The impact and management of sexual dysfunction secondary to pharmacological therapy of benign prostatic hyperplasia
Introduction
Benign prostatic hyperplasia (BPH) is a clinical syndrome which includes prostate enlargement and lower urinary tract symptoms (LUTS). The aim of BPH treatment is alleviating urination problems, preventing prostatic disease progression, and improving the quality of life. Over the past two decades, the standard of treatment has shifted from surgery to drug therapy. A greater understanding of detrusor physiology as well as the development of effective drugs for solving the dynamic and static components of the obstruction in the prostate and bladder neck have paved the path for the ubiquity of pharmacological therapy for BPH. However, clinical studies in the 1990s reported that despite the amelioration of LUTS, adverse effects of BPH drug therapy began to surface (1). These effects were noted as sexual dysfunction, which can be divided into erectile dysfunction (ED), ejaculatory dysfunction (EjD), orgasmic disorders, as well as sexual desire disorders.
Pharmacological BPH therapy protocols advocate the use of α-blockers (ABs) and 5α-reductase inhibitors (5ARI), individually or in combination, as well as phosphodiesterase-5 (PDE5) inhibitors, individually or concomitantly with ABs. Adverse sexual side effects can occur as a result of the drug group itself or specific drugs within the group (2). Watchful Waiting is usually utilized in patients with an AUA symptom score <10. ABs bind to α1-adrenoceptors and act by relaxing the smooth muscles of the prostate and bladder neck to improve urine flow and facilitate urination. They do not reduce prostatic volume or curb the natural progression of BPH. Nonetheless, ABs do vary in uroselectivity and the production of adverse effects (3). They can be nonselective (e.g., doxazosin, terazosin, and alfuzosin) and selective (e.g., tamsulosin and silodosin). 5ARIs cause the inhibition of 5α-reductase enzymes and prevent the conversion of non-active forms testosterone to DHT, the androgen steroid compound mainly responsible for the initial and subsequent enlargement of the prostate gland (4). The enzyme has two isoforms: type 1 (found in the liver and skin) and 2 (found in reproductive tissues). 5ARIs are prominently available as finasteride and dutasteride, with both exhibiting similar clinical effects. 5ARIs may lead to ED, EjD, and decreased libido compared to placebo (5). The combination of AB and 5ARI is an increasingly popular mode of treatment which incorporates the combined effect of both components, including combined sexual side effects. Lastly, PDE5 inhibitors (e.g., tadalafil) are commonly used in order to treat ED as well as inhibit PDE11, which is found in the prostate and testes (6). As of yet, PDE11 inhibition with therapeutic doses of tadalafil has shown no clinical significance (7). Nonetheless, findings from studies have revealed that PDE5 is highly expressed in the lower urinary tract and supporting vasculature, and that PDE5 inhibition potentially decreases smooth muscle cell proliferation in the prostate, relaxes smooth muscle in the prostate, bladder neck, and supporting vasculature, increases blood perfusion to the lower urinary tract, and modulates bladder afferent nerve activity (8). For these reasons, in 2011, the FDA approved tadalafil to also treat the signs and symptoms of BPH as well as a combination of BPH and ED when the conditions coincide.
Management of sexual dysfunction in men treated with BPH/LUTS should be focused and symptom-dependent. A validated questionnaire of enquiry, such as the international index of erectile function or male sexual health questionnaire, should be used to assess sexual function before beginning pharmacological therapy for BPH/LUTS. The clinician then must assess co-morbidities and concomitant medications, particularly those that affect erectile capacity, as well as address any risk factors for cardiovascular disease before initiating any sort of therapy. Also before pharmacological therapy, the physician must advise the patient on lifestyle modifications to improve sexual function, such as weight loss and an appropriate exercise regimen. The physician may even consider PDE5 inhibitors if necessary, especially if the patient presents with true symptoms of ED. If the patient has EjD, he must be advised to transform his BPH/LUTS therapy to an alternative AB or 5ARI. Once the relevant sexual dysfunction is tended to, the physician must provide the apposite counseling on the safety and tolerability of pharmacological therapies for BPH/LUTS. Follow up can be done in 4–8 weeks to check sexual function, after which 6-month checkups are adequate (9).
Adverse sexual side effects of select medications
ABs
A survey conducted in 2003 found that among 1,275 prescribers, including urologists and primary care physicians, AB monotherapy was the most common treatment regimen employed for BPH (10). ABs such as tamsulosin (Flomax) work on the dynamic component of BPH or the increased smooth muscle tone in the bladder neck and prostate responsible for obstructing urinary flow (11). This increase in smooth muscle tone in the bladder neck and prostate is mediated by sympathetic stimulation of the α1-adrenergic receptors in these tissues (11). Blockage of the α1-adrenergic receptors in the prostate and bladder neck causes smooth muscle relaxation and improves urinary flow (11). Three types of α1-adrenergic receptors are present throughout the body including α1A, α1B, and α1D (11). The majority (70%) of the α1-adrenergic receptors in the prostate are of the α1A subtype (11).
