W, Pilatz A, Diemer T, et al. Male urogenital infections: impact of infection and inflammation on ejaculate parameters. World J Urol 2013;31:717-23. [Crossref] [PubMed]
- Gimenes F, Souza RP, Bento JC, et al. Male infertility: a public health issue caused by sexually transmitted pathogens. Nat Rev Urol 2014;11:672-87. [Crossref] [PubMed]
- Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017;389:1885-906. [Crossref] [PubMed]
- Polanska K, Znyk M, Kaleta D. Susceptibility to tobacco use and associated factors among youth in five central and eastern European countries. BMC Public Health 2022;22:72. [Crossref] [PubMed]
- Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018;392:1015-35. [Crossref] [PubMed]
- Jedrychowski W. Review of recent studies from central and Eastern Europe associating respiratory health effects with high levels of exposure to “traditional” air pollutants. Environ Health Perspect 1995;103:15-21. [Crossref] [PubMed]
- Selvaraju V, Baskaran S, Agarwal A, et al. Environmental contaminants and male infertility: Effects and mechanisms. Andrologia 2021;53:e13646. [Crossref] [PubMed]
- Sansone A, Di Dato C, de Angelis C, et al. Smoke, alcohol and drug addiction and male fertility. Reprod Biol Endocrinol 2018;16:3. [Crossref] [PubMed]
- Sanocka D, Kurpisz M. Infertility in Poland--present status, reasons and prognosis as a reflection of Central and Eastern Europe problems with reproduction. Med Sci Monit 2003;9:SR16-20.
- Fu L, Sun Y, Han M, et al. Incidence Trends of Five Common Sexually Transmitted Infections Excluding HIV From 1990 to 2019 at the Global, Regional, and National Levels: Results From the Global Burden of Disease Study 2019. Front Med (Lausanne) 2022;9:851635. [Crossref] [PubMed]
- The tobacco use situation published by the World Health Organization. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/tobacco?
The Changes in Population Size Over Seventy Years of New China The Current Situation and Reflections on STD Prevention and Control in China - Zhou SH, Deng YF, Weng ZW, et al. Traditional Chinese Medicine as a Remedy for Male Infertility: A Review. World J Mens Health 2019;37:175-85. [Crossref] [PubMed]
- Wang Y, Kong F, Fu Y, et al. How can China tackle its declining fertility rate? BMJ 2024;386:e078635. [Crossref] [PubMed]
- Qiao J, Feng HL. Assisted reproductive technology in China: compliance and non-compliance. Transl Pediatr 2014;3:91-7. [Crossref] [PubMed]
- Chinese Center for Disease Control and Prevention. China’s human assisted reproductive technology annual report 2021. CDC; 2023.
World Health Organization Infertility


