Genital pain: algorithm for management
Introduction
Chronic testicular pain is a common long-term disability reported in North America and results in significant loss of work productivity and an increase in direct and indirect healthcare costs (1). Although a common complaint in Urology or Andrology, it is historically and currently a challenging disease to treat (2,3). With an increase in easily available information by the media, the last decade has seen an explosion in magazines focusing on men’s health and men’s problems. Additionally, there seems to be an increase in referral for the evaluation of testicular pain due to public awareness of testicular cancer (4). Many of these patients will see multiple physicians during the course of their evaluation, further increasing their frustration and potentially straining the physician/patient relationship (5).
Epidemiology
Chronic orchialgia (CO) is estimated to affect up to 100,000 men per year due to etiologies including 6% to 12% after vasectomy, up to 18% after inguinal hernia repair, up to 5% after scrotal surgery and up to 1% to 2% after abdominal or groin surgery (6). CO occurs at any age but the majority of the patients are in the 20–30 years of age group (7). It is the most cited urological reason for medical discharge in the US Army (8).
Etiology
CO is defined as intermittent or constant unilateral or bilateral testicular pain of more than 3 months which significantly interferes with the daily activities of the patient, prompting him to seek medical intervention (7).
Although extensive reports have been devoted to analyzing the characteristics and causes of other types of chronic pain syndromes, especially of chronic low back pain, there is little published in Urology about the etiology and treatment of CO (9). The majority of the published literature is cohort studies with limited number of patients, rarely placebo-controlled, and without a uniform standard evaluation (5).
Based on different etiologies, CO can be idiopathic or secondary with idiopathic accounting for approximately 50% of the cases (3). Holland et al. proposed that idiopathic orchialgia or ‘phantom orchialgia’ may be caused by noxious stimuli that activate an abnormal neuronal pathway causing referred pain in scrotum (10). Easily recognized and reversible causes include spermatocele, tumor, infection, varicocele, and torsion (5). Additional etiologies are testis (trauma, atrophy, scarring); epididymis (cysts, sperm granuloma); cord (vasectomy, hernia, nerve pain, iatrogenic, arteriovenous fistula); scrotal wall (hydrocele); urethral (benign prostatic hyperplasia, prostatitis, urethral stricture); urinary tract (stone, infection) (4). Nonurological causes of scrotal pain must also be entertained. These include psychosocial-stress, peritonitis, incarcerated hernia, ruptured abdominal aortic aneurysm, low back pain and referred scrotal pain (4,10).
Post-inguinal hernia repair orchialgia, one of the most common causes of CO can be due to various conditions, such as ischemic orchitis, epididymitis, edema, infection, cord fibrosis and scarring, varicocele or hydrocele formation, torsion, referred pain from radiculitis or ureteral pathology, and entrapment or disruption of the genital branch of the genitofemoral and ilioinguinal nerves, paranasal nerve fibers, or autonomic plexus within the cord (11).
Pathophysiology
During its descent in intrauterine life, the testis carries autonomic nerve supply and vasculature with it. The nerves travel along the testicular vessels to the aortic and renal plexuses. Both testis (T10–T12) and epididymis (T12–L1) are innervated from the presacral ganglia. The genital branch of genitofemoral and ilioinguinal nerve provide sensory innervation for the anterior scrotal wall. The posterior surface of the scrotum is innervated by scrotal branches of the superficial perineal nerve, via the perineal branch of the pudendal nerve (S1–S3) (4).
Through noxious stimuli, a neurogenic inflammation is elicited by activation of unmyelinated sensory neurons resulting in release of neuropeptides such as substance P and calcitonin gene related peptide (CGRP). High levels of IL-6 and TNF-α have clearly been demonstrated in patients with complex regional pan syndromes providing evidence that depression and chronic pain share common biological pathways in the serotonergic and nor-adrenergic systems (4,7).