Tamsulosin
Tamsulosin is a third generation pharmacologically uroselective antagonist of α1A-adrenoceptors in the prostate with minimal affinity for the α1B-adrenoceptors of cardiovascular smooth muscle, thus not establishing any risk for hypotension (11,12). The degree of tamsulosin’s selectivity for the α1A receptor in comparison to the α1B receptor has been estimated to be 15.8:1 (13). Tamsulosin has been reported to affect libido, erectile function, and ejaculation. Priapism has rarely been reported since market approval and is expected to occur in less than 1 in 50,000 patients (11). In one study that analyzed the results of the male sexual function-4 (MSF-4) questionnaire found that of 354 men treated with tamsulosin, 40.7% considered their sexual function as deteriorated after 6 months of therapy (14).
Based on the package insert, the rates of adverse events reported for tamsulosin were derived from two 13-week trials in the United States that included a total of 1,487 men (11). A decrease in libido was reported by 1.0% of men receiving the 0.4 mg dose of tamsulosin and 2.0% of men receiving the 0.8 mg dose of tamsulosin (11). These percentages are in comparison to a 1.2% incidence of decreased libido occurring in patients receiving placebo (11). A meta-analysis of two randomized controlled trials and two open label trials including a total of 2,743 patients demonstrated that a decrease in sexual desire occurred in between 1% and 4.7% of patients (15). These incidences were in comparison to 0–0.3% of patients receiving placebo or serenoa repens (Permixon). Decrease in erectile function was observed in between 0.6% and 6.3% of 4,789 patients included in a meta-analysis of randomized controlled and open label trials (15). In comparison, between 0% and 1.6% of patients receiving placebo experienced decreases in erectile function (15).
The results of the MSF-4 questionnaire also revealed that ejaculation disorders were the most frequently reported side effect of tamsulosin therapy (14). Abnormal ejaculation, including ejaculation failure, ejaculation disorder, and retrograde ejaculation, was reported in approximately 8.4% of men receiving the 0.4 mg dosage of tamsulosin and 18.1% of men receiving the 0.8 mg dose of tamsulosin (11). These percentages are much higher than that of the placebo group, which was 0.2% (11). These frequencies suggest a dose-related effect of tamsulosin on ejaculatory function. In other controlled clinical trials, the proportion of patients reporting EjD ranged from 0% to 26% depending on the dose of tamsulosin used and the study duration (9,15). Two studies in conducted in healthy men have suggested that tamsulosin causes decreased ejaculate volume, sometimes to the point of anejaculation (16,17). A pooled analysis of several European phase II trials found abnormal ejaculation to be more frequent in patients younger than 65 years of age (6.3%) than in patients older than 65 years of age (2.6%) (18). However, the frequency of abnormal ejaculation in both of these groups did not reach statistical significance in comparison to the placebo group (18).
In a 2015 prospective study involving 156 BPH patients, EjD was found in 64% of patients prescribed tamsulosin, with 15% of patients void of ejaculate. The effect of the uroselective tamsulosin relaxes the tone of smooth muscles of the bladder neck, thus reducing the pressure proximally to the verumontanum, leading to retrograde ejaculation. Sometimes due to the reduced amount or complete absence of the ejaculate, the extent of sensory stimuli coming from posterior urethra responsible for orgasm is reduced (19).
Alfuzosin
Alfuzosin (Uroxatral) is a clinically uroselective α1-adrenergic receptor antagonist (20). However, alfuzosin’s selectivity for the α1A receptive subtype in comparison to the α1B receptive subtype has been estimated to be much lower than that of tamsulosin (0.31:1 versus 15.8:1, respectively) (13). Still, alfuzosin exhibits selective action in the urinary tract likely due to preferential distribution into the prostate gland versus the blood and limited ability to penetrate the blood brain barrier (21). As per the prescribing information, adverse event data was obtained from three placebo-controlled clinical trials that were conducted over a three-month time period and involved 1,608 men, 473 of whom received one alfuzosin 10 mg extended-release tablet daily while the remaining 1,135 received 15 mg daily (20). Impotence was reported to occur in between 0% and 3% of patients receiving alfuzosin and not significantly more frequently than with placebo (between 0% and 1%), based on clinical trials results (22). Decrease in erectile function based on a meta-analysis of randomized controlled and open label trials has been reported to be between 0.0% and 2.8% in patients receiving alfuzosin (15). In comparison, the patients receiving a placebo in these trials reported decreases in erectile function at rates ranging from 0–2.3% (15). A meta-analysis of randomized controlled trials including a total of 1,302 patients demonstrated incidences of decreased sexual desire in 0–0.7% of patients receiving alfuzosin and 0.7% of patients receiving the placebo (15). Priapism was reported after market approval so incidence was not reported for this adverse effect (20). The reported incidence of ejaculatory disorders with the use of alfuzosin is generally less than 1.5% based on information from clinical trials (9,15). This percentage is in comparison to 0% of patients in the placebo groups reporting such adverse effects (15). No dose-effect relationship was observed with alfuzosin and the incidence of sexual side effects (23).
