
Fisetin-mediated PPAR-γ upregulation: a novel therapeutic approach for corpus cavernosum smooth-muscle-cell apoptosis and restoration of erectile function after cavernous nerve injury
Highlight box
Key findings
• Fisetin improves recovery from neurogenic erectile dysfunction (NED) after cavernous nerve injury via peroxisome proliferator-activated receptor gamma (PPAR-γ) upregulation, curbing apoptosis.
What is known and what is new?
• Fisetin is known for its anti-apoptotic and anti-fibrotic effects, and PPAR-γ is also associated with fibrosis.
• Fisetin exerts its anti-apoptotic and anti-fibrotic effects by regulating PPAR-γ.
Implications and what should change now?
• Further research and clinical trials are needed to verify the efficacy of fisetin.
Introduction
Erectile dysfunction (ED) refers to the inability of men to achieve or maintain an adequate erection for satisfactory sexual activity (1). Neurogenic erectile dysfunction (NED) (2) accounts for 10–19% (3) of all ED cases and is typically caused by cavernous nerve injury (CNI) resulting from pelvic surgeries such as radical prostatectomy for prostate cancer (4-6). Although phosphodiesterase-5 inhibitors (PDE-5is) are the first-line treatment for NED, efficacy is not as strong as expected, also in nerve sparing prostatectomy (7), while other treatment methods such as intracavernous injection, vacuum erection devices, and penile prosthesis implantation are associated with poor compliance, pain, hematoma, mechanical failure, and infection (8,9). Therefore, there is an urgent need to deepen the understanding of the pathophysiological mechanisms underlying NED and to develop safer and more effective treatment strategies. The recent NED literature suggests that after the loss of neural control in the corpora cavernosa, a series of pathophysiological responses gradually occur, including reduced blood perfusion, ischemia, hypoxia, and cellular phenotypic transformation (10-13). Among these, the increase in apoptosis of the corpus cavernous smooth muscle cells (CCSMCs) leads to penile cavernoma fibrosis, which in turn induces ED, which represents a key pathological change as well as a critical factor for treatment failure in NED (5).
Fisetin is a polyphenolic flavonoid found in various fruits and vegetables and is well-known for its antifibrotic properties (14-16). Recent studies have also highlighted its antiapoptotic effects, which are associated with the activation of peroxisome proliferator-activated receptor gamma (PPAR-γ) (17-19). PPAR-γ was initially recognized for its antifibrotic characteristics, and further research has revealed its potential antiapoptotic effects (20,21). PPAR-γ is widely distributed in various cell types, including endothelial and smooth muscle cells, and its regulatory functions are crucial for maintaining cellular function and tissue homeostasis. We hypothesized that fisetin may reduce apoptosis in CCSMCs after CNI by upregulating PPAR-γ, ultimately improving erectile function (EF). This study examined the protective effects of fisetin for CCSMCs after CNI and attempted to clarify its underlying mechanisms. We present this article in accordance with the ARRIVE and MDAR reporting checklists (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-63/rc).
Methods
Reagents and equipment
This study employed the following materials: Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12); serum-free Roswell Park Memorial Institute (RPMI 1640) medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA); Fisetin (Xu Shuo Biotechnology Co., Ltd., Shanghai, China); T0070907 (SynTheAll Pharmaceuticals Co., Ltd., Shanghai, China); cobalt chloride (CoCl2) (Sigma-Aldrich, St. Lous, MI, USA); fetal bovine serum (Natocor, Cordoba, Argentina); trypsin (Hangzhou Hu Hui Chemical Co., Ltd., Hangzhou, China); Cell Counting Kit-8 (CCK-8) (Hangzhou Zhen Bo Biotechnology Co., Ltd., Hangzhou, China); RIPA lysis buffer (Shanghai Bi Yun Tian Biotechnology Co., Ltd., Shanghai, China); anti-caspase 3, anti-caspase 9, and anti-β-actin antibodies (Cell Signaling Technology, Danvers, MA, USA); anti-Bax and anti-Bcl-2 antibodies (Abcam, Cambridge, UK); anti-HIF-1α and anti-α-SMA antibodies (Hua Xing Bio, Chaoyang, Liaoning, China); anti-PPAR-γ antibody (Hua Xing Bio); and horseradish peroxidase (HRP)-conjugated secondary antibodies (Shanghai Bi Yun Tian Biotechnology Co., Ltd., Shanghai, China). Other equipment used included CO2 incubators (Thermo Fisher Scientific, Waltham, MA, USA), a spectrophotometer (Shanghai Precision Scientific Instruments Co., Ltd., Shanghai, China), a high-speed refrigerated centrifuge (Eppendorf, Hamburg, Germany), and protein electrophoresis, transfer, and gel imaging systems (Bio-Rad Laboratories, Hercules, CA, USA).
