
Comparison of prostate cancer detection rates and complications between transrectal ultrasound-guided transperineal and transrectal biopsies: a systematic review and meta-analysis
Highlight box
Key findings
• No significant difference was observed in the cancer detection rate between the transperineal (TP) and transrectal (TR) biopsies of the prostate.
• Compared to the TR biopsy, the TP method was associated with a lower risk of rectal bleeding, urinary retention, and fever, but had a higher risk of pain.
What is known and what is new?
• The rates of prostate cancer (PCa) detection and associated complications between TR ultrasound-guided TP and TR prostate biopsies have been investigated in several studies, but the findings are inconsistent.
• In this meta-analysis, TP and TR biopsies have similar detection rates for PCa, but TP biopsy shows a significantly lower risk of rectal bleeding, urinary retention, and fever.
What is the implication, and what should change now?
• Compared to TR biopsy, TP biopsy has a relatively lower risk of associated complications.
• Given the limitations of the present study, our findings need to be verified by further studies.
Introduction
Prostate cancer (PCa) is the most frequently diagnosed solid malignancy among men and has the second highest mortality rate after lung cancer in the world (1), posing a serious threat to men’s health. A 2017 report indicated that approximately 10 million men worldwide were diagnosed with PCa, 700,000 of whom had distant metastases (2). In the early stage, most patients with PCa show no obvious clinical symptoms, while others exhibit clinical manifestations such as urinary tract obstruction (3). Remarkably, definitive treatments, such as radical prostatectomy and radiotherapy, often provide a cure in patients with localized disease. Therefore, the early detection of PCa is crucial.
Routine screening for PCa includes serum prostatic-specific antigen (PSA) measurement. If elevated, digital rectal examination (DRE) and imaging should be considered (4,5). PSA is the most commonly used tumor marker for PCa; however, other benign lesions can also cause PSA elevation (6). DRE has a limited value in the diagnosis of PCa due to its low sensitivity (7). Ultrasonography of the prostate may reveal hypoechoic nodules in the peripheral zone, but generally transrectal ultrasound (TRUS) has a low detection rate for PCa (8) and is mainly used to guide prostate biopsy. Multiparametric magnetic resonance imaging can guide biopsy decision-making by increasing the likelihood of detecting clinically significant PCa while lowering detection of insignificant disease (5). Due to the limitations of these tests, the definitive diagnosis of PCa relies on biopsies guided by TRUS (9-11). The cancer detection rate (CDR) increases with the increase in the number of biopsy specimens obtained.
Transrectal (TR) and transperineal (TP) biopsies are the two main approaches for obtaining prostate tissue samples in PCa detection (12,13). In TR biopsy, the needle is guided through the anterior rectal wall using ultrasound transducers with lateral, end-fire, or biplanar capability. Whereas, in TP biopsy, the needle passes through the perineal skin, which requires the guidance of a lateral or biplanar transducer (14). Several clinical trials have compared the effects of TRUS-guided TR and TP biopsies on CDRs and associated complications (14-18). However, the conclusions are inconsistent and even contradictory. Therefore, in this study, we performed a meta-analysis to investigate the impact of TRUS-guided TP and TR biopsies on the CDR and complications associated with the procedure in order to provide evidence-based clinical practice recommendations for the systematic biopsy of the prostate. We present this article in accordance with the MOOSE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-150/rc) (19).
Methods
Literature retrieval
In September 2024, we searched PubMed, Web of Science, Embase, Cochrane Library, China Wanfang data, and China National Knowledge Infrastructure (CNKI) and collected relevant literature on TP and TR biopsies of the prostate. The search keywords were “prostate cancer”, “prostatic neoplasms”,“prostatic adenocarcinoma”, “transrectal ultrasound”, “TRUS”, “transrectal”, and “transperineal”. The language was limited to English and Chinese. The search was conducted independently by two authors and finally cross-checked. When there was any inconsistency, agreement was reached through discussion.
