Serum testosterone levels and other determinants of sperm retrieval in microdissection testicular sperm extraction
Introduction
Infertility affects around 15% of couples, of which 20% is accounted for exclusively by male factors (1). Of the limited options that can be offered to non-obstructive azoospermia (NOA) patients (2), microdissection testicular sperm extraction (microTESE) has become the standard of care owing to its superior sperm retrieval rate (SRR), minimal structural-and-functional tissue disruption, and lower post-operative complications (3,4). Unfortunately, there remains a considerable proportion of patients who still fail to have their sperms successfully retrieved with this procedure (2). As a crucial ingredient of spermatogenesis, testosterone has been a focus of attempts aimed at optimizing the outcomes of microTESE (5,6). Whereas the critical role of testosterone in spermatogenesis has long been established (7), its role in the management of NOA patients is less clear; several reports have investigated the benefit of pre-operative testosterone optimization on microTESE outcomes. While some suggested better outcomes with testosterone optimization (5,8), others showed no effect of testosterone on SRR (6,9,10). The conflicting outcomes of published reports are likely to reflect—among other methodological and demographic variations—the complexity of the body’s physiological responses to this hormone, which has been shown to have wide inter-person variations in normal, otherwise healthy individuals (11). In patients with an underlying pathology that prevents the initiation and/or maintenance of normal spermatogenesis, this variation may be more pronounced. With such incongruent findings, further research is needed to better our understanding of the role of testosterone in the operative outcomes of microTESE in NOA. We herein present our experience with microTESE outcomes and its determinants in our population, with particular emphasis on testosterone and its association with the operative outcomes. Our primary objective was to investigate the relationship between pre-operative serum testosterone level and microTESE outcomes. The secondary objective was to identify the significant determinants of the operation’s success in our population.
Methods
Study design, population and setting
This is a retrospective medical chart review of NOA patients who underwent microTESE under the care of the Urology Department, King Faisal Specialist Hospital and Research Center (KFSHRC), a tertiary referral center, during the period Aug 2009–July 2015. As described by the World Health Organization guidelines, azoospermia was established by the absence of spermatozoa in two separate analyzed ejaculates (12). Patients with serum testosterone level ≤9.9 nmol/L were considered to have low testosterone. MicroTESE that resulted in the retrieval of at least one sperm was considered positive/successful.
Collected variables
The following variables were collected for each patient: age, body mass index (BMI), serum luteinizing hormone (LH), follicle-stimulating hormone (FSH), thyroid-stimulating hormone (TSH), total testosterone, estradiol, prolactin, chromosomal aberrations, pre-operative sonographic findings [e.g., testicular volume and varicocele presence (testicular volume was measured using the product of ultrasonographically determined dimensions of the testis, multiplied by 0.52)], previous chemotherapy or bone marrow transplantation, intraoperative sperm retrieval, sperm motility, sperm-freezing amount and post-operative histological diagnosis.
Statistical analysis
Study findings were summarized using frequencies, means and standard deviations. Between-group comparisons were conducted using Student’s t- and χ2 tests, as appropriate. Hierarchical multiple logistic regression was used to assess for potential determinants of microTESE outcomes, to identify the magnitude of each association, and to adjust for potential confounding variables between each of the examined variables and microTESE outcomes. All tests were two-tailed. A P value <0.05 was considered significant.
Ethical considerations
This study was approved by the Institutional Research Advisory Council (RAC), the institutional ethical review board. Informed consent was obtained from each patient.
Results
Sample characteristics
A total of 421 NOA patients underwent microTESE during the identified period. Of those, 371 received no prior treatment with clomiphene or human chorionic gonadotropin (HCG). The characteristics of the study population, along with microTESE outcomes and histopathological findings are shown in Table 1.