Animal model construction and experimental design
Experiments in this study were approved by the Animal Ethics Committee of Zhejiang Chinese Medical University (approval No. IACUC-202401-04) and followed the Regulations of the People’s Republic of China on the Administration of Laboratory Animals and the Animal Experiment Ethics Guide of Zhejiang Chinese Medical University for the care and use of animals. All experimental operations were performed under the approved protocol to ensure animal welfare and experimental compliance. Twenty-four specific pathogen-free grade Sprague-Dawley rats (6 weeks old, weighing 150–200 g) were purchased from the Zhejiang Chinese Medical University Animal Experiment Center. The animals were housed in a standard specific pathogen-free environment with free access to food and water under a constant temperature (23±1 °C) and a 12-hour light-dark cycle. They were raised to 8 weeks old and 350–400 g before being randomly assigned to three experimental groups: the control group, the model group, and the fisetin treatment group. The sample size was determined based on statistical requirements, anticipated experimental loss, and the need for subsequent analyses. According to statistical principles, a minimum of three replicates is required to ensure reliable and reproducible results. Additionally, we anticipated potential losses during the CNI modeling process due to surgical complications or other factors. Considering these factors and adhering to the 3R principles (Replacement, Reduction, Refinement) for animal welfare, we decided on a sample size of 8 rats per group. This number ensures statistical robustness while minimizing the number of animals used. On the day of surgery, rats were anesthetized via intraperitoneal injection of 3% pentobarbital sodium (50 mg/kg). Subsequently, a midline abdominal incision was made to expose the bilateral cavernous nerves (CNs). In the model and fisetin treatment groups, the bilateral CNs were precisely isolated and clamped twice using micro-forceps (Jin Zhong, Shanghai, China), with each clamping lasting 60 seconds and separated by a 10-second interval, to induce injury. The control group underwent only the abdominal incision and nerve exposure without clamping (22). Following the surgery, rats in the fisetin treatment group were administered fisetin intragastrically at a dose of 2.5 mg/(kg·day), starting from the day of surgery and continuing for 28 days (23). The control and model groups received an equivalent volume of isotonic saline solution daily over the same period. All animal procedures were conducted under anesthesia. During the study period, all rats were monitored for survival status, with no deaths recorded. The experimenters were aware of the group allocation. On postoperative day 28, erectile function (EF) was assessed in all rats. Immediately after EF assessment, penile tissue samples were collected, with some samples fixed in 4% formaldehyde and others frozen in liquid nitrogen and stored at −80 °C for subsequent analyses [including Western blot and real-time quantitative polymerase chain reaction (RT-qPCR)]. To minimize potential confounders, the order of treatments and measurements was randomized. A protocol was prepared before the study without registration.
EF assessment
On the 28th day of the experiment, rats were anesthetized via intraperitoneal injection 3% pentobarbital sodium (50 mg/kg dose). EF was evaluated as the ratio of intracavernous pressure to mean arterial pressure (ICP/MAP). To evaluate MAP a longitudinal incision was made parallel to the trachea, separating the neck tissues, and the right carotid artery was located through the tracheal position, with careful dissection of the right carotid artery. The proximal end was clamped, the distal end was ligated with a ligature, a small incision was made on the proximal end with ophthalmic scissors, and a polyethylene-50 (PE-50) catheter containing heparin was inserted into the carotid artery. The other side of the catheter was connected to a pressure transducer to record the changes in intra-arterial pressure. All pressure data measurements were completed using software for recording, and the experiment was conducted in a blinded manner. To evaluate the ICP, a 25-G needle filled with 100 U/mL of heparin was inserted into the right side of the penis and this needle was connected to a pressure transducer via a PE-50 catheter. Both MAP and ICP were measured with the MP160 pressure transducer. Following this, we used a silver bipolar electrode to stimulate the CN below the clamping site from the first surgery. The stimulation parameters were as follows: a voltage of 5 V, a frequency of 20 Hz, and pulse width of 5 ms. Each round of stimulation lasted for 60 seconds, with a 5-minute interval between the two rounds of stimulation (24). All pressure data measurements were completed using software for recording, and the experiment was conducted in a blinded manner.