Interestingly, data from a preliminary open-label study of 3,076 men with LUTS or BPH have shown that the use of alfuzosin 10 mg daily for one year has resulted in improvements from baseline in ED and ejaculatory disorders (P<0.001 for both conditions) (24). The mean improvements from baseline in these two conditions were greater for men with more severe LUTS and BPH at baseline than men with milder symptoms (24). Further, alfuzosin is well tolerated when used in combination with low doses of PDE5 inhibitors for ED (22). A six-month open label study conducted in 42 men demonstrated that the side effect profile of alfuzosin in combination with tadalafil was similar to that with each agent alone (25).
The effective mechanism through which the alpha1A selective antagonists cause these symptoms of sexual dysfunction is unknown (9). However, several theories have been proposed. The first theory is that these selective alpha1A antagonists may affect the emission phase of ejaculation by blocking alpha1A selective antagonists in the organs involved in this process – the seminal vesicles and the vas deferens (9). The large ratio of tamsulosin selectivity for the α1A receptor subtype over the α1B receptor subtype may explain its increased association with abnormal ejaculation in comparison to other α1-adrenergic receptor antagonists. A study in rats demonstrated that 3 and 10 µg/kg doses of tamsulosin significantly decreased the amplitude and area under the curve of seminal vesicle pressure in comparison to placebo, while 3 and 10 µg/kg doses of alfuzosin did not (26). As the seminal vesicles are a major contributor to the volume of semen, this effect may an example of some of the EjD observed with the use of tamsulosin (27). Nevertheless, the effect observed in rats may not necessarily carry over to human subjects.
The second theory is that these ABs affect the central nervous system by binding to serotonin and/or dopamine receptors and blocking signals controlling ejaculation. Unlike alfuzosin, tamsulosin crosses the blood brain barrier and may consequently cause sexual dysfunction through blockage of α1-adrenergic receptors in the central nervous system (26). Thus, patients experience retrograde ejaculation or decreased ejaculate volume. These effects are reversible with drug discontinuation (28). Effects of alpha adrenergic antagonists on blood pressure may also cause ED (15). Tamsulosin has also shown a strong affinity for the 5-HT1A and D2 receptors which are both involved in the central control of ejaculation (27). This mechanism would again explain the highly reported frequency of abnormal ejaculation with tamsulosin in comparison with other BPH medications.
It has been assumed by many researchers and healthcare providers that the EjD caused by ABs was due to retrograde ejaculation. The previously mentioned study in rats also demonstrated that tamsulosin had a significantly greater inhibitory effect on bladder neck closure and seminal vesicle contractions than alfuzosin (26). Thus, because alpha adrenergic antagonists relax the bladder neck, they may allow semen to flow into the bladder during ejaculation. However, a few studies have found evidence to contradict the idea that tamsulosin causes retrograde ejaculation. A study of 17 Korean urologists demonstrated that treatment with tamsulosin 0.2 and 0.4 mg once daily significantly decreased mean ejaculate volume (17). However, no sperm was found in mid-stream urine collected after ejaculation (17). Another study included 57 healthy volunteers treated for five days with either placebo, tamsulosin 0.8 mg once daily, or alfuzosin XL 10 mg once daily; although mean ejaculate volume decreased in 90% of patients who received tamsulosin, sperm was rarely detected in post-ejaculate urine (16). Tamsulosin also caused anejaculation in 35% of participants while anejaculation was not observed in any of the participants in the alfuzosin or placebo group.
Still, a retrospective analysis of 7,974 men with BPH found that men taking tamsulosin to treat LUTS had better scores on the Sexual Health Inventory for Men than those taking other ABs or 5ARIs, especially in those with more severe LUTS (29). These researchers postulated how direct effects of tamsulosin and alfuzosin may be responsible for improvements in sexual functioning. These ideas were based on data from animals and in vitro trials, so their relevance in humans is not definitive. In precontracted rat corpora cavernosum, alfuzosin was found to fully relax cavernosal tissue in vitro (29). Further, tamsulosin was second only to prostaglandin E1 in its enhancing effect on small muscle relaxation of the corpus cavernosum of dogs, rabbits, and humans in vitro when it was compared to other substances such as phentolamine (29). Thus, the effects of tamsulosin and other ABs on sexual health might not always be negative.
Silodosin
Silodosin (Rapaflo) is an α1-adrenergic receptor antagonist used for treating BPH. It is selective for adrenoceptors in the prostate and bladder. By causing a blockage of these receptors, there is a relaxation of smooth muscle in the bladder neck and prostate, which results in an improvement of urine flow and decreased symptoms of BPH (30). It is dosed once daily with meals, and has a dose reduction for renal impairment (CrCl <50 mL/min). In a 12-week, multicenter, double-blinded, placebo-controlled trial that included 897 patients, and the most common adverse drug reaction due to silodosin was retrograde ejaculation at 28.1%. Although there is a high incidence of retrograde ejaculation due to silodosin, there is no incidence of it causing a decrease in libido. Men using silodosin may experience a “dry orgasm” during sexual activity, due to the semen entering the bladder instead of emerging through the penis during ejaculation. Patients may become nervous if unwarned of this side effect by the physician. However, the orgasm usually stays normal. Post-orgasm urine is cloudy due to mixing of urine with ejaculate.