Another potential cause of the hypersensitivity could be from injury to the nerves leading to Wallerian degeneration (WD) in these peripheral nerves. WD is characterized as an autodestructive change in the proximal and distal nerve axon that produces an environment clear of inhibitory debris, and supportive of axon regrowth and functional recovery. Also, an immune cell response initiated by neutrophils, and cytokines and macrophages are subsequently activated (6).
Parekattil et al. retrospectively reviewed a prospective database of spermatic cord biopsy specimens from 56 men with a total of 57 procedures. Tissue biopsies were obtained from mapped regions of the spermatic cord in all cases and the biopsies were stained with hematoxylin and eosin. They also used 3 human cadaveric spermatic cords to confirm localization of the nerve distribution identified on pathological mapping. They identified a median of 25 small diameter (less than 1 mm) nerve fibers in the spermatic cord. Of the 57 procedures, 48 (84%) showed WD in 1 or more of these nerves but only 2 of 10 controls (20%) had such degeneration (6).
More recently, Oka et al. collected spermatic cords from 11 men undergoing orchiectomy for localized testicular tumors and biopsied a third of the spermatic fascia from 36 men undergoing microsurgical varicocelectomy. They used pan-neuronal marker PGP 9.5 stain which revealed dense nerve distributions in the spermatic cord and fascia. Of the nerves 50% were identified near the vas deferens and 20% were identified in the spermatic fascia. Sensory and sympathetic nerve fibers represented most of the nerves but a few parasympathetic nerve fibers were observed (12).
Nervous system plasticity which leads to CO is an entity thought to result in chronically up-regulated central and peripheral neuropathic pathways. The neurons in the peripheral and central nervous system gain the ability to change structure, gene expression, chemical and receptor profile, and/or function leading to chronic painful stimuli (8).
Diagnosis
The complaint is usually of a squeezing, deep ache in the testis ‘ like the day after you got kicked there’, often bilateral or alternating from one side to the other, intermittent and most commonly associated with low back pain. Sometimes the patient reports that if feels as if the testicle is pinched in the crotch of the underwear but trouser readjustment does not help (9).
The differences between nociceptive and neuropathic orchialgia need to be understood because their treatment differs significantly. If the pain is neuropathic in nature, it is characterized by symptoms of burning, hyper- or hypoesthesia, and radiation of the pain to the skin of the corresponding scrotum however orchialgia elicited by compression of the testis is typically a nociceptive sensation that is dull and aching. It may be accompanied by anatomical changes, such as testicular enlargement or atrophy (11).
Clinical assessment
Scrotal pain must be carefully evaluated with a full patient history and physical examination. The patient’s age, sexual history, and duration, severity, and onset (gradual vs. sudden) of pain are necessary to focus the clinician’s attention on the correct diagnostic path (10).
Laboratory tests, including urinalysis and urine and semen cultures, should be obtained when indicated (i.e., suspicion of malignancy or infection) (10). A study by Cui et al. revealed that deficiencies in Vitamin B12 and testosterone are common in chronic pain syndromes and may play a role in chronic testicular pain. The prevalence of testosterone and B12 deficiencies in their 125 patients was much higher than that reported in the general population. About 80% of patients with corrected chemical deficiencies with sufficient follow-up reported some improvement in pain (13).
From the outset it is important to be honest with the patient and make him understand that there is no ‘magic cure’ thereby gaining the patient’s trust. He is also made aware that further consultations, investigations and interventions may be necessary (4).
Treatment
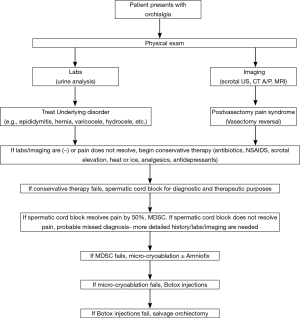
The desired goal of treatment is to allow the sufferer to return to routine activity without significant use of analgesics (7). An algorithm of management is presented in Figure 1.
