Extirpative surgery for chronic orchialgia: is there a role?
Introduction
Chronic Orchialgia is defined as intermittent or continuous scrotal content pain, lasting greater than 3 months in duration, and interfering with a patient’s activities (1). It is recognized this pain is not purely testicular in nature, but may involve the epididymis, spermatic cord, anterior scrotum, or para-testicular structures including a varicocele (2). Chronic scrotal content pain should be differentiated from extra-scrotal etiologies such as radiculitis, pelvic floor muscular dysfunction, chronic pelvic pain syndrome, prostatitis, distal ureteral stone, inguinodynia or neuropathic pain associated with changes of the ilioinguinal, iliohypogastric, and genitofemoral nerves (3-5). Pain following herniorrhaphy presents a multifactorial situation that may include neuropathic inguinodynia plus nociceptive pain due to ischemic orchitis, edema, cord fibrosis, or entrapment of the para-vasal nerve fibers (6-8). Chronic orchialgia or inguinodynia are now a more common occurrence than hernia recurrence following herniorrhaphy (8). Differentiating the nociceptive pain from the neuropathic pain can be difficult and a recent Finnish study revealed 34 testicular injuries were reported among 62 urologic complications from herniorrhaphy (6). Seventeen of the 34 testes were removed for chronic pain or necrosis.
Chronic orchialgia has been identified as the most common urologic reason for medical discharge from the US Army and to have an estimated incidence of 1% in the United Kingdom (9,10). The incidence of chronic orchialgia is increasing over time possibly related to increased willingness of providers to address this pain (11). Psychological patient factors are associated with chronic orchialgia and may contribute to the rising incidence. Major depression is seen in 27% of patients with chronic orchialgia, 50% have a non-genital chronic pain syndrome and 56% of patients have a somatization disorder (12). As patient stressors increase, these psychological factors may contribute to the patient’s experience of chronic scrotal content pain.
An estimated 18.6% of men with chronic scrotal pain never receive a satisfactory explanation for their pain even after visiting an average of 4.5 urologists and undergoing an average of 4.7 to 7.2 procedures (13). The exact etiology of chronic scrotal pain is often not identified and the pathophysiology not fully understood (1,2,4,11,14). Twenty-five percent to 50% of cases are noted to be idiopathic. These factors create a frustrating situation for patients and physicians when determining the best treatment course. Most treatment algorithms rely on a thorough history and physical exam to identify specific causes of chronic orchialgia and guide therapeutic decisions. When the pain is determined idiopathic, treatment with empirical medical therapy and micro-denervation of the spermatic cord are universally suggested prior to consideration of extirpative surgery with orchiectomy (1,2,11,15,16).
Prior to considering orchiectomy, the patient and physician should feel comfortable understanding the risks and potential benefits of extirpative surgery. This edition of Therapeutic Advances in Urology thoroughly discusses these alternatives prior to orchiectomy. When the situation dictates or the patient and physician feel they are at the “last resort”, this review will help guide the decision prior to orchiectomy.
Effects of chronic orchialgia
As previously discussed, chronic orchialgia is a frustrating issue for patients and physicians. Orchialgia is associated with significant psychological burden. A precipitating event prior to development of pain has been suggested by several authors (9,13). This event may have been prior sexual abuse, trauma, or emotional disturbance. These psychological aspects may need treated for successful resolution of the pain, often involving a multidisciplinary team. Quallich and Arslanian-Engoren (13) found that few studies in the literature utilized psychologic or psychiatric evaluation in the treatment algorithm potentially limiting pain resolution success in cases of somatization. There is inadequate literature to document the impact of chronic orchialgia on quality of life, emotional suffering, avoidance behaviors, or lost productivity. In general, men with chronic orchialgia do exhibit characteristics of other chronic pain syndromes. These specific characteristics include pain as a chief complaint, history of unsuccessful treatments, and lack of correspondence between symptoms and objective medical findings (13). An Institute of Medicine report documenting the annual cost of a chronic pain patient compared with a patient without pain showed an increased cost of $4,500 for moderate pain and $7,700 for severe pain.
A 2011 study assessing the effect of orchialgia on male sexual function found a significant impact on International Index of Erectile Function (IIEF) scores (17). This study is unique in the inclusion of a control group of 50 men without orchialgia as a comparison. Significant impairments in orgasmic function, libido, overall sexual satisfaction and overall IIEF scores were found in the patients with orchialgia. No significant difference was found in erectile function between the two groups. Nearly 80% of patients with orchialgia were bothered by a decrease in frequency of sexual activity.