The major cause of this ejaculatory disorder is considered to be due to the contraction of the seminal vesicle and ejaculatory duct at the time of ejaculation (31). Of the alpha receptor subtypes (α1A, α1B, and α1D) (Table 1) that are present in the seminal vesicle and prostate, α1A is considerably more prominent, accounting for 75% of the receptors. It has also been determined that α1A subtype is also responsible for the contraction of the ejaculatory duct (39). In one study conducted in Japan, the ejaculatory process was observed using Doppler technology in males with and without oral silodosin. In the three males that were receiving silodosin, Doppler studies showed that the bladder neck never completely closed during ejaculation, causing the seminal fluid to flow into the bladder, instead of exiting through the urethra. In only one of these three cases did the patient have a small amount of semen exit through the urethral orifice as well as into the bladder. There was no documentation of any seminal fluid traveling into the bladder in those males not using silodosin (40).
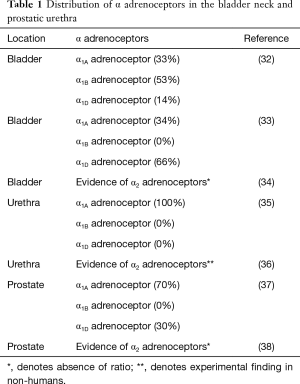
Full table
5α-reductase inhibitors
Dutasteride
Dutasteride (Avodart) is a competitive 5ARI that antagonizes both isoforms of 5α-reductase (41). Dutasteride is indicated alone or in combination with the α1A-adrenergic receptor antagonist tamsulosin for the treatment of men with an enlarged prostate with symptomatic BPH, for symptom improvement, reduction in risk of acute urinary retention (AUR), and reduction in risk for BPH-related surgery (e.g., transurethral resection of the prostate and prostatectomy). The oral formulation of dutasteride is a 0.5 mg capsule administered as a once daily dose, without regard to meals, with or without tamsulosin. Dutasteride is contraindicated in hypersensitivity, pregnancy, women of childbearing potential and in pediatrics, and should not be handled by most of these populations since dutasteride is absorbed through the skin and could results in fetal exposure.
Based on the package insert, common adverse reactions from clinical trials [ARIA 3001, ARIA 3002 (both United States), and ARIA 3003 (19 countries)] in 4,325 men, 2,167 receiving dutasteride, were impotence, decrease in libido, breast enlargement/tenderness, and EjD (41,42). In the combination trial with tamsulosin (CombAT trial), these adverse events occurred more frequently in the combination therapy group with than either monotherapy alone (41). Although ED was the number one cause of treatment discontinuation in all study arms, discontinuation rates were low at only 1–1.5%.
Finasteride
Finasteride (Proscar) is the another prominent 5ARI, although finasteride selectively inhibits 5α-reductase type 2, found predominantly in the prostate gland (43,44). Finasteride is indicated alone for the treatment of symptomatic BPH for symptom improvement, reduction in risk of AUR, and reduction in need for surgery, as well as in combination with the α1-selective antagonist doxazosin (Cardura), together indicated to slow the progression of symptomatic BPH. Finasteride is available in 5 mg tablets taken once daily without regard to meals with or without doxazosin. Like dutasteride, finasteride also has the following contraindications: hypersensitivity, pregnancy, women of childbearing age, and pediatrics (43).
In clinical trials, the adverse effects reported were mainly related to sexual function and included impotence, decreased libido, decrease ejaculate volume, EjD, and breast enlargement/tenderness. However, findings of studies may vary based on setup and criteria. A 2015 study found reduced ejaculation strength and orgasmic function but no significant decrease in sexual desire, which is usually a notorious side effect of 5ARIs (19). A 5-year prospective analysis by Kaplan et al. found that the incidence of ED, EjD, and reduced libido was higher with dutasteride (5.1%, 2.4%, 2.7%) compared to finasteride use (2.1%, 1.8%, 1.4%) despite their equality in effectively treating LUTS (45).
Combined therapy of ABs and 5α-reductase inhibitors
In a 2015 study, complete absence of ejaculation was experienced by 23% of patients on combined therapy, but only 15% on tamsulosin and 5% on finasteride (19). In the same study, it was found that erection improved in all three treatment groups. Patients with severe urinary symptoms often identified relief in the act of urination with improved erectile function.
