Implications of ejaculatory sperm DNA fragmentation on male infertility management
Latest guidelines from the World Health Organization (WHO) on the laboratory examination of human semen still focus on traditional semen analysis (SA) (1). However, the more recent recommendations from the major societies in the andrology field suggest to utilize new functional tests in certain conditions (2,3). Although the American Urological Association (AUA) best practice statement (2) and the joint guideline course of European Academy of Andrology (EAA) and the European Association of Urology (EAU) on clinical andrology (2) stated that routine use of ejaculatory sperm DNA fragmentation (SDF) in all infertile couples is not supported by current literature, there is a general consensus that some of the infertile couples may benefit from sperm DNA integrity evaluation. Abnormal SDF, is characterized by more than 15% (fair sperm DNA integrity) to more than 30% (poor sperm integrity) fragmented DNA in ejaculatory sperm (4).
Agarwal and colleagues extensively reviewed three decades of the literature on the methodology of sperm DNA integrity testing as well as its clinical indications and significance in reproductive medicine (5). The first part of their article focused on different laboratory methods of SDF testing. The very detailed laboratory information is more applicable for laboratory specialists than the clinicians. However, a practical comparison of eight different laboratory methods including the principles, advantages and disadvantages of each test is appropriately presented in a summarized table, which can be very useful (5). Sperm chromatin structure assay (SCSA) is considered the most reliable test, if the flow cytometry instrument and skilled technicians are available (6). This test can be performed on both fresh and frozen semen samples with a reasonable cost. There are laboratories in the United States which provide collection kits with dry shipper sending to patient address with a physician order. There is also a current procedural terminology (CPT) code for SCSA which is acceptable (CPT #88182) by some insurance companies.
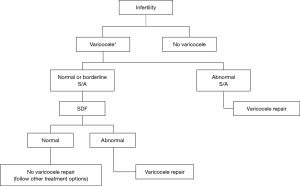
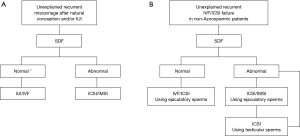
In the second part, the authors have focused on clinical indications for SDF testing based on the existing evidences in the literature and expert opinions (5). Case presentation under different clinical scenarios seems to be an excellent educational idea. However, the high volume of information provided by authors may need repetitive reading of this manuscript to understand everything truly and extract the best clinical applications. Therefore, we have made three flow charts, based on valuable work of Agarwal et al., other relative recent publications and our clinical experiences to summarize the potential clinical applications of SDF testing (Figures 1,2).
Clinical varicocele is usually graded from I to III (8). Making decision for surgical repair, especially in low grade varicocele and borderline abnormal SA, is often challenging. Abnormal SDF, in this group of patients makes varicocelectomy more justified (Figure 1).
Agarwal et al. used three terminologies of “recurrent abortion”, “recurrent pregnancy loss” and “recurrent miscarriage” in different parts of their review (5). However, it looks that their main focus is on losing three consecutive pregnancies, either natural or after intrauterine insemination (IUI), prior to 20 weeks from the last menstrual period. This affects approximately 1% to 2% of women (9). In approximately half of these patients, etiology of recurrent miscarriages remains unexplained (9). SDF evaluation can help to choose the most appropriate type of assisted reproductive technology (ART) (10) including IUI, traditional in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI) or intracytoplasmic morphologically selected sperm injection (IMSI) in this group of patients (Figure 2A). Another challenge is unexplained recurrent ART failures when there is not any known male or female factor. The integrity of sperm DNA is considered to be vital for normal fertilization, embryonal development, successful implantation and pregnancies in both natural and assisted reproduction. Based on animal and also limited clinical data, using testicular sperm can be a solution when ejaculatory sperms have high level of SDF (11,12) (Figure 2B). Testicular sperm can be harvested either through a needle or by open incisional biopsy.
Another clinical scenario, in which SDF testing can be helpful, is to choose between IUI and IVF/ICSI in infertile couples who otherwise are candidate for both versions of ART. In this situation, abnormal SDF suggests to apply higher levels of ART treatment, i.e., IVF/ICSI.
Future prospective multicenter clinical trials, using SDF tests, will help the field of sperm DNA integrity to grow. We look forward to have a reliable and standard sperm function test to evaluate SDF with specific clinical indications to increase the success rate of treatment for infertile couples.
Acknowledgements
We appreciate Professor Stuart Howards for reviewing our flowcharts and providing his suggestions on this commentary.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- World Health Organization. WHO laboratory manual for the examination and processing of human semen. Fifth edition. Geneva, WHO, 2010.
- Jarow J, Sigman M, Kolettis PN, et al. The Optimal Evaluation of the Infertile Male: AUA Best Practice Statement. Washington: American Urological Association Education and Research, Inc, 2011. Available online: https://www.auanet.org/education/guidelines/male-infertility-d.cfm
- Björndahl L, Giwercman A, Tournaye H, et al. editors. Clinical Andrology: EAU/ESAU Course Guidelines. 1 edition. CRC Press, 2010.
- Bungum M. Sperm DNA integrity assessment: a new tool in diagnosis and treatment of fertility. Obstet Gynecol Int 2012;2012:531042. [Crossref] [PubMed]
- Agarwal A, Majzoub A, Esteves SC, et al. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol 2016;5:935-50. [Crossref] [PubMed]
- Gillan L, Evans G, Maxwell WM. Flow cytometric evaluation of sperm parameters in relation to fertility potential. Theriogenology 2005;63:445-57. [Crossref] [PubMed]
- Dinelli L, Courbière B, Achard V, et al. Prognosis factors of pregnancy after intrauterine insemination with the husband's sperm: conclusions of an analysis of 2,019 cycles. Fertil Steril 2014;101:994-1000. [Crossref] [PubMed]
- Velasquez M, Tanrikut C. Surgical management of male infertility: an update. Transl Androl Urol 2014;3:64-76. [PubMed]
- Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol 2009;2:76-83. [PubMed]
- Simopoulou M, Gkoles L, Bakas P, et al. Improving ICSI: A review from the spermatozoon perspective. Syst Biol Reprod Med 2016;62:359-71. [Crossref] [PubMed]
- Esteves SC, Sanchez-Martin F, Sanchez-Martin P, et al. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril 2015;104:1398-405. [Crossref] [PubMed]
- Zini A, Bach PV, Al-Malki AH, et al. Use of testicular sperm for ICSI in oligozoospermic couples: how far should we go? Hum Reprod 2017;32:7-13. [PubMed]