
Age-related changes in lncRNA expression in sperm
Highlight box
Key findings
• Significant differences in long noncoding RNA (lncRNA) and messenger RNA (mRNA) expression were identified between sperm from older fathers (≥40 years) and younger controls (<40 years). A total of 8,154 differentially expressed lncRNAs and 2,930 differentially expressed mRNAs were detected.
• Functional enrichment analysis revealed involvement in metabolic pathways, RNA transport, and protein hydrolysis.
• A coexpression network of 178 lncRNA-mRNA pairs was constructed, suggesting regulatory roles.
What is known and what is new?
• Advanced paternal age (APA) is known to increase risks of genetic disorders and neurodevelopmental issues in offspring.
• Our study provides new insights into lncRNA and mRNA expression changes in sperm from older fathers and their potential impact on offspring health.
What is the implication, and what should change now?
• The findings suggest lncRNAs and mRNAs in sperm may link APA to offspring health.
• Future research should explore the molecular mechanisms and clinical relevance of these lncRNAs and mRNAs in intergenerational health.
Introduction
Over the past few decades, owing to increasing life expectancy, development in social and economic status, increased stress, and increased opportunities for assisted reproductive technology, the age of parenthood has become increasingly delayed (1). An increasing number of researchers have focused on the impact of advanced maternal age on offspring, especially on early-life survival and offspring health. Study has shown that advanced maternal age is associated with increased rates of embryo chromosomal abnormalities, intrauterine growth restriction (IUGR), premature birth, stillbirth, and birth defects (2). Reports have also indicated that advanced maternal age is associated with a range of negative health outcomes in offspring, from physical issues such as increased body mass index (BMI), blood pressure (3), and height to psychiatric disorders such as autism (4), bipolar disorder (5), depression, stress and anxiety (6), as well as impaired social functioning (7). However, the impacts of advanced paternal age (APA) on fertility, early development, and offspring health have not received sufficient attention. Currently, although no consensus on the definition of APA has been reached, some professional associations define APA as 40 years of age or older (8).
Some recent studies have confirmed the negative impact of APA on reproductive health. Sperm damage, sperm abnormalities, sperm dysfunction, and damage to supporting cells and interstitial cells are more common in the testes of elderly males (9). In addition, increasing evidence suggests that APA increases the risk of certain genetic diseases in offspring. Miller et al. reported an association between APA and schizophrenia (10). Polga et al. reported that the risk of bipolar affective disorder in children increases with increasing paternal age (11). Buizer-Voskamp and colleagues recently reported that the odds of having a child with autism for elderly males (≥45 years old) are 3.3 times greater than those for younger males (12). Furthermore, study has also revealed associations between APA and stillbirth, musculoskeletal syndromes, cleft palate, acute lymphoblastic leukemia, and retinoblastoma (13). However, the mechanisms underlying these associations remain to be elucidated.
Long noncoding RNAs (lncRNAs) have various functions, such as regulating genes involved in chromatin modification, directly binding genes to induce X chromosome inactivation, inhibiting antisense messenger RNA (mRNA) expression, promoting antisense mRNA degradation, acting as competing endogenous RNAs (ceRNAs) to inhibit microRNAs (miRNAs), and inhibiting the interactions of DNA-binding proteins with target genes, have received widespread attention in mammals (14). lncRNAs also regulate various cellular processes, such as differentiation, proliferation, and apoptosis. Zhang et al. reported that lncRNAs are important components of the RNA-protein regulatory network involved in animal sperm development (15). Qiu and colleagues investigated the expression patterns of stage-specific lncRNAs during early human embryonic development, emphasizing their substantial role in this critical process. In addition, An et al. reported significant differences in the expression of lncRNAs and mRNAs in sperm between mice with obesity induced by a high-fat diet and normal-weight mice, and these differences were also detected in their male offspring, which maintained an expression pattern similar to that of their parents, indicating that lncRNAs are carriers of obesity-induced paternal inheritance (16). Many studies have revealed a link between APA and a range of health issues in offspring. While advanced maternal age has been linked to alterations in lncRNA expression by Bouckenheimer et al. (17), particularly in oocytes and early embryos, changes in lncRNA expression in sperm as males age have not been studied, and the relationship between these changes and offspring health has yet to be explored further. Our objective is to investigate the expression profile characteristics of lncRNAs that are differentially expressed in the sperm of elderly versus young men, and to elucidate their potential relationship with the health of their offspring. We present this article in accordance with the MDAR reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-31/rc).