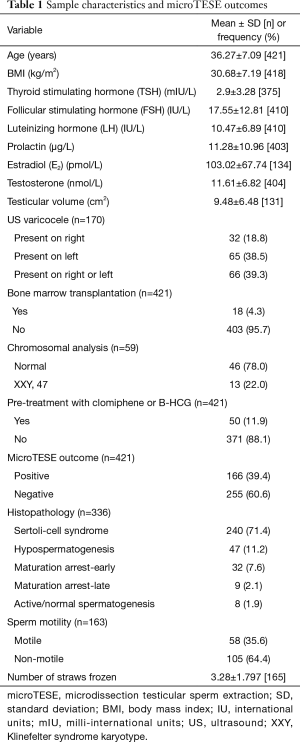
Full table
Testosterone and microTESE success
Out of the 421 operations done, sperms were successfully retrieved in 166 cases (39.4%). There was no significant difference in serum testosterone levels between NOA patients with positive- and negative-microTESE outcomes for whom no prior treatment with clomiphene or HCG was initiated (P=0.599) (Table 2). Similarly, the difference in serum testosterone level between positive and negative microTESE groups was not significant when all patients (including those with prior treatment) were examined (P=0.820). The distribution of positive and negative microTESE outcomes was not significantly different between normal (n=223, SRR =38.6%) and low (n=181, SSR =40.3%) testosterone groups (P=0.718); normal and non-treated/naive low (n=131, SRR =42.0%) testosterone groups (P=0.526); or normal and pre-treated low (n=50, SRR =36.0%) testosterone groups (P=0.736).
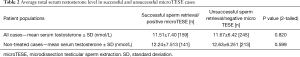
Full table
Testosterone vs. extracted sperm amount and motility
We investigated the relationship of serum testosterone level with the motility and amount of sperms extracted from microTESE. No significant difference was found in sperm motility (P=0.777) or average number of vials that could be frozen after microTESE (P=0.276) between normal and low testosterone groups. Likewise, there was no significant difference between normal and non-treated/naive low testosterone groups in either sperm motility (P=0.987) or average number of frozen straws/vials (P=0.570). The lack of significant difference in sperm motility (P=0.462) and frozen straws/vials (P=0.145) was also applicable to normal vs. pre-treated low testosterone groups.
MicroTESE-outcome determinants
In order to identify the potential determinants of microTESE outcomes, we conducted hierarchical multiple logistic regression, in which patients’ age and BMI; hormonal profile (FSH and prolactin); and total testosterone and histopathology were entered sequentially in this order. Of all variables, only age (P=0.044) and histopathology (P<0.001) were found to have significant relationship with microTESE outcomes. With each year increase in age, there was an estimated 4% increase in the odds of having a positive microTESE results (aOR =1.039; 95% CI, 1.001–1.077; P=0.044). Hypospermatogenesis was associated with over a 3-fold increase in the odds of having a successful retrieval of sperms than sertoli-cell only syndrome (aOR =4.380; 95% CI, 2.099–9.141; P<0.001). When only naïve NOA were included in the model, only tissue histopathology retained its statistical significance (P<0.001), with hypospermatogenesis conferring around a 4-fold increase in the odds of having a successful sperm retrieval than sertoli-cell only syndrome (aOR =4.961; 95% CI, 2.197–11.202; P<0.001). Of note, testosterone remained a non-significant determinant of microTESE outcomes even after adjusting for all other variables.
Discussion
To our knowledge, this is the first study of microTESE outcomes in relation to testosterone and other factors in our population. In this study, neither serum testosterone level nor FSH was found to be significant predictors of microTESE success. To date, the predictive significance of serum hormonal levels remains controversial (13). While some reports suggested a potential predictive role (10,14-18), the majority found no association between hormonal profiles and outcomes of microTESE (6,13,19-25), which is consistent with the findings reported in this study.
One possible explanation to the lack of relationship between microTESE outcomes and serum testosterone level is the robustness of microTESE itself. Because of the feedback loops, it is expected that derangements in hormonal levels would reflect, at least in part, the extent of the disease within the testicular tissues; the fewer the sperm-production areas that have a normal response, the more extreme the serum hormonal values would be. However, microTESE involves meticulous dissection that makes it resilient to fluctuations in the number of areas of spermatogenesis (26). The active search for islands of spermatogenesis makes sperm retrieval in microTESE less dependent on random chance, which would be otherwise affected by the total number of sperm production areas that are present within the testicular tissue. In other words, a blinded aspiration procedure may be more successful when a higher number of areas with active spermatogenesis are presented within the testicular tissue, but this may be less applicable to microTESE. This may explain, in part, why variations in hormonal levels may not be significantly different between successful and non-successful microTESE procedures. However, such hormonal derangements may affect certain qualities and stages of maturation of the retrieved sperms (e.g., elongated vs. round spermatids) and thus the likelihood of fertilization, clinical pregnancies, and live births (27).