Eligibility criteria
The inclusion criteria for the literature were as follows: (I) a randomized controlled trial (RCT), case-control study, or cohort study design; (II) a population of patients undergoing systematic biopsy of the prostate; (III) a test group that underwent TRUS-guided TP biopsy and a control group that underwent TRUS-guided TR biopsy; (IV) CDR as the outcome; and (V) complications of rectal bleeding, urinary retention, gross hematuria, fever, and post-biopsy pain evaluated.
The exclusion criteria for the literature were as follows: (I) abstracts, reviews, and preclinical studies; (II) insufficient data; and (III) biopsy not guided by TRUS.
Literature bias risk assessment and data extraction
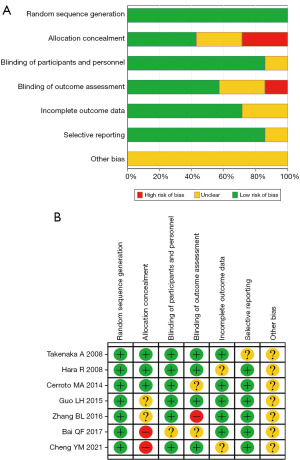
Two authors independently assessed the risk of bias in the literature and extracted the data. The methodological quality of RCTs was assessed using the Cochrane Risk Assessment Scale (20) which consists of the following seven items: random sequence generation, allocation hiding, participant and investigator blinding method, blind outcome assessment, integrity of the resulting data, selective reporting of research findings, and other risks of bias. The trial quality was rated as low risk, high risk, or unclear. The Newcastle-Ottawa scale (NOS), consisting of eight questions, totaling nine points, with ≥6 points indicative of high-quality research, was applied to determine the quality of cohort or case-control studies (21).
Information extracted from the literature included first author, year, country, age and number of patients, study design, serum PSA level, CDR, and post-biopsy complications.
Statistical analysis
Statistical data were analyzed using Stata 15.0 software (StataCorp., San Diego, CA, USA). The relative risk (RR) and 95% confidence interval (CI) were used to assess differences in CDR and complications between the groups. The Chi-squared test and I2 statistic were used to verify the heterogeneity hypothesis. The fixed-effects model (Mantel-Haenszel method) and random-effects model (DerSimonian-Laird method) were applied to combine the data. If high heterogeneity was detected (I2≥50% or P≤0.05), a random-effects model was used; otherwise, a fixed-effects model was used. A funnel plot of CDR was created, and the P value of the Egger test was calculated to evaluate whether publication bias was present. The stability of the results was evaluated via sensitivity tests. P<0.05 was considered statistically significant.
Results
Literature search results and quality assessment
Employing our screening criteria, a total of 20 articles (14-18,22-36) were finally included in this meta-analysis (Figure 1), comprised of seven RCTs, four prospective cohort studies (PCSs), and nine retrospective cohort studies (RCSs). There were 2,979 patients with PCa in the TP group and 2,610 patients in the TR group. The basic characteristics of the included studies are shown in Table 1. The results of RCT bias risk assessment are illustrated in Figure 2A,2B. None of the NOS scores of the PCSs and RCSs were lower than 6, indicating high quality.