Methods
Human sperm samples
Sperm samples were collected from volunteers (n=6) who met the following criteria: (I) a sperm concentration >15×106/mL, forward motility ≥32%, and normal sperm morphology in >4% of sperm and (II) no other diseases, including diseases of the reproductive glands, acute and chronic gonadal inflammation, and diseases involving increased immune factors. Volunteers were excluded if they exhibited: (I) severe semen abnormalities, including oligozoospermia, asthenozoospermia, or azoospermia; (II) current smoking or any tobacco use within the preceding 6 months; (III) significant lifestyle irregularities or substantial dietary changes during the 6-month pre-enrollment period; or (IV) occupational exposure or chronic contact with environmental pollutants (heavy metals, organic solvents, ionizing radiation, etc.). After abstaining from ejaculation for 2–7 days, semen samples were collected by masturbation and placed in sterile containers. The samples were sent to the laboratory within 1 hour after ejaculation. The sperm samples were liquefied by incubation at 37 ℃ for 30 minutes and analyzed via computer-assisted sperm analysis (CASA). The sperm samples were subsequently purified on a Percoll density gradient and stored in liquid nitrogen until RNA purification. All participants provided informed consent for the study. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Women’s Hospital of Nanjing Medical University (No. 2022KY-039).
Library construction and transcriptome sequencing
The total RNA was extracted from the human sperm using the TRIzol method. A specific library was constructed by removing ribosomal RNA, and the transcriptome was sequenced (the transcriptome library was constructed and sequenced by Beijing Boao Jingdian Biotechnology Co., Ltd.). In this experiment, high-throughput sequencing of multiple samples was carried out using the Illumina HiSeq sequencing platform in double-terminal sequencing mode. Skewer software was used to remove joint sequences and low-quality fragments from the 3' end of the sequencing data. FastQC software was used to analyze the quality of the preprocessed data and calculate the Q20 and Q30 base ratios. BWA software was used to compare the preprocessed data to the rRNA sequence database. The preprocessed sequences from each sample were compared with the rat reference genome via STAR software. After alignment of sequence reads to the reference genome, StringTie software was used to guide the assembly of transcripts on the basis of files containing the locations of known transcripts in the genome, filter transcripts for which expression was equal to zero, and reassemble the transcripts assembled by StringTie for all samples. LncRNAs were identified by screening the assembled transcripts with StringTie.
Screening and functional analysis of differentially expressed mRNAs and lncRNAs
To further analyze the differences in mRNA and lncRNA expression levels in spermatozoa between the APA group and the young group, fragments per kilobase of transcript per million fragments mapped (FPKM) values were used to calculate the expression of mRNA and lncRNA transcripts. Differential expression analysis was performed with the criteria |log2fold change (FC)| ≥1 and P<0.05, and significant differences in lncRNA and mRNA transcripts between the APA group and young group were identified using Differential Expression analysis for Sequence data (DESeq) software. The software programs coding potential calculator (CPC), Coding-Non-Coding Index (CNCI), and Hidden Markov ModelER (HMMER) were used to predict coding potential, and lncRNAs predicted to have no coding potential by all three software programs were considered novel lncRNAs.
Analysis of lncRNA target genes
The results of the coexpression analysis were used to predict target genes. To comprehensively analyze the relationships between the lncRNAs and mRNAs at three levels (the expression level, their relative positions, and the sequence level), lncTar software was used to determine whether the lncRNA-mRNA pairs identified by coexpression analysis engaged in targeted regulation.
Functional enrichment analysis of the target genes of differentially expressed mRNAs and lncRNAs
Gene Ontology (GO) functional enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were carried out with the target gene set (mRNAs and lncRNAs).
Real-time quantitative PCR analysis
Collects total RNA from all samples, selects eight genes from differentially expressed lncRNAs, with three biological replicates set for each DE-lncRNA, and performs real-time quantitative PCR analysis. The primers are shown in Table 1.
Table 1
| Primer | Sequences 5'-3' |
|---|---|
| MERGE.76562.24 | TCATTTACCGAGATGTCAAGCC |
| MERGE.74639.34 | TTGGAGGGAGTGGAGAAGTGC |
| MERGE.109971.5 | CCACCTGTGCCTGGTATTTGAG |
| MERGE.229873.6 | CCAGGCACGAGCTATGTTGAG |
| MERGE.66303.11 | CTGGAGGAGGTTTATCTGCTGG |
| MERGE.176104.2 | GAATTGGTAATGGCAGATAGGGT |
| MERGE.106596.7 | GTTCACCGAGATGCCGACTG |
| NONHSAT206684.1 | CTTGCCTGACCTCTTGCTTTC |
| GAPDH | GGAAGCTTGTCATCAATGGAAATC |
lncRNA, long noncoding RNA.