Our findings suggest histopathology to be predictive of microTESE outcomes. This is consistent with the previous reports that examined this relationship (10,20,28,29). In fact, to date, histopathology remains the single most significant predictive factor of microTESE sperm retrieval in NOA (13). Of the different histopathological subtypes, sertoli-cell only has been consistently suggested to yield the least microTESE retrieval rates (19). We also found, and as noted by previous reports, the most favorable outcomes to be present in tissues showing hypospermatogenesis (10,19). Unfortunately, histopathology would be of limited practical usefulness as a predictor, since it is determined intra- or post-operatively. Alternatively, performing pre-operative biopsies as a standalone procedure is invasive and may expose the testicular tissue to unnecessary complications (30), especially that multiple biopsies are often needed to reflect the true status of spermatogenesis within the testes (31). Interestingly, higher age at which microTESE was performed was associated with better microTESE outcomes. Data exploring the effect of age on microTESE outcomes are scarce. Bernie et al. suggested better chances of microTESE success in older age, but no clear association was identified (13). Albeit counterintuitive, the need for microTESE at an earlier age may reflect a more severe disease, and thus, lower probability of success rates.
Conclusions
Serum testosterone level appears to have no significant association with microTESE outcomes in NOA. Among the clinicopathological patient characteristics, the underlying histological pattern is the most significant determinant of the procedure’s success.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Research Advisory Council (RAC), the institutional ethical review board (RAC#2141091). Informed consent was obtained from each patient.
References
- Stephen EH, Chandra A. Declining estimates of infertility in the United States: 1982-2002. Fertil Steril 2006;86:516-23. [Crossref] [PubMed]
- Shin DH, Turek PJ. Sperm retrieval techniques. Nat Rev Urol 2013;10:723-30. [Crossref] [PubMed]
- Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod 1999;14:131-5. [Crossref] [PubMed]
- Deruyver Y, Vanderschueren D, Van der Aa F. Outcome of microdissection TESE compared with conventional TESE in non-obstructive azoospermia: a systematic review. Andrology 2014;2:20-4. [Crossref] [PubMed]
- Hussein A, Ozgok Y, Ross L, et al. Optimization of spermatogenesis-regulating hormones in patients with non-obstructive azoospermia and its impact on sperm retrieval: a multicentre study. BJU Int 2013;111:E110-4. [Crossref] [PubMed]
- Reifsnyder JE, Ramasamy R, Husseini J, et al. Role of optimizing testosterone before microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol 2012;188:532-6. [Crossref] [PubMed]
- Wang RS, Yeh S, Tzeng CR, et al. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev 2009;30:119-32. [Crossref] [PubMed]
- Ravizzini P, Carizza C, Abdelmassih V, et al. Microdissection testicular sperm extraction and IVF-ICSI outcome in nonobstructive azoospermia. Andrologia 2008;40:219-26. [Crossref] [PubMed]
- Modarresi T, Hosseinifar H, Daliri Hampa A, et al. Predictive factors of successful microdissection testicular sperm extraction in patients with presumed sertoli cell-only syndrome. Int J Fertil Steril 2015;9:107-12. [PubMed]
- Yildirim ME, Koc A, Kaygusuz IC, et al. The association between serum follicle-stimulating hormone levels and the success of microdissection testicular sperm extraction in patients with azoospermia. Urol J 2014;11:1825-8. [PubMed]
- Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 2013;369:1011-22. [Crossref] [PubMed]
- World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge University Press, 1999.