Fluorescence staining
Tissue sections from the midportion of the penile corpus cavernosum were deparaffinized via a soak in xylene and then rehydrated through a graded ethanol series (100%, 95%, 70%, 50%, 30%) for 5 minutes each, which was followed by a gentle rinse in phosphate-buffered saline (PBS) for 5 minutes. The sections were placed in 1× citrate antigen retrieval solution and microwaved at a low temperature for 15 minutes for antigen retrieval. After retrieval, the sections were rinsed three times with PBS for 5 minutes each. The sections were then incubated in 5% goat serum at room temperature for 30 minutes to block nonspecific binding. Appropriate amounts of α-SMA primary antibody (1:200) and TUNEL reagent were applied for double staining, and the sections were incubated overnight at 4 °C. After retrieval, the sections were warmed to 37 °C for 1 hour and then rinsed three times with PBS for 5 minutes each. Subsequently, an appropriate amount of fluorescently labeled secondary antibody (1:500) was applied, and the sections were incubated at 37 °C in the dark for 1 hour. DAPI (1:500) was used for nuclear staining, and the sections were incubated in the dark for 1 hour before being rinsed three times with PBS for 5 minutes each. An appropriate amount of mounting medium was applied to seal the sections, which were then stored at 4 °C. The double-staining results of α-SMA and TUNEL were observed under a fluorescence microscope. The same method was used for immunofluorescence staining of PPAR-γ (1:200).
Masson staining for penile cavernous tissue analysis
Penile corpus cavernosum tissue samples were fixed in 4% formaldehyde at room temperature for 24 hours, which was followed by paraffin embedding and preparation of 5-µm-thick sections. The sections were deparaffinized in xylene and rehydrated through an ethanol gradient (100%, 95%, 70%, 50%, 30%), with each ethanol step lasting 5 minutes, and finally rinsed with distilled water. The sections were treated with Bouin solution at 56 °C for 1 hour to enhance staining, which was followed by nuclear staining with Weigert iron hematoxylin solution for 10 minutes. Subsequently, the sections were stained in Biebrich scarlet-acid fuchsin solution for 15 minutes and then treated with phosphotungstic acid/phosphomolybdic acid solution for 10 minutes to achieve differentiation of tissue structures. Finally, the sections were stained in aniline blue solution for 10 minutes to visualize collagen fibers. The stained sections were dehydrated through an ethanol gradient, cleared in xylene, and mounted with synthetic resin. The mounted sections were observed under a light microscope, with collagen fibers appearing blue, muscle fibers red, and cell nuclei black (25).
Cell culture and treatment
We prepared 3 normal sexually mature rats, independent of the 24 rats used in the main experiment, to isolate CCSMCs for subsequent in vitro experiments (26,27). These rats were adapted to feeding before the experiment and managed under the same conditions as the main experiment. CCSMCs were evenly seeded in 96-well plates at a density of 2×103 cells per well. After incubation in a cell culture incubator for 24 hours, the cells were treated with various concentrations of CoCl2 (12.5, 25, 50, 100, 200, 400, and 600 µmol/L) and fisetin (0.5, 1, 2, 4, and 8 µmol/L) alone or in combination for 24 hours. Each concentration was tested in six replicate wells. Subsequently, 10 µL of CCK-8 reagent was added to each well and incubated for an additional 4 hours. Absorbance was measured at 450 nm using Biotek Cytation 1. The cell survival rate was calculated based on the PBS-treated control group to determine the optimal concentrations of CoCl2 and fisetin. There were four groups in the cell experiment, each with six replicates: the control group: the control group, the model group (CoCl2), the fisetin group (CoCl2 + fisetin), and the T0070907 group (CoCl2 + fisetin + T0070907). T0070907 can effectively bind to PPAR-γ and inhibit its activation (28), and its safety has been confirmed in numerous experimental studies.
Western blotting
After penile tissue and cell samples were collected, total proteins were lysed in RIPA solution containing protease and phosphatase inhibitors for extraction. The quantity of protein was determined using a bicinchoninic acid (BCA) assay. Equal amounts of protein, approximately 5 µg per sample, were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were then blocked with a 5% bovine serum albumin (BSA) solution for 1 hour. Subsequently, the membranes were incubated overnight at 4 °C with specific primary antibodies. The primary antibodies used included anti-caspase 3 (1:1,000), anti-caspase 9 (1:1,000), anti-Bax (1:800), anti-Bcl-2 (1:1,000), anti-α-SMA (1:1,000), anti-Col-1 (1:1,000), anti-Col-3 (1:1,000), and anti-β-actin (1:1,000), with β-actin serving as a loading control. On the following day, the membranes were incubated for 1 hour with appropriate secondary antibodies diluted to 1:20,000 and conjugated with HRP (29). Protein bands were detected and captured using enhanced chemiluminescence (ECL) with a digital imaging device. To ensure equal protein loading across all samples, β-actin was used as a loading control. Quantitative assessment was performed using ImageJ software (US National Institutes of Health, Bethesda, MD, USA).
RT-qPCR
Total RNA was extracted from penile tissue and cultured CCSMCs at subzero temperatures via TRIzol reagent. The extracted RNA was then reverse transcribed into complement DNA (cDNA) using a reverse transcription kit. RT-qPCR was performed on the cDNA with Hieff qPCR SYBR Green Master Mix. β-actin was used as an endogenous control to ensure uniform protein loading (30). Table 1 lists the specific primers used in this study.