Statistical analysis
GraphPad Prism 6.0 was used to analyze all the data, and Student’s t-test was used to assess statistical significance. P<0.05 was used to indicate a statistically significant difference.
Results
Characterization of the lncRNAs
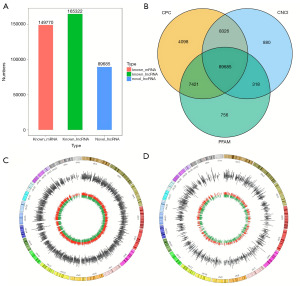
We analyzed lncRNAs in sperm samples from the young group (males <40 years old) and the APA group (males ≥40 years old) using the Illumina HiSeq sequencing platform. A total of 255,007 lncRNAs and 149,770 mRNAs were detected; among the lncRNAs were 165,322 known lncRNAs and 89,685 newly discovered lncRNAs (Figure 1A). A Venn diagram of the lncRNAs predicted with the three software programs (intersection on the Venn diagram indicated novel lncRNAs) shows the lncRNAs in the sperm of the young group and that of the APA group (Figure 1B). The chromosomal distributions of the differentially expressed lncRNAs and mRNAs are shown in Figure 1C,1D, respectively.
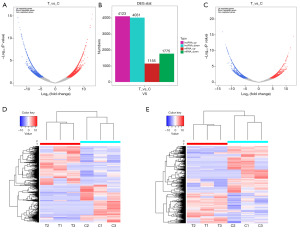
Differential expression of lncRNAs and mRNAs between the APA group and young group
Differentially expressed genes were screened with the criteria |log2FC| ≥1 and P<0.05. DESeq software was used to screen lncRNA and mRNA transcripts with significantly different expression between the APA group and the young group. Compared with their expression in the young group, 8,154 lncRNAs were significantly differentially expressed in the APA group: these lncRNAs included 4,031 downregulated lncRNAs and 4,123 upregulated lncRNAs (Figure 2A,2B). Furthermore, a total of 2,930 mRNAs were significantly differentially expressed; these mRNAs included 1,155 upregulated and 1,775 downregulated mRNAs (Figure 2B,2C). The results of clustering analysis of the differentially expressed lncRNAs and mRNAs between the APA group and the young group are shown in Figure 2D,2E.
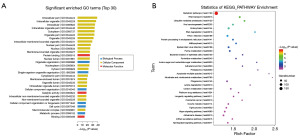
Functional enrichment analysis of the differentially expressed lncRNAs
GO enrichment analysis of the differentially expressed lncRNA target genes revealed 11,623 GO terms with significant enrichment in the lncRNAs, including 8,506 lncRNAs enriched in biological process (BP) terms, 1,123 lncRNAs enriched in cellular component (CC) terms, and 1,994 lncRNAs enriched in molecular function (MF) terms; these GO terms included mainly intracellular components and organelles (Figure 3A). As shown in Figure 3B, KEGG enrichment analysis of the target genes of the differentially expressed lncRNAs showed their enrichment mainly in metabolic pathways and pathways related to RNA transport, ubiquitin-mediated protein degradation, viral carcinogenesis, endocytosis, RNA degradation, and HTLV-I infection.
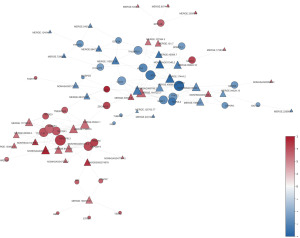
Comprehensive analysis of the lncRNA-mRNA network
With the results of the lncRNA and mRNA expression analysis, we constructed a coexpression network containing 178 lncRNA-mRNA pairs (that satisfied the following criteria: |r|>0.99 and a P value <0.05) (Figure 4).
Experimental verification of differentially expressed lncRNAs
We selected eight DE-lncRNAs for RT-qPCR validation, including four upregulated lncRNAs (MERGE.76562.24, MERGE.74639.34, MERGE.109971.5, and MERGE.229873.6) and four downregulated lncRNAs (MERGE.66303.11, MERGE.176104.2, MERGE.106596.7, and NONHSAT206684.1). The RT-qPCR results are consistent with our sequencing results, indicating that the expression profile of lncRNAs is reliable (Figure 5). The characteristics of the eight DE-lncRNAs are shown in Table 2.