Conservative therapy
Modifying predisposing exertional and postural habits, and the use of scrotal suspension, have been recommended. Some advise a minimum 1-month treatment with NSAIDS, with the possible addition of oral antibiotics. While the clinical relationship between depression and chronic pain is unclear, many patients will improve on a trial of low-dose antidepressants (9).
Surgical options
Surgical treatment may ultimately be necessary if medical management proves unsuccessful (9). There is no Level 1 evidence to support the use of a specific procedure for the treatment for idiopathic CO (8).
Theoretically, if the pain is truly testicular and not referred, spermatic cord block or division of the scrotal and spermatic branches of the genitofemoral and ilioinguinal nerves should relieve the pain (9).
Patients who have undergone vasectomy should consider vasectomy reversal if pain appears congestive in nature with the fullness of epididymis and association of pain with sexual intercourse. Diagnosis of a varicocele should lead to a varicocelectomy in patients with CO (2).
Microsurgical denervation of spermatic cord
Patients who have failed other surgical options could be offered microsurgical spermatic cord denervation (MSCD). It has been described since the late 1970s (1). Although denervation of the spermatic cord risks possible hydrocele formation, testicular atrophy and increased pain, these risks do not outweigh the consequences of orchiectomy. Furthermore, the psychological repercussions of genital injury leading to castration anxiety have been well documented and certainly may contribute to heightened attention to perceived pain in this area (14).
Procedure
The goal of the operation is to divide all structures that may carry neural fibers but to preserve the arteries (testicular, cremasteric, deferential), several lymphatics to reduce the likelihood of hydrocele, and the vas deferens to prevent obstruction and preserve fertility (15). The rationale of this approach is that by interrupting the neural pathways to and from the scrotal contents, this decreases afferent nerve stimulation and downregulates pain centres (1).
The key selection measure for this procedure is a positive response to a spermatic cord block. Levine et al. demonstrated at their center a strong correlation between a positive response to a spermatic cord block, defined as >50% temporary reduction of pain, and permanent relief of pain following microsurgical denervation of the spermatic cord (MDSC) (15). Benson et al. revealed that men with CO who have a positive response to a spermatic cord block are likely to have durable and complete resolution of symptoms after undergoing MDSC (16). Success rates may vary based on the individual surgeon, and informed consent is critical, as the pain may persist but rarely worsens (15).
Outcomes
Marconi et al. prospectively evaluated 50 patients with chronic scrotal content pain (CSP) who had a positive response to a spermatic cord block test with local anesthesia. They assessed pain severity using an analog visual pain scale. They observed no intraoperative complications or testicular units loss but performed two reoperations for hematocele and hydrocele. Their study revealed that six months after surgery, 40 patients (80%) were pain-free, 6 (12%) had persistent pain and 4 (8%) had no change in pain (17).
A retrospective review of 772 patients who underwent MDSC between 2008 and 2016 was done by Parekattil et al. with the primary outcome measure being level of pain. Pain was assessed preoperatively and postoperatively using two assessment tools: (I) the subjective visual analog scale (VAS) and (II) an objective standardized externally validated pain assessment tool (PIQ-6, QualityMetric Inc., Lincoln, RI). The review revealed an 84% significant reduction in pain (complete resolution in 425 patients while 291 patients reported a greater than 50% reduction in pain). Using the objective PIQ-6 outcomes, a significant reduction in pain was seen in 67% of patients at 6 months and 68% at 1 year post-op (18).
Last resort surgical options
Epididymectomy and orchiectomy are last resort surgical options with mixed results. One study showed greater outcomes (92% patient satisfaction) in those patients with pain from epididymal cysts rather than patients with epididymitis or epididymalgia (43% patient satisfaction). Another long-term study showed a 90% patient satisfaction from epididymectomy in patients with postvasectomy epididymal pain or obstructive pain from previous hernia repair (2). Orchiectomy has been shown to significantly decrease pain in 40–75% if patients (15).