Table 1
| Name | Forward primer sequence 5'-3' | Reverse primer sequence 5'-3' |
|---|---|---|
| α-SMA | CAGTCGCCATCAGGAACCTC | TTGGCCCATTCCAACCATCA |
| β-actin | CCACCAGTTCGCCATGGAT | CAGTTGGTGACAATGCCGTG |
| HIF-1α | CATCAAGTCAGCAACGTGGA | GCACGTCATAGGCGGTTTCT |
| Caspase 3 | GGACCTGTGGACCTGAAAAA | GCATGCCATATCATCGTCAG |
| Caspase 9 | CTGAGCCAGATGCTGTCCCATA | GACACCATCCAAGGTCTCGATGTA |
| Bax | GGCGATGAACTGGACAACAAC | CCACACGGAAGAAGACCTCTC |
| Bcl-2 | CTGAACCGGCATCTGCACAC | TGAGCAGCGTCTTCAGAGACA |
| PPAR-γ | GGAGCCTAAGTTTGAGTTTGCTGTG | TGCAGCAGGTTGTCTTGGATG |
HIF, hypoxia-inducible factor; PPAR, peroxisome proliferator activated receptor; SMA, smooth muscle actin.
Molecular docking
To explore the potential interaction between fisetin and PPAR-γ, molecular docking simulations were conducted using AutoDock Vina (version 1.2.0, The Scripps Research Institute, La Jolla, CA, USA). The three-dimensional structure of PPAR-γ (PDB ID: 2PRG) was downloaded from the Protein Data Bank (https://www.rcsb.org/), and the structure of fisetin (CID: 5281614) was retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Prior to docking, the protein structure was prepared by removing water molecules and adding polar hydrogen atoms, while the ligand structure was optimized using the MMFF94 force field. The docking simulations were performed with the following parameters: grid box size of 60×60×60 Å centered on the ligand-binding pocket, exhaustiveness set to 20, and the Lamarckian genetic algorithm as the search method. The binding affinity was calculated based on the lowest-energy binding pose, and the results were visualized and analyzed using PyMOL (version 2.5.2, Schrödinger, LLC) to determine the specific interaction characteristics, including hydrogen bonds and hydrophobic interactions, between fisetin and PPAR-γ.
Statistical analysis
Statistical and graphical analyses were performed with GraphPad Prism software version 9.5 (GraphPad Software, San Diego, CA, USA), and the data are presented as the mean ± standard error of the mean (SEM). For comparisons across multiple groups, one-way analysis of variance (ANOVA) was first performed to detect overall differences. If a significant effect was found (P<0.05), post hoc pairwise comparisons were conducted using Tukey’s Honestly Significant Difference (HSD) test to control the family-wise error rate. For experiments involving only two groups, an unpaired Student’s t-test was applied. A corrected P value <0.05 was considered statistically significant for all analyses.
Results
Fisetin effectively inhibited the apoptosis of CCSMCs in NED rats following CNI
Compared to those in the control group, the protein and messenger RNA (mRNA) expression levels of caspase 3, caspase 9, and Bax in CCSMCs of the model rats were significantly increased, while the protein and mRNA expression levels of Bcl-2 showed a decreasing trend, although this was not significantly significant (Figure 1A-1C). In the fisetin group, the protein and mRNA expression levels of caspase 3 and Bax were significantly reduced (Figure 1A-1C). The mRNA level of caspase-9 was also significantly decreased, the protein level was decrease (Figure 1A-1C). The protein and mRNA expression levels of Bcl-2 in the fisetin group were elevated, but these increases were not statistically significant (Figure 1A-1C). The ratio of Bax protein to Bcl-2 protein in the model rats’ penile cavernosum was increased, but not significantly so, while the ratio of Bax mRNA to Bcl-2 mRNA was significantly increased (Figure 1D,1E). In the fisetin group, the expression ratio of Bax protein to Bcl-2 protein was decreased, but this change was not statistically significant (Figure 1D). The ratio of Bax mRNA to Bcl-2 mRNA was significantly reduced after fisetin treatment (Figure 1E). TUNEL staining experiments revealed apoptosis in CCSMCs of the model group, and Fisetin intervention significantly reduced the occurrence of apoptosis (Figure 1F).
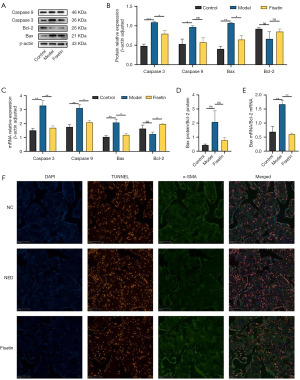
Fisetin improved cavernous tissue fibrosis after bilateral CNI
Western blot analysis revealed that the levels of Col-1 and Col-3 in the penile corpora cavernosa of the model rats were significantly increased (Figure 2A,2B). Masson trichrome staining also indicated an elevated ratio of collagen to smooth muscle in the penile corpora cavernosa tissue (Figure 2C,2D). After 28 days of intragastric administration via gavage of 2.5 mg/(kg·day) of fisetin, the content of Col-1 and Col-3 was reduced (Figure 2A,2B). Masson staining similarly showed a decreased ratio of collagen to smooth muscle (Figure 2C,2D).