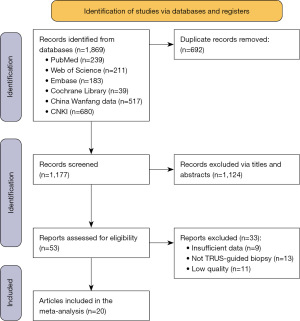
Table 1
| First author (reference) | Year | Country | Study design | Patients | Mean age (years) | PSA level (ng/mL) | NOS score | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|
| TP | TR | TP | TR | |||||||
| Emiliozzi P (22) | 2003 | Italy | PCS | 108 | 107 | 68.0 | 8.20 | 8.20 | 9 | ① |
| Watanabe M (15) | 2005 | Japan | PCS | 402 | 402 | 72.5 | 10.30 | 10.30 | 9 | ① |
| Takenaka A (16) | 2008 | Japan | RCT | 100 | 100 | 71.0 (TP)/72.0 (TR) | 17.10 | 19.60 | NA | ①②③⑤ |
| Hara R (23) | 2008 | Japan | RCT | 126 | 120 | 71.0 | 8.34 | 8.48 | NA | ①③④⑤ |
| Abdollah F (24) | 2011 | Italy | PCS | 140 | 140 | 66.3 | 9.70 | 10.00 | 8 | ① |
| Cerruto MA (25) | 2014 | Italy | RCT | 54 | 54 | 66.5 (TP)/67.3 (TR) | 15.95 | 12.36 | NA | ①②③④⑤ |
| Tian X (29) | 2014 | China | RCS | 175 | 107 | 63.0 (TP)/64.0 (TR) | 1.91–112.52 | 1.45–108.27 | 7 | ①③④⑤⑥ |
| Guo LH (14) | 2015 | China | RCT | 173 | 166 | 67.0 | 8.81 | 10.48 | NA | ①②④⑤⑥ |
| Zhang BL (30) | 2016 | China | RCT | 56 | 55 | 69.0 (TP)/70.3 (TR) | 33.15 | 29.69 | NA | ① |
| Bai QF (31) | 2017 | China | RCT | 80 | 80 | 63.5 (TP)/64.1 (TR) | 4.30–99.80 | 4.50–100.00 | NA | ①②③④⑤ |
| Di Franco CA (26) | 2017 | Italy | RCS | 111 | 108 | 68.0 (TP)/66.0 (TR) | 7.80 | 6.90 | 7 | ① |
| Huang GL (27) | 2019 | China | PCS | 130 | 108 | 66.6 (TP)/67.1 (TR) | 9.30 | 10.90 | 9 | ①③④⑤ |
| Zhai ZX (32) | 2020 | China | RCS | 157 | 162 | 69.9 (TP)/70.8 (TR) | 34.20 | 37.40 | 6 | ①②③④⑤⑥ |
| Gu XL (34) | 2021 | China | RCS | 61 | 60 | 60.0 (TP)/60.1(TR) | 4.74 | 36.02 | 6 | ①②③④⑤⑥ |
| Cheng YM (33) | 2021 | China | RCT | 60 | 60 | 63.0 (TP)/63.2 (TR) | 17.19 | 18.09 | NA | ①②③④⑤ |
| Jiang K (35) | 2021 | China | RCS | 80 | 80 | 63.3 (TP)/61.6 (TR) | 9.80 | 9.60 | 7 | ①②③④ |
| Chen HX (36) | 2022 | China | RCS | 139 | 139 | 67.7 (TP)/66.9 (TR) | 15.27 | 15.06 | 6 | ① |
| He J (18) | 2022 | China | RCS | 540 | 237 | 69.2(TP)/70.7 (TR) | 9.4–89.2 | 6.60–47.10 | 8 | ①③⑤ |
| Lu M (28) | 2023 | China | RCS | 207 | 245 | 70.3 (TP)/70.0 (TR) | 20.60 | 23.20 | 8 | ① |
| Eltafahny A (17) | 2024 | Kuwait | RCS | 80 | 80 | 65.8 (TP)/65.1 (TR) | 14.20 | 23.70 | 8 | ①③④⑤ |
①: CDR; ②: rectal bleeding; ③: urinary retention; ④: hematuria; ⑤: fever; ⑥: pain. PSA level: some data are presented as a range. CDR, cancer detection rate; NA, not applicable; NOS, Newcastle-Ottawa scale; PCS, prospective cohort study; PSA, prostate-specific antigen; RCS, retrospective cohort study; RCT, randomized controlled trial; TP, transperineal; TR, transrectal.
Principal results of the meta-analysis
Heterogeneity analysis
The heterogeneity was not high in the combined analysis of CDR (I2=24.0%; P=0.16; Figure 3), rectal bleeding (I2=16.2%; P=0.30; Figure 4A), urinary retention (I2=0.0%; P=0.78; Figure 4B), fever (I2=0.0%; P=0.90; Figure 4C), pain (I2=22.9%; P=0.27; Figure 5A), and hematuria (I2=43.0%; P=0.06; Figure 5B), and the fixed-effects model was therefore used for further analysis.