Phosphodiesterase-5 inhibitors
Tadalafil
A study conducted by Hellstrom et al. states that tadalafil (Cialis) does not induce any detrimental effects on spermatogenesis or testicular function (46). Tadalafil is commonly prescribed for men with ED along with LUTS secondary to BPH (47). McVary and McKenna report a multicenter, randomized, double-blind placebo-controlled study involving 281 men reported to have LUTS secondary to BPH were randomly assigned to once daily 5 mg for 6 weeks, proceeded by an increase in dosage to 20 mg for 6 weeks (12 weeks of placebo) (47). They state that there are decreases in International Prostate Symptom Score (IPSS) in regards to a mean change from baseline to 6 weeks of −2.8 with tadalafil 5 mg compared to −1.2 with the placebo (P<0.003), and −3.8 at 12 weeks with tadalafil 5/20 mg compared to −1.7 with the placebo (P<0.001) (47). A similar study by Roehrborn et al. was conducted using a larger sample size of men with LUTS secondary to BPH and 2.5, 5, 10, and 20 mg of tadalafil (48). An improvement of baseline to endpoint after 12 weeks, IPSS mean change was reported to be −3.9 of 2.5 mg of tadalafil (P<0.015), −4.9 for 5 mg of tadalafil (P<0.001), −5.2 for 10 mg of tadalafil, and −5.2 for 20 mg of tadalafil (P<0.001), compared to −2.3 for the placebo (48). Another study showed a small but statistically significant median maximum urinary flow rate improvement for tadalafil versus placebo (49). The dosage recommended for individuals experiencing LUTS secondary to BPH is 5 mg of tadalafil (48). In fact, a 2015 clinical study observed improvement in approximately two-thirds of their patients, with over 50% reporting after 1 week of therapy and more than 70% after 4 weeks (50). No unexpected adverse events have been reported; no meaningful adverse effects have been observed in visual, auditory, or cardiovascular systems. Tadalafil is also effective in men of different ages, disease severity, prior AB exposure, and prostatic volumes (51). The noted changes in IPSS may have been induced by an increased concentration of the cGMP, resulting in a decrease of prostate muscle tension (7). The effects of nitric oxide (NO) on the smooth muscle of the bladder and the inhibition of PDE in the prostate and the prostatic urethra is documented but not well studied (52). Though the current literature lacks an explicit description of the effect of tadalafil on the prostate, bladder, penis, and LUTS (52), proposed mechanisms for how tadalafil may ameliorate BPH-associated LUTS include: upregulating NO/cyclic guanosine monophosphate activity (for decreasing smooth muscle tension in the prostatic stroma and capsule and attenuating cellular proliferation associated with prostate/bladder hypertrophy), downregulating Rho-kinase and endothelin-1 activity (for increasing smooth muscle relaxation to decrease bladder outlet obstruction and restore erection), modulating autonomic hyperactivity and afferent nerve activity, reducing inflammation, as well as increasing pelvic perfusion and reducing ischemia (to reverse pelvic organ atherosclerosis) (9,53).
Administering tadalafil concomitantly with ABs have been reported to increase hypotension or orthostatic hypotension (54). The PDE5-inhibiting mechanism of tadalafil is similar to that of ABs in regards to peripheral vasodilation. In a study by Kloner et al., the additive effects of tadalafil on two commonly prescribed ABs, doxazosin and tamsulosin, were compared (55). The hypotensive effects of doxazosin were increased by 10 mmHg, while there was no significant change when tamsulosin was taken with tadalafil (55). This hypotensive effect may diminish the ability to produce or maintain an erection. Kloner et al. determined that tamsulosin is a safe AB when combined with tadalafil (55). When concurrently administrating other ABs with tadalafil, a great deal of precaution must be taken. An alternative management approach is combining tadalafil with finasteride; a 2015 study found the combination therapy had clinically meaningful improvement in symptoms, great treatment satisfaction, and no report of adverse side effects (56). This combination therapy is well-tolerated, regardless of the presence/absence of ED at treatment initiation (57).
Due to the fact that sexual dysfunction occurs around the same age as BPH symptoms in most men, it is difficult to definitively determine the degree to which different medications used in the treatment of BPH contribute to some symptoms of sexual dysfunction (14). Further, it appears to be difficult to predict which patients will experience sexual dysfunction as a result of the use of medications for BPH (14). Studies that attempted to identify correlation between patient characteristics such as age and prostatic volume have failed to find any associations (14). Direct comparative trials of the various agents used for the treatment of BPH would also be helpful in order to provide more definitive conclusions can be made regarding which agents pose the lowest risk of causing sexual side effects, particularly impotence and decreased libido. Incidence rates of adverse sexual side effects have found to be higher at year 1 of follow-up compared to thereafter for finasteride and the finasteride-doxazosin combination (58), suggesting that the effects early in the course of treatment may later subside. This would be an important precaution for the physician to address to the patient. Also noteworthy is that adverse side effects may be linked to comorbidities typically tied to BPH patients (58). Adverse sexual side effects of select ABs and 5ARIs have been summarized in Table 2.