Risks of orchiectomy for chronic orchialgia
Risks associated with orchiectomy can be due to loss of testicular tissue or more specifically related to the loss of a testicle due to chronic pain. Immediate potential complications following unilateral orchiectomy include infection, acute surgical pain, bleeding, reactions to anesthesia, lowered serum testosterone, lowered fertility potential, loss of libido, loss of muscle mass, hot flashes, weight gain, erectile dysfunction, or even osteoporosis. It is recognized that many of these potential complications relate to the risk of hypogonadism. When bilateral orchiectomy is considered, a loss of testosterone and fertility is guaranteed.
Psychological alterations following orchiectomy should be discussed prior to proceeding with orchiectomy. Unfortunately, there is a lack of research regarding psychologic changes related to loss of a testicle for benign disease. Additionally, patients may be reluctant to consider a psychological component to their chronic pain prior to considering surgery (13). Following surgery, a loss of testosterone may lead to moodiness and increase risk of depression. Loss of libido may occur and alter sexual function. Patients may exhibit decreased satisfaction with the cosmetic appearance of their genitalia. If orchiectomy is considered early in the treatment algorithm, men may subsequently lament the loss of an organ to an unclear etiology and regret not attempting less invasive treatments.
Risks and complications of surgery should be discussed with patients and documented clearly prior to proceeding with orchiectomy. In addition to the above risks, it is important to inform the patient that pain may persist following orchiectomy. Several studies have shown residual pain following orchiectomy (1,9,18). One study identified 10 men who had persistent pain for more than six months following orchiectomy (18). Mean age of these men was 35 years and ranged from 25 to 78 years. Initial cause of testicular pain was inguinal herniorrhaphy in 40% and vasectomy in 30%. All men initially attempted pain management. Three men were suicidal and under psychiatric care when evaluated for further treatment options. All ten men underwent exploration of the external inguinal ring to identify a neuroma on the distal end of the genital branch of the genitofemoral nerve. The authors noted resection of a neuroma resulted in 80% of patients achieving “excellent” relief of pain. It should be noted that pain may migrate to the contralateral side following unilateral orchiectomy. Without the treating physician discussing the potential for residual pain or migrating pain following orchiectomy, the patient may inappropriately conclude that pain cannot continue because the presumed painful testicle is removed.
Outcomes of orchiectomy for chronic orchialgia
Recognizing that complete resolution of chronic orchialgia following orchiectomy is not universally achieved and there are significant risks, it is imperative the treating physician provide the patient with an accurate expectation for success with orchiectomy. Level one evidence based studies do not exist to provide this guidance. Identifying nociceptive and neuropathic properties of the pain experience may allow a more specific treatment, particularly in cases of inguinodynia. Documenting likelihood of success as discussed with the patient should be included in the patient’s medical record.
Davis et al. reviewed 31 surgically treated patients with chronic orchialgia (1). Following initial epididymectomy, only one of ten patients did not subsequently undergo orchiectomy. They noted that 55% of patients who underwent scrotal orchiectomy had complete pain relief and another 33% had partial pain relief. Following inguinal orchiectomy, 73% of patients reported complete pain relief and 27% reported partial pain relief. All patients following inguinal orchiectomy noted at least partial improvement in pain. The authors concluded that inguinal orchiectomy should be used when conservative measures were unsuccessful. It should be noted that micro-denervation of the spermatic cord was described but not as thoroughly studied at the time of this conclusion.
Yamamoto and colleagues identified four of 12 idiopathic chronic orchialgia patients who were non-responders to cord block and psychiatric consultation suggested an organic cause for pain (14). These patients underwent inguinal orchiectomy, with three of four noting complete pain relief. The other patient noted partial pain relief. Follow up of these patients was between 4 and 9 months.
Other studies have not shown the same success post-orchiectomy (2,9). Costabile and colleagues found 80% of patients continued to have pain following orchiectomy for idiopathic chronic orchialgia (Table 1). Treatment algorithms for chronic orchialgia reflect the variable success noted in these orchiectomy studies. Most algorithms suggest utilization of orchiectomy as the treatment of last resort (2,4,11,12,16) but several authors suggest orchiectomy is not indicated in idiopathic cases of chronic orchialgia (9,15). When considering orchiectomy, it is clear a higher ligation is most effective. This is possibly due to greater ability to address the neuropathic component related to ilioinguinal or genital branch of genitofemoral nerve dysfunction.
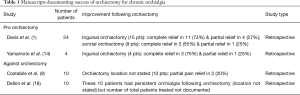
Full table
Limitations of studies assessing orchiectomy for chronic orchialgia
Studies reviewing utility of orchiectomy for chronic orchialgia have many limitations. Most studies are retrospective in nature and do not include a comparison group of men. The majority of studies have a relatively small number of patients. The severity of pain both pre-surgery and post-surgery are most commonly evaluated with a visual analog scale but may not account for psychological, emotional, and quality of life domains.