Fisetin improved EF in NED rats
In this study, fisetin was administered orally at a dose of 2.5 mg/(kg·day) for a total of 28 consecutive days. After 28 days of intragastric administration via gavage of 2.5 mg/(kg·day) of fisetin, the EF of rats in the fisetin group was assessed using ICP and MAP. The results indicated that 28 days after CNI, the maximum ICP (ICPmax), the change in ICP (ΔICP), and the ICP/MAP ratio were significantly reduced in the rats. Following fisetin treatment, these parameters showed improvement, with increases in ICPmax, ΔICP, and ICP/MAP ratio, but were still below normal levels (Figure 3A-3C).

The antiapoptotic effect of fisetin on CCSMCs in NED rats was associated with PPAR-γ
RT-qPCR analysis revealed that compared to that in the control group, the mRNA expression of PPAR-γ in the penile tissue of the model group was significantly reduced (Figure 4A). Additionally, immunofluorescence labeling indicated a decrease in PPAR-γ level in the penile corpora cavernosa tissue following CNI (Figure 4B). After treatment with fisetin, this reduction in expression was significantly reversed (Figure 4A,4B). The molecular docking results indicated that the binding energy between fisetin and PPAR-γ was −7.8 kcal/mol (Figure 4C). The detailed methodology for the molecular docking study is described in the Methods section.
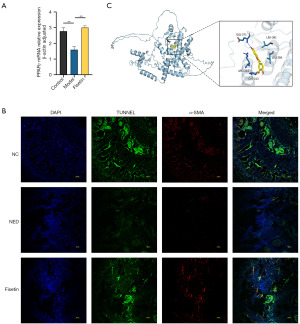
Downregulation of PPAR-γ expression in oxygen-deprived CCSMCs in vitro
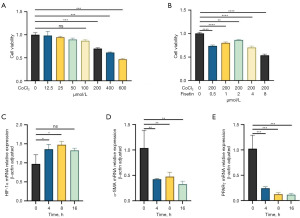
The results indicated that at a concentration of 200 µmol/L CoCl2, the mortality rate of CCSMCs reached 75%, while 2 µmol/L of fisetin restored the viability of CCSMCs to nearly 90% of normal levels (Figure 5A,5B). Subsequent experiments were conducted with CoCl2 and fisetin concentrations set at 200 and 2 µmol/L, respectively. After intervention with 200 µmol/L of CoCl2 for 4, 8, and 16 hours, the mRNA levels of HIF-1α in CCSMCs significantly increased over time, while α-SMA significantly decreased, and the mRNA levels of PPAR-γ also significantly decreased over time (Figure 5C-5E).
Fisetin inhibited the apoptosis of CCSMCs after CNI via the upregulation of PPAR-γ
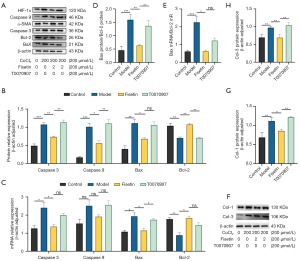
According to Western blot data analysis, T0070907 significantly reversed the reduction in caspase 3 and caspase 9 protein levels and the increase in Bcl-2 protein level induced by fisetin treatment (Figure 6A,6B). Bax protein level increased, but this change was not statistically significant (Figure 6A,6B). The RT-qPCR results further confirmed that T0070907 could significantly increase Bax mRNA levels, while the mRNA levels of caspase 3, caspase 9, and Bcl-2 all decreased (Figure 6C). Additionally, Western blot results indicated that T0070907 led to a significant increase in ratio of Bax to Bcl-2 protein expression (Figure 6D), while RT-qPCR results indicated an increase in the ration of Bax to Bcl-2 mRNA (Figure 6E). In hypoxic model cells, we further examined the protein levels of Col-1 and Col-3 and found that the protein levels of both collagens were significantly elevated after PPAR-γ inhibition (Figure 6F-6H).