Full table
Conclusions
Before the pharmacological therapy for BPH is initiated, it is crucial that the physician discusses potential side effects with the patient so they can comprehend the risks. In some cases, patients may prefer to continue treatment with ABs or 5ARIs regardless of experiencing the sexual side effects. Other options available would be either to decrease the dosage of medication, decrease the frequency, or terminate the treatment completely and instead opt for surgery. Based on the patient’s presentation of LUTS and possible sexual dysfunction, the physician may evaluate an alternative class of BPH therapy drugs. The effect of AB on libido and erectile function is similar to that of a placebo, while having different effects on ejaculation. 5ARIs produce sexual side effects and increased risk for ED, EjD, and decreased libido compared to a placebo. Combination therapy with AB and 5ARI triples the risk for EjD incidence compared to that of AB or 5ARI used individually. The phosphodiesterase-5 inhibitor tadalafil is presently a new treatment alternative to other established drugs for LUTS, such as the aforementioned ABs or 5ARIs. However, it is not just an alternative, since sexual adverse events associated with ABs and 5ARIs are avoided; tadalafil is the only drug that can treat both ED and LUTS simultaneously. Nevertheless, objective improvement on LUTS has been met with controversy. Further studies are imperative in gauging the long-term the role of combined therapy of phosphodiesterase-5 inhibitors and ABs or 5-ARIs in the management LUTS/BPH (59).
Acknowledgements
The authors are thankful to Drs. Kelly Warren, Todd Miller, and Peter Brink for departmental support, as well as Mrs. Wendy Isser and Ms. Grace Garey for literature retrieval.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Seftel AD, de la Rosette J, Birt J, et al. Coexisting lower urinary tract symptoms and erectile dysfunction: a systematic review of epidemiological data. Int J Clin Pract 2013;67:32-45. [Crossref] [PubMed]
- Giuliano F, Droupy S. Sexual side effects of pharmacological treatments. Prog Urol 2013;23:804-10. [Crossref] [PubMed]
- Yokoyama T, Hara R, Fukumoto K, et al. Effects of three types of alpha-1 adrenoceptor blocker on lower urinary tract symptoms and sexual function in males with benign prostatic hyperplasia. Int J Urol 2011;18:225-30. [Crossref] [PubMed]
- Hamilton RJ, Andriole GL, Freedland SJ. 5α-reductase inhibitors: preventing the treatable. Eur Urol 2012;62:242-4; discussion 244-5. [Crossref] [PubMed]
- Nickel JC, Gilling P, Tammela TL, et al. Comparison of dutasteride and finasteride for treating benign prostatic hyperplasia: the Enlarged Prostate International Comparator Study (EPICS). BJU Int 2011;108:388-94. [Crossref] [PubMed]
- Briganti A, Salonia A, Gallina A, et al. Drug Insight: oral phosphodiesterase type 5 inhibitors for erectile dysfunction. Nat Clin Pract Urol 2005;2:239-47. [Crossref] [PubMed]
- Coward RM, Carson CC. Tadalafil in the treatment of erectile dysfunction. Ther Clin Risk Manag 2008;4:1315-30. [Crossref] [PubMed]
- Yokoyama O, Igawa Y, Takeda M, et al. Tadalafil for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a review of clinical data in Asian men and an update on the mechanism of action. Ther Adv Urol 2015;7:249-64. [Crossref] [PubMed]
- Mirone V, Sessa A, Giuliano F, et al. Current benign prostatic hyperplasia treatment: impact on sexual function and management of related sexual adverse events. Int J Clin Pract 2011;65:1005-13. [Crossref] [PubMed]
- Seftel A, Rosen R, Kuritzky L. Physician perceptions of sexual dysfunction related to benign prostatic hyperplasia (BPH) symptoms and sexual side effects related to BPH medications. Int J Impot Res 2007;19:386-92. [Crossref] [PubMed]
- Henkel R, Bastiaan HS, Schuller S, et al. Leucocytes and intrinsic ROS production may be factors compromising sperm chromatin condensation status. Andrologia 2010;42:69-75. [Crossref] [PubMed]
- Song SH, Son H, Kim KT, et al. Effect of tamsulosin on ejaculatory function in BPH/LUTS. Asian J Androl 2011;13:846-50. [Crossref] [PubMed]
- Martin DJ, Lluel P, Guillot E, et al. Comparative alpha-1 adrenoceptor subtype selectivity and functional uroselectivity of alpha-1 adrenoceptor antagonists. J Pharmacol Exp Ther 1997;282:228-35. [PubMed]
- Zlotta AR, Teillac P, Raynaud JP, et al. Evaluation of male sexual function in patients with Lower Urinary Tract Symptoms (LUTS) associated with Benign Prostatic Hyperplasia (BPH) treated with a phytotherapeutic agent (Permixon), Tamsulosin or Finasteride. Eur Urol 2005;48:269-76. [Crossref] [PubMed]