Minimally invasive interventions
Ultrasound guided targeted microcryoablation (UTM)
Studies have shown that nerves are sensitive to freezing injury and could be desensitized when exposed to −15 to −20 °C.
Procedure
UTM is usually performed under general anesthesia. The patient is placed in a supine position with the inguinal and genital region prepared for the procedure. A spermatic cord block is performed. An ultrasound probe is used to identify the spermatic cord at the external inguinal ring. An EndocareTM 1.7 mm round ice Cryoprobe is percutaneously placed into the peri-spermatic tissue on the medial and lateral aspect of the spermatic cord under ultrasound guidance at the external inguinal ring. The tissue is cooled to −40 °C for 90 s. The cryoprobe is passively thawed and removed (2).
Outcomes
A cohort of 221 patients underwent UTM from November 2012 to July 2016. Almost all patients had failed prior MDSC. About 75% of patients experience a significant reduction in pain (5% complete resolution, 70% reported a >50% reduction in pain) at a median follow-up of 5 months. At 6-month and 1-year follow-up, objective PIQ-6 was completed with the following outcomes: significant reduction in pain in 53% of patients at 6 months and 51% at 1 year postoperative. The only complications were two wound infections and four patients who developed penile pain (19).
Botox injection
Botulinum-A toxin has been shown to modulate the release of neuropeptides (substance P and calcitonin gene-related peptide) leading to inhibition of neurogenic inflammation and chronic pain. Neurogenic inflammation leading to WD of the neural fibers in the spermatic cord has been discussed as a possible pathologic cause of pain in patients with CO. The inhibitory effect of Botox on neurogenic inflammation provides could provide an antinociceptive effect. Botox has been used in orthopedics to treat intra-articular pain and in neurology for migraine headaches.
Procedure
Totally 100 units of Botox were diluted in 10 cc of saline. The mixture is injected medial and lateral to the spermatic cord at the external inguinal ring. This is performed with the patient under conscious sedation (2).
Outcomes
A cohort of 44 patients underwent Botox injection. Based on visual analog scale, 62.5% of patients experienced a significant reduction in pain (7.5% complete resolution, 55% reported a >50% reduction in pain) with a median follow-up of 7 months. Analysis with the more objective PIQ-6 questionnaire revealed a significant reduction in pain in 27% of patients at 6 months and 40% at 1 year postoperative. There were no complications in this cohort (20).
AmnioFix injection
Injectable dehydrated amniotic/chorionic membrane allograft (AmnioFix®) is substance derived from human amniotic membrane. AmnioFix has been shown to reduce scar tissue formation, reduce inflammation, and enhance healing. Patients with orchialgia have been shown to have neurogenic inflammation around the spermatic cord. Because of its anti-inflammatory properties, it may offer another noninvasive treatment option for difficult orchialgia patients.
Procedure
AmnioFix injectable form is injected medial and lateral to the spermatic cord at the level of the external inguinal ring (2).
Outcomes
A cohort of 15 patients underwent treatment with AmnioFix injection over a mean follow-up period of 10 months. All patients had undergone conservative treatments, spermatic cord blocks, and microsurgical neurolysis prior to attempt with AmnioFix. AmnioFix was injected medial and lateral to cord at the external inguinal ring. The injection was done under ultrasound guidance. Over this period, 62% of patients expressed a significant reduction in their pain after treatment with AmnioFix. There were no complications in this cohort of patients.
Conclusions
As treatment options for chronic orchialgia have advanced, the application of a structured algorithm may be helpful for physicians providing care for these patients. The above is a possible treatment approach for this condition.