Discussion
In our study, we first examined the therapeutic effects of fisetin on erectile function and tissue pathology in a rat model of CNI-induced neurogenic erectile dysfunction (NED). We observed significant improvements in apoptosis and fibrosis in the penile tissue of bilateral CNI rats treated with fisetin. Quantitative analysis of TUNEL staining and apoptotic markers, including caspase 3, caspase 9, Bax, and Bcl-2 at the both protein and mRNA levels, followed by assessment of fibrosis via Masson staining and protein levels of Col-1 and Col-3, revealed that fisetin markedly ameliorated the apoptotic status and fibrosis of CNI rats. Additionally, RT-qPCR indicated that fisetin could reverse the downregulation of PPAR-γ mRNA expression in the penile tissue of rats after CNI, while molecular docking experiments demonstrated effective binding between fisetin and PPAR-γ. Subsequently, in a hypoxia model induced by CoCl2 in CCSMCs, we observed reduced mRNA levels of PPAR-γ and α-SMA, which were reversed by fisetin treatment; we also observed the upregulation of caspase 3, caspase 9, Bax, Col-1, and Col-3 and the downregulation of α-SMA and Bcl-2. The application of the PPAR-γ inhibitor T0070907 reversed these changes, further confirming the pivotal role of PPAR-γ in fisetin’s effect. These findings suggest that fisetin may improve EF by upregulating PPAR-γ expression, inhibiting CCSMCs apoptosis, and ameliorating corporal fibrosis.
This study is the first to find evidence indicating that fisetin can improve EF by inhibiting apoptosis in CCSMCs and improving collagen deposition. A substantial body of research has established that fisetin possesses antiapoptotic and antifibrotic effects (19,31,32). For instance, Yang et al. treated lead-induced synaptic dysfunction, neuroinflammation, and neurodegeneration in mice with fisetin and found a significant increase in Bcl-2 and a decrease in Bax and caspase 3 in their brain tissues (31). Moreover, Chen et al. reported that fisetin exerted a potent protective effect against angiotensin II-induced apoptosis in H9c2 cells and SHR models (33).
PPAR-γ is a subtype of the PPAR family, which belongs to the nuclear receptor superfamily of ligand-activated transcription factors (34). Previous studies have shown that the activation of PPAR-γ can effectively suppress the increases in proapoptotic protein levels and decreases in antiapoptotic protein levels (35,36). Wu et al. found that the overexpression of PPAR-γ in a model of oxygen-glucose deprivation in N2-A cells could effectively maintain mitochondrial membrane potential and prevent apoptosis (37). In our study, we further discovered that the ant-apoptotic properties of fisetin in ED from CNI are mediated through the activation of PPAR-γ. This is consistent with the findings of Garg et al. (18), who were first to report on the potential interaction between fisetin and PPAR-γ through molecular docking experiments in their study on the protective mechanism of fisetin against cardiac ischemia-reperfusion injury in a Wistar rat model. Further enzyme-linked immunosorbent assay (ELISA) and Western blot experiments also confirmed that fisetin alleviated apoptosis by upregulating PPAR-γ, and these protective effects could be reversed by a PPAR-γ inhibitor (18). It is important to note that the role of PPAR-γ in apoptosis is complex and multifaceted. For instance, Ji et al.’s research on ischemia–reperfusion injury in mouse liver demonstrated that the inhibition of PPAR-γ can lead to a reduction in hepatocyte apoptosis (38). This suggests that PPAR-γ may exert distinct biological functions across various disease models. There is a scarcity of literature on the relationship between PPAR-γ and EF. Hu et al. found in their study on the improvement of diabetic ED found that rats receiving 500 mg/(kg·day) of probucol had significantly higher ICP and MAP values than did the control rats group, with a marked reduction in fibrosis as shown by Masson staining, which was accompanied by an increase in PPAR-γ levels (39). These findings underscore the necessity for further research into how PPAR-γ affects EF and to establish its ability to serve as a therapeutic target in the treatment of ED.
Our study has several limitations. First, the use of a rat model may not fully replicate the complexity of human NED, and our findings need validation in larger and more diverse models. Second, while we focused on PPAR-γ, the broader signaling pathways involved in fisetin’s effects were not fully explored. Third, the sample size and 28-day study duration may limit the generalizability of our results. Future studies should consider longer durations or multiple time points to better assess the long-term effects of fisetin. Lastly, the absence of a group treated with fisetin alone without inducing CNI limits our understanding of its independent effects. Future research should address these limitations to provide a more comprehensive evaluation of fisetin’s therapeutic potential.
In summary, our findings indicate that fisetin, by upregulating the expression of PPAR-γ, can effectively prevent bilateral CNI-induced CCSMC apoptosis and ameliorate fibrosis, exerting a positive therapeutic impact on NED. Although our experimental results are promising, the safety and clinical efficacy of fisetin as a potential treatment for NED need to be further validated in a larger patient population. Future studies should further clarify the molecular mechanisms by which PPAR-γ exerts a therapeutic effect for CNI-induced ED, with a particular focus on antiapoptotic pathways, to more definitively contribute to the scientific development of NED treatment strategies.