Other limitations are not study specific but related to the identification and documentation of the orchialgia. Specific definitions of success are not universally available. Distinguishing between neuropathic and nociceptive components can be difficult. Psychological factors related to somatization and coping are undervalued. Finally, comparisons between studies may be difficult due to lack of an agreed upon standard algorithm for conservative treatment.
Conclusions
Chronic orchialgia is a debilitating process for patients but the decision to proceed with orchiectomy for this condition should not be considered lightly. There are significant potential risks for the patient including the continuation or migration of the pain experience. When the determination to proceed with orchiectomy has been decided, the complications and potential for failure should be discussed with the patient and well documented in the medical record. Inguinal orchiectomy with high ligation appears to have the best results for resolution of scrotal pain possibly related to that ability to address the sensory component from ilioinguinal and genitofemoral nerves. Currently the evidence supports the use of orchiectomy as a procedure of last resort or if specific patient situations suggest this is in the best interest of the patient.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author is the Consultant for American Medical Systems & Coloplast Corp.
References
- Davis BE, Noble MJ, Weigel JW, et al. Analysis and management of chronic testicular pain. J Urol 1990;143:936-9. [PubMed]
- Levine L. Chronic orchialgia: evaluation and discussion of treatment options. Ther Adv Urol 2010;2:209-14. [Crossref] [PubMed]
- Holland JM, Feldman JL, Gilbert HC. Phantom orchalgia. J Urol 1994;152:2291-3. [PubMed]
- Kumar P, Mehta V, Nargund VH. Clinical management of chronic testicular pain. Urol Int 2010;84:125-31. [Crossref] [PubMed]
- Chen DC, Hiatt JR, Amid PK. Operative management of refractory neuropathic inguinodynia by a laparoscopic retroperitoneal approach. JAMA Surg 2013;148:962-7. [Crossref] [PubMed]
- Rönkä K, Vironen J, Kokki H, et al. Role of orchiectomy in severe testicular pain after inguinal hernia surgery: audit of the Finnish Patient Insurance Centre. Hernia 2015;19:53-9. [Crossref] [PubMed]
- Chen DC, Amid PK. Persistent orchialgia after inguinal hernia repair: diagnosis, neuroanatomy, and surgical management: Invited comment to: Role of orchiectomy in severe testicular pain and inguinal hernia surgery: audit of Finnish patient insurance centre. Rönka K, Vironen J, Kokki H, Liukkonen T, Paajanen H. DOI . Hernia 2015;19:61-3. [Crossref]
- Amid PK, Hiatt JR. New understanding of the causes and surgical treatment of postherniorrhaphy inguinodynia and orchalgia. J Am Coll Surg 2007;205:381-5. [Crossref] [PubMed]
- Costabile RA, Hahn M, McLeod DG. Chronic orchialgia in the pain prone patient: the clinical perspective. J Urol 1991;146:1571-4. [PubMed]
- Strebel RT, Leippold T, Luginbuehl T, et al. Chronic scrotal pain syndrome: management among urologists in Switzerland. Eur Urol 2005;47:812-6. [Crossref] [PubMed]
- Tojuola B, Layman J, Kartal I, et al. Chronic orchialgia: Review of treatments old and new. Indian J Urol 2016;32:21-6. [Crossref] [PubMed]
- Heidelbaugh JJ, Llanes M, Weadock WJ. An algorithm for the treatment of chronic testicular pain. J Fam Pract 2010;59:330-6. [PubMed]
- Quallich SA, Arslanian-Engoren C. Chronic testicular pain in adult men: an integrative literature review. Am J Mens Health 2013;7:402-13. [Crossref] [PubMed]
- Yamamoto M, Hibi H, Katsuno S, et al. Management of chronic orchialgia of unknown etiology. Int J Urol 1995;2:47-9. [Crossref] [PubMed]
- Kavoussi PK, Costabile RA. Orchialgia and the chronic pelvic pain syndrome. World J Urol 2013;31:773-8. [Crossref] [PubMed]
- Singh V, Sinha RJ. Idiopathic chronic orchialgia - a frustrating issue for the clinician and the patient. Indian J Surg 2008;70:107-10. [Crossref] [PubMed]
- Ciftci H, Savas M, Gulum M, et al. Evaluation of sexual function in men with orchialgia. Arch Sex Behav 2011;40:631-4. [Crossref] [PubMed]
- Dellon AL, Hashemi SS, Tollstrupt TH. Orchialgia after orchiectomy. Plast Reconstr Surg 2014;134:998e-9e. [Crossref] [PubMed]