Acknowledgements
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cassidy DJ. Early experience with microsurgical spermatic cord denervation for chronic orchialgia at a Canadian centre. Can Urol Assoc J 2015;9:e72-4. [Crossref] [PubMed]
- Tojuola B, Layman J, Kartal I, et al. Chronic orchialgia: Review of treatments old and new. Indian J Urol 2016;32:21-6. [Crossref] [PubMed]
- Tu XA, Yu JW. Updated diagnosis and management of chronic orchialgia. Zhonghua Nan Ke Xue 2016;22:195-9. [PubMed]
- Kumar P, Mehta V, Nargund VH. Clinical management of chronic testicular pain. Urol Int 2010;84:125-31. [Crossref] [PubMed]
- Levine L. Chronic orchialgia: evaluation and discussion of treatment options. Ther Adv Urol 2010;2:209-14. [Crossref] [PubMed]
- Parekattil SJ, Gudeloglu A, Brahmbhatt JV, et al. Trifecta nerve complex: potential anatomical basis for microsurgical denervation of the spermatic cord for chronic orchialgia. J Urol 2013;190:265-70. [Crossref] [PubMed]
- Singh V, Sinha RJ. Idiopathic chronic orchialgia - a frustrating issue for the clinician and the patient. Indian J Surg 2008;70:107-10. [Crossref] [PubMed]
- Kavoussi PK, Costabile RA. Orchialgia and the chronic pelvic pain syndrome. World J Urol 2013;31:773-8. [Crossref] [PubMed]
- Masarani M, Cox R. The aetiology, pathophysiology and management of chronic orchialgia. BJU Int 2003;91:435-7. [Crossref] [PubMed]
- Gordhan CG, Sadeghi-Nejad H. Scrotal pain: evaluation and management. Korean J Urol 2015;56:3-11. [Crossref] [PubMed]
- Narita M, Moriyoshi K, Hanada K, et al. Successful treatment for patients with chronic orchialgia following inguinal hernia repair by means of meshoma removal, orchiectomy and triple-neurectomy. Int J Surg Case Rep 2015;16:157-61. [Crossref] [PubMed]
- Oka S, Shiraishi K, Matsuyama H. Microsurgical anatomy of the spermatic cord and spermatic fascia: distribution of lymphatics, and sensory and autonomic nerves. J Urol 2016;195:1841-7. [Crossref] [PubMed]
- Cui T, Terlecki R. Prevalence of relative deficiencies in testosterone and vitamin B12 among patients referred for chronic orchialgia: implications for management. Am J Mens Health 2016. [Epub ahead of print].
- Levine LA, Matkov TG, Lubenow TR. Microsurgical denervation of the spermatic cord: a surgical alternative in the treatment of chronic orchialgia. J Urol 1996;155:1005-7. [Crossref] [PubMed]
- Levine LA, Hoeh MP. Evaluation and management of chronic scrotal content pain. Curr Urol Rep 2015;16:36. [Crossref] [PubMed]
- Benson JS, Abern MR, Larsen S, et al. Does a positive response to spermatic cord block predict response to microdenervation of the spermatic cord for chronic scrotal content pain? J Sex Med 2013;10:876-82. [Crossref] [PubMed]
- Marconi M, Palma C, Troncoso P, et al. Microsurgical spermatic cord denervation as a treatment for chronic scrotal content pain: a multicenter open label trial. J Urol 2015;194:1323-7. [Crossref] [PubMed]
- Tojuola B, Kartal I, Brahmbhatt J, et al. Targeted Robotic assisted microsurgical denervation of the spermatic cord for the treatment of chronic scrotal content pain: single center, large series review. J Urol 2015;193:e836.
- Calixte N, Tojuola B, Kartal I, et al. Salvage Ultrasound Guided Targeted Microcryoablation of the peri-spermatic cord for persistent Chronic Scrotal Content Pain after microsurgical denervation of the spermatic cord. Available online: http://sesaua.org/docs/meetings/ses1703/2017-program-book.aspx
- Tojuola B, Kartal I, Brahmbhatt J, et al. SCROTOX: Salvage Peri-spermatic cord Botulinum-A Toxin injections for patients with refractory chronic scrotal content pain after microsurgical denervation of the spermatic cord. J Urol 2015;193:e905. [Crossref]