Conclusions
This study found that fisetin effectively modulates the PPAR-γ signaling pathway, enhances the expression of Bcl-2, and suppresses the expression of caspase 3, caspase 9, and Bax, thereby significantly alleviating fibrosis in penile cavernous tissue. These findings provide new targets and therapeutic strategies for the treatment of NED and new directions for clinical research.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE and MDAR reporting checklists. Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-63/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-63/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-2025-63/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-2025-63/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments in this study were approved by the Animal Ethics Committee of Zhejiang Chinese Medical University (approval No. IACUC-202401-04) and followed the Regulations of the People’s Republic of China on the Administration of Laboratory Animals and the Animal Experiment Ethics Guide of Zhejiang Chinese Medical University for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shamloul R, Ghanem H. Erectile dysfunction. Lancet 2013;381:153-65. [Crossref] [PubMed]
- Thomas C, Konstantinidis C. Neurogenic Erectile Dysfunction. Where Do We Stand? Medicines (Basel) 2021;8:3. [Crossref] [PubMed]
- Lee M, Sharifi R. Non-invasive Management Options for Erectile Dysfunction When a Phosphodiesterase Type 5 Inhibitor Fails. Drugs Aging 2018;35:175-87. [Crossref] [PubMed]
- Burnett AL. Erectile dysfunction following radical prostatectomy. JAMA 2005;293:2648-53. [Crossref] [PubMed]
- Song G, Hu P, Song J, et al. Molecular pathogenesis and treatment of cavernous nerve injury-induced erectile dysfunction: A narrative review. Front Physiol 2022;13:1029650. [Crossref] [PubMed]
- Svennersten K, Reus C. Etiology, surgical anatomy, and pathophysiology of male erectile dysfunction. International Journal of Reconstructive Urology 2024;2:4-10.
- Goh HJ, Sung JM, Lee KH, et al. Efficacy of phosphodiesterase type 5 inhibitors in patients with erectile dysfunction after nerve-sparing radical prostatectomy: a systematic review and meta-analysis. Transl Androl Urol 2022;11:124-38. [Crossref] [PubMed]
- Karakus S, Burnett AL. The medical and surgical treatment of erectile dysfunction: a review and update. Can J Urol 2020;27:28-35.
- Langarizadeh MA, Salary A, Tavakoli MR, et al. An overview of the history, current strategies, and potential future treatment approaches in erectile dysfunction: a comprehensive review. Sex Med Rev 2023;11:253-67. [Crossref] [PubMed]
- Gu X, Thakker PU, Matz EL, et al. Dynamic Changes in Erectile Function and Histological Architecture After Intracorporal Injection of Human Placental Stem Cells in a Pelvic Neurovascular Injury Rat Model. J Sex Med 2020;17:400-11. [Crossref] [PubMed]
- Hassanin AM, Abdel-Hamid AZ. Cavernous smooth muscles: innovative potential therapies are promising for an unrevealed clinical diagnosis. Int Urol Nephrol 2020;52:205-17. [Crossref] [PubMed]
- Ying CC, Yang M, Wang Y, et al. Neural-like cells from adipose-derived stem cells for cavernous nerve injury in rats. Neural Regen Res 2019;14:1085-90. [Crossref] [PubMed]
- Zhou K, Ma K, Ye M, et al. Application of laser speckle blood perfusion imaging in the evaluation of erectile function in rats. Andrologia 2022;54:e14264. [Crossref] [PubMed]
- Antika LD, Dewi RM. Pharmacological aspects of fisetin. Asian Pacific Journal of Tropical Biomedicine 2021;11:1-9.
- Choi MS, Choi JY, Kwon EY. Fisetin Alleviates Hepatic and Adipocyte Fibrosis and Insulin Resistance in Diet-Induced Obese Mice. J Med Food 2020;23:1019-32. [Crossref] [PubMed]
- Kwon TH. A novel strategy employing the flavonoid fisetin to halt the progression of renal fibrosis in obstructive nephropathy. Kidney Res Clin Pract 2023;42:282-5. [Crossref] [PubMed]
- Adeli OA, Heidari-Soureshjani S, Rostamian S, et al. Effects and Mechanisms of Fisetin against Ischemia-reperfusion Injuries: A Systematic Review. Curr Pharm Biotechnol 2024;25:2138-53. [Crossref] [PubMed]
- Garg S, Khan SI, Malhotra RK, et al. The molecular mechanism involved in cardioprotection by the dietary flavonoid fisetin as an agonist of PPAR-γ in a murine model of myocardial infarction. Arch Biochem Biophys 2020;694:108572. [Crossref] [PubMed]
- Prem PN, Kurian GA. Fisetin attenuates renal ischemia/reperfusion injury by improving mitochondrial quality, reducing apoptosis and oxidative stress. Naunyn Schmiedebergs Arch Pharmacol 2022;395:547-61. [Crossref] [PubMed]
- Kawai T, Masaki T, Doi S, et al. PPAR-γ agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-β. Laboratory Investigation. 2009;89:47-58. [Crossref] [PubMed]
- Vetuschi A, Pompili S, Gaudio E, et al. PPAR-γ with its anti-inflammatory and anti-fibrotic action could be an effective therapeutic target in IBD. Eur Rev Med Pharmacol Sci 2018;22:8839-48. [Crossref] [PubMed]
- Ma K, Zhao F, Ye MY, et al. Neuroprotective effect of Hongjing I granules on erectile dysfunction in a rat model of bilateral cavernous nerve injury. Biomed Pharmacother 2020;130:110405. [Crossref] [PubMed]
- Ding H, Li Y, Chen S, et al. Fisetin ameliorates cognitive impairment by activating mitophagy and suppressing neuroinflammation in rats with sepsis-associated encephalopathy. CNS Neurosci Ther 2022;28:247-58. [Crossref] [PubMed]
- Pan F, Zhang J, Liu Y, et al. Intracavernosal Pressure Recording to Evaluate Erectile Function in Rodents. J Vis Exp 2018;56798. [Crossref] [PubMed]
- Foot NC. The Masson trichrome staining methods in routine laboratory use. Stain Technology 1933;8:101-10.