- Bernie AM, Ramasamy R, Schlegel PN. Predictive factors of successful microdissection testicular sperm extraction. Basic Clin Androl 2013;23:5. [Crossref] [PubMed]
- Tsujimura A, Matsumiya K, Miyagawa Y, et al. Prediction of successful outcome of microdissection testicular sperm extraction in men with idiopathic nonobstructive azoospermia. J Urol 2004;172:1944-7. [Crossref] [PubMed]
- Ballescá JL, Balasch J, Calafell JM, et al. Serum inhibin B determination is predictive of successful testicular sperm extraction in men with non-obstructive azoospermia. Hum Reprod 2000;15:1734-8. [Crossref] [PubMed]
- Cetinkaya M, Onem K, Zorba OU, et al. Evaluation of Microdissection Testicular Sperm Extraction Results in Patients with Non-Obstructive Azoospermia: Independent Predictive Factors and Best Cutoff Values for Sperm Retrieval. Urol J 2015;12:2436-43. [PubMed]
- Ziaee SA, Ezzatnegad M, Nowroozi M, et al. Prediction of successful sperm retrieval in patients with nonobstructive azoospermia. Urol J 2006;3:92-6. [PubMed]
- Kalsi JS, Shah P, Thum Y, et al. Salvage micro-dissection testicular sperm extraction; outcome in men with non-obstructive azoospermia with previous failed sperm retrievals. BJU Int 2015;116:460-5. [Crossref] [PubMed]
- Seo JT, Ko WJ. Predictive factors of successful testicular sperm recovery in non-obstructive azoospermia patients. Int J Androl 2001;24:306-10. [Crossref] [PubMed]
- Tournaye H, Verheyen G, Nagy P, et al. Are there any predictive factors for successful testicular sperm recovery in azoospermic patients? Hum Reprod 1997;12:80-6. [Crossref] [PubMed]
- Martin-du-Pan RC, Bischof P. Increased follicle stimulating hormone in infertile men. Is increased plasma FSH always due to damaged germinal epithelium? Hum Reprod 1995;10:1940-5. [PubMed]
- Kim ED, Gilbaugh JH 3rd, Patel VR, et al. Testis biopsies frequently demonstrate sperm in men with azoospermia and significantly elevated follicle-stimulating hormone levels. J Urol 1997;157:144-6. [Crossref] [PubMed]
- Vernaeve V, Tournaye H, Schiettecatte J, et al. Serum inhibin B cannot predict testicular sperm retrieval in patients with non-obstructive azoospermia. Hum Reprod 2002;17:971-6. [Crossref] [PubMed]
- von Eckardstein S, Simoni M, Bergmann M, et al. Serum inhibin B in combination with serum follicle-stimulating hormone (FSH) is a more sensitive marker than serum FSH alone for impaired spermatogenesis in men, but cannot predict the presence of sperm in testicular tissue samples. J Clin Endocrinol Metab 1999;84:2496-501. [PubMed]
- Tunc L, Kirac M, Gurocak S, et al. Can serum Inhibin B and FSH levels, testicular histology and volume predict the outcome of testicular sperm extraction in patients with non-obstructive azoospermia? Int Urol Nephrol 2006;38:629-35. [Crossref] [PubMed]
- Ramasamy R, Lin K, Gosden LV, et al. High serum FSH levels in men with nonobstructive azoospermia does not affect success of microdissection testicular sperm extraction. Fertil Steril 2009;92:590-3. [Crossref] [PubMed]
- Zitzmann M, Nordhoff V, von Schönfeld V, et al. Elevated follicle-stimulating hormone levels and the chances for azoospermic men to become fathers after retrieval of elongated spermatids from cryopreserved testicular tissue. Fertil Steril 2006;86:339-47. [Crossref] [PubMed]
- Su LM, Palermo GD, Goldstein M, et al. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia: testicular histology can predict success of sperm retrieval. J Urol 1999;161:112-6. [Crossref] [PubMed]
- Ezeh UI, Moore HD, Cooke ID. A prospective study of multiple needle biopsies versus a single open biopsy for testicular sperm extraction in men with non-obstructive azoospermia. Hum Reprod 1998;13:3075-80. [Crossref] [PubMed]
- Schlegel PN, Su LM. Physiological consequences of testicular sperm extraction. Hum Reprod 1997;12:1688-92. [Crossref] [PubMed]
- Gottschalk-Sabag S, Weiss DB, Folb-Zacharow N, et al. Is one testicular specimen sufficient for quantitative evaluation of spermatogenesis? Fertil Steril 1995;64:399-402. [Crossref] [PubMed]