- van Dijk MM, de la Rosette JJ, Michel MC. Effects of alpha(1)-adrenoceptor antagonists on male sexual function. Drugs 2006;66:287-301. [Crossref] [PubMed]
- Hellstrom WJ, Sikka SC. Effects of acute treatment with tamsulosin versus alfuzosin on ejaculatory function in normal volunteers. J Urol 2006;176:1529-33. [Crossref] [PubMed]
- Hisasue S, Furuya R, Itoh N, et al. Ejaculatory disorder caused by alpha-1 adrenoceptor antagonists is not retrograde ejaculation but a loss of seminal emission. Int J Urol 2006;13:1311-6. [Crossref] [PubMed]
- Chapple CR, Baert L, Thind P, et al. Tamsulosin 0.4 mg once daily: tolerability in older and younger patients with lower urinary tract symptoms suggestive of benign prostatic obstruction (symptomatic BPH). The European Tamsulosin Study Group. Eur Urol 1997;32:462-70. [PubMed]
- Stojanović N, Ignjatović I, Djenić N, et al. Adverse Effects of Pharmacological Therapy of Benign Prostatic Hyperplasia on Sexual Function in Men. Srp Arh Celok Lek 2015;143:284-9. [Crossref] [PubMed]
- Uroxatral package insert [online]. Available online: (Accessed 2013 Nov 18).http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021287s013lbl.pdf
- Martin DJ. Preclinical pharmacology of alpha1-adrenoceptor antagonists. Eur Urol 1999;36 Suppl 1:35-41; discussion 65. [Crossref] [PubMed]
- Roehrborn CG, Rosen RC. Medical therapy options for aging men with benign prostatic hyperplasia: focus on alfuzosin 10 mg once daily. Clin Interv Aging 2008;3:511-24. [Crossref] [PubMed]
- Rosen RC, Giuliano F, Carson CC. Sexual dysfunction and lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH). Eur Urol 2005;47:824-37. [Crossref] [PubMed]
- van Moorselaar RJ HR, Emberton M, Harving N, et al. Alfuzosin 10 mg once daily improves sexual function in men with lower urinary tract symptoms and concomitant sexual dysfunction. BJU Int 2005;95:603-8. [Crossref] [PubMed]
- Yassin A, Diede HE. Combination therapy: alpha1-adrenceptor blockade and tadalafil in BPH population. Int J Impot Res 2003;15:S4-S5. [Crossref]
- Giuliano F, Bernabe J, Droupy S, et al. A comparison of the effects of tamsulosin and alfuzosin on neurally evoked increases in bladder neck and seminal vesicle pressure in rats. BJU Int 2004;93:605-8. [Crossref] [PubMed]
- Giuliano F. Impact of medical treatments for benign prostatic hyperplasia on sexual function. BJU Int 2006;97 Suppl 2:34-8; discussion 44-5. [Crossref] [PubMed]
- Lee M. Alfuzosin hydrochloride for the treatment of benign prostatic hyperplasia. Am J Health Syst Pharm 2003;60:1426-39. [PubMed]
- Barqawi AB, Myers JB, O'Donnell C, et al. The effect of alpha-blocker and 5alpha-reductase inhibitor intake on sexual health in men with lower urinary tract symptoms. BJU Int 2007;100:853-7. [Crossref] [PubMed]
- Kapoor A. Benign prostatic hyperplasia (BPH) management in the primary care setting. Can J Urol 2012;19:10-7. [PubMed]
- Sakata K, Morita T. Investigation of ejaculatory disorder by silodosin in the treatment of prostatic hyperplasia. BMC Urology 2012;12:29. [Crossref] [PubMed]
- Giuliano F. Neurophysiology of erection and ejaculation. J Sex Med 2011;8 Suppl 4:310-5. [Crossref] [PubMed]
- Malloy BJ, Price DT, Price RR, et al. Alpha1-adrenergic receptor subtypes in human detrusor. J Urol 1998;160:937-43. [Crossref] [PubMed]
- Levin RM, Ruggieri MR, Wein AJ. Identification of receptor subtypes in the rabbit and human urinary bladder by selective radio-ligand binding. J Urol 1988;139:844-8. [PubMed]
- Nasu K, Moriyama N, Fukasawa R, et al. Quantification and distribution of alpha1-adrenoceptor subtype mRNAs in human proximal urethra. Br J Pharmacol 1998;123:1289-93. [Crossref] [PubMed]
- Kunisawa Y, Kawabe K, Niijima T, et al. A pharmacological study of alpha adrenergic receptor subtypes in smooth muscle of human urinary bladder base and prostatic urethra. J Urol 1985;134:396-8. [PubMed]
- Walden PD, Durkin MM, Lepor H, et al. Localization of mRNA and receptor binding sites for the alpha 1a-adrenoceptor subtype in the rat, monkey and human urinary bladder and prostate. J Urol 1997;157:1032-8. [Crossref] [PubMed]
- Perälä M, Hirvonen H, Kalimo H, et al. Differential expression of two alpha 2-adrenergic receptor subtype mRNAs in human tissues. Brain Res Mol Brain Res 1992;16:57-63. [Crossref] [PubMed]