- Chung H, Jung SH, Ryu JK, et al. Isolation and characterization of smooth muscle cells from rat corpus cavernosum tissue for the study of erectile dysfunction. Korean J Urol 2012;53:556-63. [Crossref] [PubMed]
- Pilatz A, Schultheiss D, Gabouev AI, et al. Isolation of primary endothelial and stromal cell cultures of the corpus cavernosum penis for basic research and tissue engineering. Eur Urol 2005;47:710-8; discussion 718-9. [Crossref] [PubMed]
- Lee G, Elwood F, McNally J, et al. T0070907, a selective ligand for peroxisome proliferator-activated receptor gamma, functions as an antagonist of biochemical and cellular activities. J Biol Chem 2002;277:19649-57. [Crossref] [PubMed]
- Meftahi GH, Bahari Z, Zarei Mahmoudabadi A, et al. Applications of western blot technique: From bench to bedside. Biochem Mol Biol Educ 2021;49:509-17. [Crossref] [PubMed]
- Bustin SA. Improving the quality of quantitative polymerase chain reaction experiments: 15 years of MIQE. Mol Aspects Med 2024;96:101249. [Crossref] [PubMed]
- Yang W, Tian ZK, Yang HX, et al. Fisetin improves lead-induced neuroinflammation, apoptosis and synaptic dysfunction in mice associated with the AMPK/SIRT1 and autophagy pathway. Food Chem Toxicol 2019;134:110824. [Crossref] [PubMed]
- Zhang L, Tong X, Huang J, et al. Fisetin Alleviated Bleomycin-Induced Pulmonary Fibrosis Partly by Rescuing Alveolar Epithelial Cells From Senescence. Front Pharmacol 2020;11:553690. [Crossref] [PubMed]
- Chen YP, Sivalingam K, Shibu MA, et al. Protective effect of Fisetin against angiotensin II-induced apoptosis by activation of IGF-IR-PI3K-Akt signaling in H9c2 cells and spontaneous hypertension rats. Phytomedicine 2019;57:1-8. [Crossref] [PubMed]
- Mal S, Dwivedi AR, Kumar V, et al. Role of Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) in Different Disease States: Recent Updates. Curr Med Chem 2021;28:3193-215. [Crossref] [PubMed]
- Fong WH, Tsai HD, Chen YC, et al. Anti-apoptotic actions of PPAR-gamma against ischemic stroke. Mol Neurobiol 2010;41:180-6. [Crossref] [PubMed]
- Gensch C, Clever YP, Werner C, et al. The PPAR-gamma agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells. Atherosclerosis 2007;192:67-74. [Crossref] [PubMed]
- Wu JS, Lin TN, Wu KK. Rosiglitazone and PPAR-gamma overexpression protect mitochondrial membrane potential and prevent apoptosis by upregulating anti-apoptotic Bcl-2 family proteins. J Cell Physiol 2009;220:58-71. [Crossref] [PubMed]
- Ji J, Wu L, Feng J, et al. Cafestol preconditioning attenuates apoptosis and autophagy during hepatic ischemia-reperfusion injury by inhibiting ERK/PPARγ pathway. Int Immunopharmacol 2020;84:106529. [Crossref] [PubMed]
- Hu LL, Zhang KQ, Tian T, et al. Probucol improves erectile function via Activation of Nrf2 and coordinates the HO-1 / DDAH / PPAR-γ/ eNOS pathways in streptozotocin-induced diabetic rats. Biochem Biophys Res Commun 2018;507:9-14. [Crossref] [PubMed]
(English Language Editor: J. Gray)