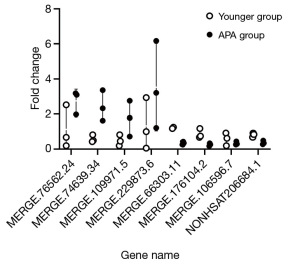
Table 2
| Running number | Regulation | P value |
|---|---|---|
| MERGE.76562.24 | Up | 0.000 |
| MERGE.74639.34 | Up | 0.000 |
| MERGE.109971.5 | Up | 0.003 |
| MERGE.229873.6 | Up | 0.000 |
| MERGE.66303.11 | Down | 0.000 |
| MERGE.176104.2 | Down | 0.013 |
| MERGE.106596.7 | Down | 0.015 |
| NONHSAT206684.1 | Down | 0.001 |
lncRNA, long noncoding RNA.
Discussion
Besides morphological studies conducted decades ago, the biology of mammalian sperm production has been widely explored only in the past one to two decades. In recent years, our understanding of the speed of sperm production has increased exponentially. The production of sperm is a continuous process that occurs throughout the entire reproductive lifespan or season of an animal. With increasing age, the sperm concentration, total sperm count, and sperm motility in semen decrease gradually, whereas the numbers of abnormal sperm and sperm with damaged DNA increase. Moreover, the risk of genetic mutations in sperm also increases. APA may lead to the accumulation of chromosomal abnormalities, single nucleotide polymorphisms (SNPs), and gene mutations, thereby increasing the risk of certain genetic diseases in offspring (18). Furthermore, Perrin et al. reported that epigenetic changes caused by paternal aging can be transmitted to offspring, increasing the susceptibility of offspring to related diseases (19).
Plant and animal studies have shown that lncRNAs are highly expressed in a cell- and tissue-specific manner in reproductive organs. Sexual reproduction is a complex process that involves cell fate determination and specialized cell division, which require the precise coordination of gene expression in response to internal and external signals. As major regulators of gene expression and chromatin organization, lncRNAs may be particularly suitable for coordinating and controlling the molecular processes involved in sexual reproduction, including sex determination (20), meiosis (21), spermatogenesis (22), and imprinting (23). Increasing evidence suggests that lncRNAs play a role in regulating gene expression in various BPs at the epigenetic, transcriptional, and posttranscriptional levels through DNA methylation, histone modification, and chromatin remodeling. The dysregulation of lncRNA levels might lead to disease. Variations in certain lncRNAs are associated with genetic susceptibility to metabolic diseases. For example, Tsai et al. reported that variations in the ANRIL gene are associated with an increased risk of type 2 diabetes (24). We characterized the lncRNA expression profiles of elderly males and compared them to those of young males. A total of 8,154 differentially expressed lncRNAs (4,031 downregulated and 4,123 upregulated lncRNAs) were identified. Through KEGG enrichment analysis, the target genes of these lncRNAs were found to be enriched mainly in metabolic pathways and pathways related to RNA transport, ubiquitin-mediated proteolysis, viral carcinogenesis, endocytosis, and RNA degradation, among other pathways. An increasing number of studies have confirmed that aging is accompanied by a decline in function and a series of critical alterations, including genetic and epigenetic changes that can affect the risk of metabolic diseases (25). Some studies have shown that the offspring of older fathers have an increased risk of metabolic diseases. The potential molecular mechanisms that lead to intergenerational disease risk include genetic (DNA sequences) and epigenetic factors in sperm (26). For example, Zhao et al. demonstrated that epigenetic reprogramming induced by the aging of sperm may lead to abnormal glucose metabolism in intergenerational and transgenerational offspring (27). Our study has revealed that the target genes of these differentially expressed lncRNAs are enriched in multiple key biological pathways, including metabolic pathways. Further research is needed to explore the relationships between these changes and metabolic diseases in offspring.