- Moriyama N, Nasu K, Takeuchi T, et al. Quantification and distribution of α1-adrenoceptor subtype mRNAs in human vas deferens: comparison with those of epididymal and pelvic portions. Br J Pharmacol 1997;122:1009-14. [Crossref] [PubMed]
- Nagai A, Hara R, Yokoyama T, et al. Ejaculatory dysfunction caused by the new α1-blocker silodosin: A preliminary study to analyze human ejaculation using color Doppler ultrasonography. Int J Urol 2008;15:915-8. [Crossref] [PubMed]
- Solutions CP. Avodart(R) [Package Insert]. Somerset, NJ, 2012. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021319s028s029lbl.pdf
- Marihart S, Harik M, Djavan B. Dutasteride: a review of current data on a novel dual inhibitor of 5alpha reductase. Rev Urol 2005;7:203-10. [PubMed]
- Merk & Co. I. Proscar(R) [Package Insert]. Whitehouse Station, NJ, 2011. Available online: http://www.merck.com/product/usa/pi_circulars/p/proscar/proscar_pi.pdf
- Steers WD. 5alpha-reductase activity in the prostate. Urology 2001;58:17-24; discussion 24. [Crossref] [PubMed]
- Kaplan SA, Chung DE, Lee RK, et al. A 5-year retrospective analysis of 5alpha-reductase inhibitors in men with benign prostatic hyperplasia: finasteride has comparable urinary symptom efficacy and prostate volume reduction, but less sexual side effects and breast complications than dutasteride. Int J Clin Pract 2012;66:1052-5. [Crossref] [PubMed]
- Hellstrom WJ, Overstreet JW, Yu A, et al. Tadalafil has no detrimental effect on human spermatogenesis or reproductive hormones. J Urol 2003;170:887-91. [Crossref] [PubMed]
- McVary KT, McKenna KE. The relationship between erectile dysfunction and lower urinary tract symptoms: epidemiological, clinical, and basic science evidence. Curr Urol Rep 2004;5:251-7. [Crossref] [PubMed]
- Roehrborn CG, McVary KT, Elion-Mboussa A, et al. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol 2008;180:1228-34. [Crossref] [PubMed]
- Roehrborn CG, Chapple C, Oelke M, et al. Effects of tadalafil once daily on maximum urinary flow rate in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. J Urol 2014;191:1045-50. [Crossref] [PubMed]
- Oelke M, Shinghal R, Sontag A, et al. Time to onset of clinically meaningful improvement with tadalafil 5 mg once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: analysis of data pooled from 4 pivotal, double-blind, placebo controlled studies. J Urol 2015;193:1581-9. [Crossref] [PubMed]
- Nishizawa O, Yoshida M, Takeda M, et al. Tadalafil 5 mg once daily for the treatment of Asian men with lower urinary tract symptoms secondary to benign prostatic hyperplasia: analyses of data pooled from three randomized, double-blind, placebo-controlled studies. Int J Urol 2015;22:378-84. [Crossref] [PubMed]
- Uckert S, Kuthe A, Jonas U, et al. Characterization and functional relevance of cyclic nucleotide phosphodiesterase isoenzymes of the human prostate. J Urol 2001;166:2484-90. [Crossref] [PubMed]
- Hatzimouratidis K. A review of the use of tadalafil in the treatment of benign prostatic hyperplasia in men with and without erectile dysfunction. Ther Adv Urol 2014;6:135-47. [Crossref] [PubMed]
- Kloner RA, Mitchell M, Emmick JT. Cardiovascular effects of tadalafil in patients on common antihypertensive therapies. Am J Cardiol 2003;92:47M-57M. [Crossref] [PubMed]
- Kloner RA, Jackson G, Emmick JT, et al. Interaction between the phosphodiesterase 5 inhibitor, tadalafil and 2 alpha-blockers, doxazosin and tamsulosin in healthy normotensive men. J Urol 2004;172:1935-40. [Crossref] [PubMed]
- Roehrborn CG, Casabe A, Glina S, et al. Treatment satisfaction and clinically meaningful symptom improvement in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia: Secondary results from a 6-month, randomized, double-blind study comparing finasteride plus tadalafil with finasteride plus placebo. Int J Urol 2015;22:582-7. [Crossref] [PubMed]
- Glina S, Roehrborn CG, Esen A, et al. Sexual function in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia: results of a 6-month, randomized, double-blind, placebo-controlled study of tadalafil coadministered with finasteride. J Sex Med 2015;12:129-38. [Crossref] [PubMed]
- Kaplan SA, Lee JY, Meehan AG, et al. Time Course of Incident Adverse Experiences Associated with Doxazosin, Finasteride and Combination Therapy in Men with Benign Prostatic Hyperplasia: The MTOPS Trial. J Urol 2016;195:1825-9. [Crossref] [PubMed]
- La Torre A, Giupponi G, Duffy D, et al. Sexual Dysfunction Related to Drugs: a Critical Review. Part V: alpha-Blocker and 5-ARI Drugs. Pharmacopsychiatry 2016;49:3-13. [PubMed]