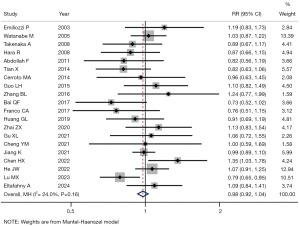


Comparison of CDR
The detection rate of PCa was reported in all 20 studies (Figure 3). There was no statistically significant difference in CDR between the TP and TR biopsy groups (RR =0.98; 95% CI: 0.92–1.04; P=0.46).
Comparison of complications
Rectal bleeding (Figure 4A), urinary retention (Figure 4B), and fever (Figure 4C) were analyzed in 9, 12, and 12 studies, respectively. The fixed-effects model indicated that compared with the TR biopsy approach, the TP approach involved a lower risk of rectal bleeding (RR =0.05; 95% CI: 0.02–0.13; P<0.001), urinary retention (RR =0.70; 95% CI: 0.49–0.99; P=0.046), and fever (RR =0.24; 95% CI: 0.15–0.39; P<0.001).
Pain (Figure 5A) and hematuria (Figure 5B) were analyzed in 4 and 11 studies, respectively. The risk of pain was higher in the TP approach group than in the TR approach group (RR =2.04; 95% CI: 1.47–2.82; P<0.001). No significant difference in hematuria was observed between the two groups (RR =1.05; 95% CI: 0.91–1.22; P=0.52).
Detection of publication bias
The funnel plot of CDR was symmetric (Figure 6), and the P value of the Egger test was greater than 0.05 (P=0.88), indicating no publication bias in the meta-analysis.
Sensitivity analysis
Figure S1 shows the results of sensitivity analysis. A significant difference was observed in the analysis of urinary retention when seven studies (17,18,23,25,27,33,35) were excluded. However, no statistically significant difference occurred in CDR, rectal bleeding, hematuria, fever, or pain when any study was omitted (Figure S1A-S1F). Therefore, in general, the overall findings of this meta-analysis were considered to be robust.
Discussion
To clarify the effects of TRUS-guided TP and TR biopsies of the prostate on the detection rate of PCa and associated complications, we conducted this meta-analysis. At present, the commonly used puncture biopsy routes are TP and TR (37). TP biopsy was the first to appear, but the procedure is relatively complicated and is substantially associated with pain. In 1989, Hodge et al. (38) first adopted the standard six-core biopsy method via TR biopsy. Due to the advantages of a more simple procedure that is well-tolerated by patients, TR biopsy has gradually become a widely used method of prostate biopsy in clinical practice (39).
After the application of strict inclusion and exclusion criteria, a total of 20 articles were included in this meta-analysis. The results suggested that CDR was essentially comparable between the TP and TR methods, without significant difference. The meta-analysis of complications showed that the TP biopsy approach was associated with a lower risk of rectal bleeding, urinary retention, and fever compared to the TR biopsy approach, whereas TP biopsy was associated with a higher risk of post-procedure pain. No significant difference was observed in the risk of hematuria between the two groups. The main complications of prostate biopsy are infection, hematuria, bloody stool, and lower urinary tract symptoms, among others (40,41). Although most of the complications related to needle biopsy are minor, there is the possibility of sepsis, severe bleeding, and even death in rare cases (40). The main manifestations of infection are fever and bacteremia. Compared with the TP route, which does not pass through the rectum, the TR route has been suggested to cause a higher risk of infection (41), although not all investigations have supported this (42).
The funnel plot of CDR was symmetric, and the P value of the Egger test was greater than 0.05. These findings suggest no significant publication bias in this meta-analysis. Consistent with those of our study, a 2019 meta-analysis conducted by Xiang et al. that included 11 articles (43) found that TR and TP biopsies had similar diagnostic accuracy for PCa, with the TP method being associated with a lower risk of rectal bleeding and fever.