Recently, Safi-Stibler reported that long-term changes in gene expression and protein abundance induced by chronic stress can be inherited by offspring, leading to impaired recovery from stress (28). Sperm RNA serves as a vertical carrier of information. Martin and colleagues discovered that the assessment of the risk of autism in offspring can be achieved through the quantification of male sperm chimera (29). Whether changes in lncRNAs in the sperm of older males are correlated with the risk of mental illness in offspring has not been studied. Through comprehensive analysis of lncRNA and mRNA expression data, we constructed a coexpression network containing 178 lncRNA-mRNA pairs. A search for mRNAs that are targeted by the lncRNAs revealed CSNK1G1, which is widely expressed in many tissue types, including the brain, where it regulates the phosphorylation of N-methyl-D-aspartate receptors and plays a role in synaptic transmission. Studies have shown that the CSNK1G1 gene may cause developmental delays and ASD (30). In addition, Cfap97d1 is essential for maintaining the sperm flagellar structure, and abnormal expression of the Cfap97d1 gene can lead to sperm motility defects (asthenozoospermia) and decreased male fertility (31). APA may be associated with reproductive system issues and decreased fertility in offspring. Lafuente et al. reported a negative correlation between sperm telomere length and progressive sperm motility (32). Ferlin and colleagues reported a positive correlation between paternal age and offspring telomere length (33). This change may be passed on to offspring and impact their fertility. However, the specific mechanism still needs to be further studied.
This study has several important limitations that warrant consideration. First, the modest sample size (n=6) may introduce selection bias and limit the statistical power of our findings, particularly given the known heterogeneity in sperm quality among aging males. Second, the age categorization (<40 vs. ≥40 years) lacked granularity to detect potential nonlinear age effects. Importantly, our transcriptomic approach focused solely on expression profiling and thus cannot provide mechanistic insights into post-transcriptional regulation or functional validation of the identified lncRNAs. Finally, the absence of direct offspring health data prevents definitive conclusions about the clinical relevance of these findings. While these limitations constrain immediate translation, they highlight critical considerations for future larger-scale studies incorporating longitudinal designs, standardized phenotyping, and functional validation.
Conclusions
In conclusion, with increasing paternal age, a decrease in sperm quality has adverse effects on male fertility. LncRNAs may play important roles in male fertility. Therefore, a deeper understanding of the relationship between lncRNAs in sperm from males of APA and offspring health may have substantial implications for future reproductive medicine research and disease prevention.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-31/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-31/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-31/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-31/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All participants provided informed consent for the study. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Women’s Hospital of Nanjing Medical University (No. 2022KY-039).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goisis A, Håberg SE, Hanevik HI, et al. The demographics of assisted reproductive technology births in a Nordic country. Hum Reprod 2020;35:1441-50. [Crossref] [PubMed]
- Rizzo M, Ducheyne KD, Deelen C, et al. Advanced mare age impairs the ability of in vitro-matured oocytes to correctly align chromosomes on the metaphase plate. Equine Vet J 2019;51:252-7. [Crossref] [PubMed]
- Carslake D, Tynelius P, van den Berg G, et al. Associations of parental age with health and social factors in adult offspring. Methodological pitfalls and possibilities. Sci Rep 2017;7:45278. [Crossref] [PubMed]
- Lee BK, McGrath JJ. Advancing parental age and autism: multifactorial pathways. Trends Mol Med 2015;21:118-25. [Crossref] [PubMed]
- Menezes PR, Lewis G, Rasmussen F, et al. Paternal and maternal ages at conception and risk of bipolar affective disorder in their offspring. Psychol Med 2010;40:477-85. [Crossref] [PubMed]
- Tearne JE, Robinson M, Jacoby P, et al. Older maternal age is associated with depression, anxiety, and stress symptoms in young adult female offspring. J Abnorm Psychol 2016;125:1-10. [Crossref] [PubMed]
- Weiser M, Reichenberg A, Werbeloff N, et al. Advanced parental age at birth is associated with poorer social functioning in adolescent males: shedding light on a core symptom of schizophrenia and autism. Schizophr Bull 2008;34:1042-6. [Crossref] [PubMed]