It is important to note that TR and TP biopsies had a similar CDR and that the TP method involved lower risks of rectal bleeding, urinary retention, and fever. Nevertheless, the TR approach remains more popular worldwide (44,45). This is because compared to the TP method, TR prostate biopsy is less time-consuming, relatively simple to perform, and does not typically require substantial anesthesia. The American Urological Association and Society of Urologic Oncologists recommend the use of either TR or TP biopsy (46). However, in terms of the CDR, it has been found in clinical practice that prostate apical tumors can escape through the TR route (47), resulting in the missed diagnosis of some apical tumors. TP biopsy thus involves a higher CDR in the prostatic apex than does TR biopsy (48,49). While adjusting the angle of TR biopsies for better apical sampling is possible, TP biopsy can be considered a useful alternative (46).
The meta-analysis involved several limitations that should be discussed. First, the included studies and sample sizes in some of the studies were relatively small, which might reduce the robustness of the conclusions. Second, the included studies were only from four countries, China, Japan, Italy, and Kuwait, which might reduce the generalizability of the findings to populations in other countries/regions. Third, only published studies in the English and Chinese languages were eligible in this meta-analysis, and those published in other languages, as well as unpublished studies, were omitted, which might have introduced a degree of publication bias.
Conclusions
Our meta-analysis indicates that TRUS-guided TP and TR biopsies have similar detection rates for PCa. Nonetheless, compared with the TR approach, TP biopsy was found to be associated with a lower risk of rectal bleeding, urinary retention, and fever but a higher risk of pain. Our findings of this meta-analysis should be verified by high-quality clinical trials and/or meta-analysis in the future.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-150/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-150/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-150/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. [Crossref] [PubMed]
- Choudhury M, Thomas SS, Cain A, et al. Timing of High-Dose-Rate Brachytherapy With External Beam Radiation Therapy in Patients With Intermediate- and High-Risk Localized Prostate Cancer and Its Effects on Toxicity and Quality of Life: A Randomized Controlled Trial (THEPCA). Int J Radiat Oncol Biol Phys 2024;119:90-9. [Crossref] [PubMed]
- Chiu PK, Roobol MJ, Teoh JY, et al. Prostate health index (PHI) and prostate-specific antigen (PSA) predictive models for prostate cancer in the Chinese population and the role of digital rectal examination-estimated prostate volume. Int Urol Nephrol 2016;48:1631-7. [Crossref] [PubMed]
- Wei JT, Barocas D, Carlsson S, et al. Early Detection of Prostate Cancer: AUA/SUO Guideline Part I: Prostate Cancer Screening. J Urol 2023;210:46-53. [Crossref] [PubMed]
- Naji L, Randhawa H, Sohani Z, et al. Digital Rectal Examination for Prostate Cancer Screening in Primary Care: A Systematic Review and Meta-Analysis. Ann Fam Med 2018;16:149-54. [Crossref] [PubMed]
- Huang Y, Li ZZ, Huang YL, et al. Value of free/total prostate-specific antigen (f/t PSA) ratios for prostate cancer detection in patients with total serum prostate-specific antigen between 4 and 10 ng/mL: A meta-analysis. Medicine (Baltimore) 2018;97:e0249. [Crossref] [PubMed]
- Song JM, Kim CB, Chung HC, et al. Prostate-specific antigen, digital rectal examination and transrectal ultrasonography: a meta-analysis for this diagnostic triad of prostate cancer in symptomatic korean men. Yonsei Med J 2005;46:414-24. [Crossref] [PubMed]
- Brock M, von Bodman C, Palisaar J, et al. Detecting Prostate Cancer. Dtsch Arztebl Int 2015;112:605-11. [Crossref] [PubMed]
- Ramacciotti LS, Strauss D, Cei F, et al. Transperineal versus Transrectal MRI/TRUS fusion-guided prostate biopsy in a large, ethnically diverse, and multiracial cohort. Int Braz J Urol 2024;50:616-28. [Crossref] [PubMed]
- De Visschere P, Oosterlinck W, De Meerleer G, et al. Clinical and imaging tools in the early diagnosis of prostate cancer, a review. JBR-BTR 2010;93:62-70. [Crossref] [PubMed]
- Xiao Y, Han L, Wang H, et al. Transperineal prostate biopsy guided by which ultrasound transducer: transrectal or transperineal: a retrospective study. PeerJ 2024;12:e18424. [Crossref] [PubMed]
- Oderda M, Diamand R, Abou Zahr R, et al. Transrectal versus transperineal prostate fusion biopsy: a pair-matched analysis to evaluate accuracy and complications. World J Urol 2024;42:535. [Crossref] [PubMed]
- Guo LH, Wu R, Xu HX, et al. Comparison between Ultrasound Guided Transperineal and Transrectal Prostate Biopsy: A Prospective, Randomized, and Controlled Trial. Sci Rep 2015;5:16089. [Crossref] [PubMed]
- Watanabe M, Hayashi T, Tsushima T, et al. Extensive biopsy using a combined transperineal and transrectal approach to improve prostate cancer detection. Int J Urol 2005;12:959-63. [Crossref] [PubMed]
- Takenaka A, Hara R, Ishimura T, et al. A prospective randomized comparison of diagnostic efficacy between transperineal and transrectal 12-core prostate biopsy. Prostate Cancer Prostatic Dis 2008;11:134-8. [Crossref] [PubMed]
- Eltafahny A, Alshamlan Y, Almazeedi A, et al. Transperineal biopsy as a new technique versus well-established transrectal biopsy for diagnosis of prostate cancer - A comparative study. Urol Ann 2024;16:155-9. [Crossref] [PubMed]
- He J, Guo Z, Huang Y, et al. Comparisons of efficacy and complications between transrectal and transperineal prostate biopsy with or without antibiotic prophylaxis. Urol Oncol 2022;40:191.e9-191.e14. [Crossref] [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Emiliozzi P, Corsetti A, Tassi B, et al. Best approach for prostate cancer detection: a prospective study on transperineal versus transrectal six-core prostate biopsy. Urology 2003;61:961-6. [Crossref] [PubMed]
- Hara R, Jo Y, Fujii T, et al. Optimal approach for prostate cancer detection as initial biopsy: prospective randomized study comparing transperineal versus transrectal systematic 12-core biopsy. Urology 2008;71:191-5. [Crossref] [PubMed]
- Abdollah F, Novara G, Briganti A, et al. Trans-rectal versus trans-perineal saturation rebiopsy of the prostate: is there a difference in cancer detection rate? Urology 2011;77:921-5. [Crossref] [PubMed]
- Cerruto MA, Vianello F, D'Elia C, et al. Transrectal versus transperineal 14-core prostate biopsy in detection of prostate cancer: a comparative evaluation at the same institution. Arch Ital Urol Androl 2014;86:284-7. [Crossref] [PubMed]
- Di Franco CA, Jallous H, Porru D, et al. A retrospective comparison between transrectal and transperineal prostate biopsy in the detection of prostate cancer. Arch Ital Urol Androl 2017;89:55-9. [Crossref] [PubMed]
- Huang GL, Kang CH, Lee WC, et al. Comparisons of cancer detection rate and complications between transrectal and transperineal prostate biopsy approaches - a single center preliminary study. BMC Urol 2019;19:101. [Crossref] [PubMed]
- Lu M, Luo Y, Wang Y, et al. Transrectal versus transperineal prostate biopsy in detection of prostate cancer: a retrospective study based on 452 patients. BMC Urol 2023;23:11. [Crossref] [PubMed]
- Tian X, Zhu CY, Li TQ, et al. Comparison of the clinical value of transperineal and transrectal prostate biopsy guided by transrectal ultrasonography in diagnosis of prostate cancer. China Journal of Modern Medicine 2014;24:80-2.
- Zhang BL, Zeng DL, Wang W, et al. Comparison of value between transrectal ultrasound-guided transperineal biopsy and transrectal biopsy for diagnosis of prostatic cancer. Guangxi Medical Journal 2016;38:792-5.
- Bai QF. Comparative analysis on the value of ultrasound guided transperineal puncture and transrectal puncture in the diagnosis of prostate cancer. Chinese Journal of Biochemical Pharmaceutics 2017;37:360-2.