- Brandt JS, Cruz Ithier MA, Rosen T, et al. Advanced paternal age, infertility, and reproductive risks: A review of the literature. Prenat Diagn 2019;39:81-7. [Crossref] [PubMed]
- Dong S, Chen C, Zhang J, et al. Testicular aging, male fertility and beyond. Front Endocrinol (Lausanne) 2022;13:1012119. [Crossref] [PubMed]
- Miller B, Messias E, Miettunen J, et al. Meta-analysis of paternal age and schizophrenia risk in male versus female offspring. Schizophr Bull 2011;37:1039-47. [Crossref] [PubMed]
- Polga N, Macul Ferreira de Barros P, Farhat LC, et al. Parental age and the risk of bipolar disorder in the offspring: A systematic review and meta-analysis. Acta Psychiatr Scand 2022;145:568-77. [Crossref] [PubMed]
- Buizer-Voskamp JE, Laan W, Staal WG, et al. Paternal age and psychiatric disorders: findings from a Dutch population registry. Schizophr Res 2011;129:128-32. [Crossref] [PubMed]
- Nybo Andersen AM, Urhoj SK. Is advanced paternal age a health risk for the offspring? Fertil Steril 2017;107:312-8. [Crossref] [PubMed]
- Fang Y, Fullwood MJ. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genomics Proteomics Bioinformatics 2016;14:42-54. [Crossref] [PubMed]
- Zhang C, Gao L, Xu EY. LncRNA, a new component of expanding RNA-protein regulatory network important for animal sperm development. Semin Cell Dev Biol 2016;59:110-7. [Crossref] [PubMed]
- An T, Zhang T, Teng F, et al. Long non-coding RNAs could act as vectors for paternal heredity of high fat diet-induced obesity. Oncotarget 2017;8:47876-89. [Crossref] [PubMed]
- Bouckenheimer J, Assou S, Riquier S, et al. Long non-coding RNAs in human early embryonic development and their potential in ART. Hum Reprod Update 2016;23:19-40. [Crossref] [PubMed]
- Kong A, Frigge ML, Masson G, et al. Rate of de novo mutations and the importance of father's age to disease risk. Nature 2012;488:471-5. [Crossref] [PubMed]
- Perrin M, Kleinhaus K, Messinger J, et al. Critical periods and the developmental origins of disease: an epigenetic perspective of schizophrenia. Ann N Y Acad Sci 2010;1204:E8-13. [Crossref] [PubMed]
- Mulvey BB, Olcese U, Cabrera JR, et al. An interactive network of long non-coding RNAs facilitates the Drosophila sex determination decision. Biochim Biophys Acta 2014;1839:773-84. [Crossref] [PubMed]
- Yamashita A. meiRNA, A Polyvalent Player in Fission Yeast Meiosis. Noncoding RNA 2019;5:45. [Crossref] [PubMed]
- Shichino Y, Yamashita A, Yamamoto M. Meiotic long non-coding meiRNA accumulates as a dot at its genetic locus facilitated by Mmi1 and plays as a decoy to lure Mmi1. Open Biol 2014;4:140022. [Crossref] [PubMed]
- Andergassen D, Muckenhuber M, Bammer PC, et al. The Airn lncRNA does not require any DNA elements within its locus to silence distant imprinted genes. PLoS Genet 2019;15:e1008268. [Crossref] [PubMed]
- Tsai FJ, Yang CF, Chen CC, et al. A genome-wide association study identifies susceptibility variants for type 2 diabetes in Han Chinese. PLoS Genet 2010;6:e1000847. [Crossref] [PubMed]
- Wang K, Liu H, Hu Q, et al. Epigenetic regulation of aging: implications for interventions of aging and diseases. Signal Transduct Target Ther 2022;7:374. [Crossref] [PubMed]
- Su L, Patti ME. Paternal Nongenetic Intergenerational Transmission of Metabolic Disease Risk. Curr Diab Rep 2019;19:38. [Crossref] [PubMed]
- Zhao WL, Gu NH, Li ZZ, et al. Autism-like behaviors and abnormality of glucose metabolism in offspring derived from aging males with epigenetically modified sperm. Aging (Albany NY) 2020;12:19766-84. [Crossref] [PubMed]
- Safi-Stibler S, Gabory A. Epigenetics and the Developmental Origins of Health and Disease: Parental environment signalling to the epigenome, critical time windows and sculpting the adult phenotype. Semin Cell Dev Biol 2020;97:172-80. [Crossref] [PubMed]
- Breuss MW, Antaki D, George RD, et al. Autism risk in offspring can be assessed through quantification of male sperm mosaicism. Nat Med 2020;26:143-50. [Crossref] [PubMed]
- Gold NB, Li D, Chassevent A, et al. Heterozygous de novo variants in CSNK1G1 are associated with syndromic developmental delay and autism spectrum disorder. Clin Genet 2020;98:571-6. [Crossref] [PubMed]
- Oura S, Kazi S, Savolainen A, et al. Cfap97d1 is important for flagellar axoneme maintenance and male mouse fertility. PLoS Genet 2020;16:e1008954. [Crossref] [PubMed]
- Lafuente R, Bosch-Rue E, Ribas-Maynou J, et al. Sperm telomere length in motile sperm selection techniques: A qFISH approach. Andrologia 2018;
- Ferlin A, Rampazzo E, Rocca MS, et al. In young men sperm telomere length is related to sperm number and parental age. Hum Reprod 2013;28:3370-6. [Crossref] [PubMed]