- Zhai ZX, Zhong GP, Yang L, et al. Comparison of Ultrasound-guided Transrectal and Transperineal Prostate Biopsy. Chinese Journal of Minimally Invasive Surgery 2020;20:405-8.
- Cheng YM, Mo YL, Tan J, et al. Comparative analysis of the efficacy and safety of transrectal and transperineal biopsy guided by rectal ultrasound in the diagnosis of prostate cancer. Hainan Medical Journal 2021;32:2227-30.
- Gu XL, He BX, Zhu ZH. Consistency between ultrasound-guided transperineal and transrectal prostate biopsy in the diagnosis of prostate cancer. Journal of Molecular Imaging 2021;44:489-91.
- Jiang K, Hang ZY, Ma HX, et al. Comparison of the detection rate and safety of transrectal and transper-ineal puncture biopsy for prostate cancer. China Medical Herald 2021;18:103-6.
- Chen HX, Ren J, Fang ZL. Comparison of the clinical diagnostic efficacy between free-hand transperineal prostate biopsy and transrectal prostate biopsy. Journal of China-Japan Friendship Hospital 2022;36:199-202.
- Liu JX, Wang ZY, Niu SX, et al. Transrectal versus transperineal prostate biopsy for cancer detection in patients with gray-zone prostate-specific antigen: a multicenter, real-world study. Asian J Androl 2024;26:377-81. [Crossref] [PubMed]
- Hodge KK, McNeal JE, Terris MK, et al. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol 1989;142:71-4; discussion 74-5. [Crossref] [PubMed]
- Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 2014;65:124-37. [Crossref] [PubMed]
- Loeb S, Vellekoop A, Ahmed HU, et al. Systematic review of complications of prostate biopsy. Eur Urol 2013;64:876-92. [Crossref] [PubMed]
- Steensels D, Slabbaert K, De Wever L, et al. Fluoroquinolone-resistant E. coli in intestinal flora of patients undergoing transrectal ultrasound-guided prostate biopsy--should we reassess our practices for antibiotic prophylaxis? Clin Microbiol Infect 2012;18:575-81. [Crossref] [PubMed]
- Mian BM, Feustel PJ, Aziz A, et al. Complications Following Transrectal and Transperineal Prostate Biopsy: Results of the ProBE-PC Randomized Clinical Trial. J Urol 2024;211:205-13. [Crossref] [PubMed]
- Xiang J, Yan H, Li J, et al. Transperineal versus transrectal prostate biopsy in the diagnosis of prostate cancer: a systematic review and meta-analysis. World J Surg Oncol 2019;17:31. [Crossref] [PubMed]
- Parkin CJ, Gilbourd D, Grills R, et al. Transrectal ultrasound-guided prostate needle biopsy remains a safe method in confirming a prostate cancer diagnosis: a multicentre Australian analysis of infection rates. World J Urol 2022;40:453-8. [Crossref] [PubMed]
- Osama S, Serboiu C, Taciuc IA, et al. Current Approach to Complications and Difficulties during Transrectal Ultrasound-Guided Prostate Biopsies. J Clin Med 2024;13:487. [Crossref] [PubMed]
- Wei JT, Barocas D, Carlsson S, et al. Early Detection of Prostate Cancer: AUA/SUO Guideline Part II: Considerations for a Prostate Biopsy. J Urol 2023;210:54-63. [Crossref] [PubMed]
- Mabjeesh NJ, Lidawi G, Chen J, et al. High detection rate of significant prostate tumours in anterior zones using transperineal ultrasound-guided template saturation biopsy. BJU Int 2012;110:993-7. [Crossref] [PubMed]
- Yan W, Li H, Zhou Y, et al. Prostate carcinoma spatial distribution patterns in Chinese men investigated with systematic transperineal ultrasound guided 11-region biopsy. Urol Oncol 2009;27:520-4. [Crossref] [PubMed]
- Moran BJ, Braccioforte MH. Stereotactic transperineal prostate biopsy. Urology 2009;73:386-8. [Crossref] [PubMed]
(English Language Editor: J. Gray)



