Chronic scrotal pain and microsurgical spermatic cord denervation: tricks of the trade
Introduction
Chronic orchialgia is a common urologic complaint accounting for ~2.5% of new patient visits (1). Despite this, its etiology and pathophysiology are poorly understood and, as a result, many patients fail to receive adequate treatment.
Defined as an intermittent, or constant pain that interferes with activities of daily living for at least 3 months or more, patients with chronic orchialgia frequently localize pain to other scrotal structures, such as the epididymis, spermatic cord, and other para-testicular components (2). As such, some experts have proposed the use of the term chronic scrotal content pain (CSCP) to be a more appropriate term for this condition (3,4). CSCP can manifest at any age; however, the majority of patients initially present in their mid to late thirties and will frequently see multiple urologists during the course of their workup (5,6).
Pain in the scrotum can either be the result of a direct process (tumor, hydrocele, torsion etc.) or it may be referred from structures with shared innervation (i.e., a mid-ureteral stone, vascular aneurysm, indirect inguinal hernia). Consequently, a comprehensive understanding of the afferent innervation supplying the scrotal contents is essential. Primarily, the ilioinguinal nerve and the genital branch of the genitofemoral nerve provide somatic innervation of the scrotal contents (5,7,8). Autonomic fibers from the parasympathetic ganglia of T10–12 supply the testis and fibers from the T10–L1 ganglia supply the epididymis and vas deferens (8,9). These fibers then converge within the spermatic cord into three separate structures to form what has been described as the “trifecta nerve complex” consisting of the peri-vasal complex, the posterior peri-arterial/lipomatous complex, and the intra-cremasteric complex (10). The unmyelinated C fibers and myelinated A fibers within these nerves then carry the pain message cephalad via the dorsal horn of the spinal cord, along the medial and lateral spinothalamic tracts, and then finally to the brain (3). Appreciation of this architecture provides the foundational understanding upon which microsurgical intervention is based.
The process by which acute pain becomes chronic is poorly understood. It is currently believed that progress from acute to chronic pain occurs in discrete, pathophysiologic steps (11). Traditionally, noxious stimuli lessen as healing progresses. This leads to decreases in perceived pain until finally, resolution is achieved. Persistent, painful stimuli are known to activate secondary mechanisms both at the periphery and within the central nervous system that cause allodynia, hyperalgesia, and hyperpathia. These signals can then induce physical changes in the sensory nerves including Wallerian degeneration that predisposes the patient to feel pain more easily (10). From a psychological perspective, the patient becomes extremely sensitized to any painful stimulus that occurs at, or near the region of the scrotum. This creates a ‘cycle of pain’ whereby initial painful stimuli makes the patient hyper-aware of any negative sensation in the region and thus, much more likely to notice any subsequent discomfort. This ‘re-setting’ of the pain commences the cycle anew, and potentially results in a decreased pain threshold and increased pain sensitivity.
Patient evaluation
Chronic pain is a complex process with multiple contributing factors ranging from the anatomic to the psychosocial. Accordingly, evaluation of a patient with CSCP should proceed in a methodical and step-wise fashion. Initial efforts should be focused on ruling out underlying medical and anatomic causes such as a tumors, intermittent torsion, active infection, or varicocele (Figure 1).
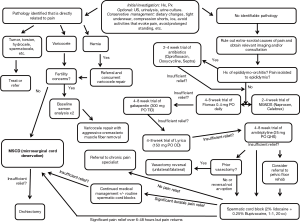
A comprehensive history and physical should fully describe the onset, location, duration, character, severity, and radiation of the patient’s pain (Figure 1). A dull, aching, persistent pain is considered to be classic for CSCP while severe, intermittent pain may be more indicative epididymitis, orchitis or even intermittent torsion (12). Practitioners should also endeavor to identify aggravating and alleviating factors. Many patients with genital pain experience worsening of their symptoms with prolonged sitting and constipation. Any prior surgeries (especially scrotal, inguinal, retroperitoneal, and spinal surgeries) should be thoroughly investigated. If a new, or recurrent hernia has been identified then referral to general surgery should be the first step. A history of prior vasectomy is particularly important as anywhere from 6–30% of vasectomized patients will develop post-vasectomy pain syndrome (PVPS) (13,14). In these cases, developing a link between the time of the vasectomy and the development of the pain is critical. Appropriate questioning regarding the patient’s social circumstances and the current level of disability caused by his pain can be helpful in identifying the potential for secondary gain. A thorough past medical history should reveal the presence of any other history of chronic pain, injury, or psychiatric illnesses such as depression.
In all cases, a frank discussion should be had with the patient regarding the level of bother he associates with his pain. How does his pain affect his day-to-day life? How long has he been seeking care for this problem? Is he willing to undergo surgery? Questions such as these can paint a more global picture regarding the patient’s current situation and may help guide practitioners when deciding how quickly to proceed/or advance through the steps of medical and surgical management (Figure 1).
Physical exam should focus on the genitalia and pelvic floor. If the patient’s pain is unilateral, the contralateral side should be initially examined to divert the focus away from the pain. Mentioning this to the patient prior to examination is important as to allow them to brace themselves. Special attention should then be paid to the testes, epididymis, and spermatic cord. A rectal exam and examination of the pelvic floor musculature is indicated as up to 63% of men with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) will report comorbid scrotal content pain (4,15-17). This determination is important as it affects what treatments are available to the patient.
Patients can undergo scrotal duplex ultrasound to rule out anatomic causes for pain while patients with a history of radiating back pain may warrant additional imaging in the form of CT or MRI. Urinalysis with urine culture should be utilized to rule out infection. Although each of these steps are critical for ruling out reversible causes of scrotal pain, it is worth noting that up to 50% of men with CSCP will have no identifiable etiology (2,18).
Medical management
Medical management of CSCP is often made more challenging by the paucity of clear guidelines and research in the medical literature. Initial treatment should focus on conservative management such as tighter-fitting underwear, scrotal support, ice/compression and avoidance of activities that evoke pain.
Following failure of conservative strategies, a single 2–4 weeks course of empiric antibiotics is a reasonable first step for men with signs and symptoms consistent with infectious epididymo-orchitis. Quinolone antibiotics and trimethoprim/sulfamethoxazole are preferred agents as their lipophilic nature that allows them to thoroughly penetrate both the testis as well as the epididymis. Although a negative urine culture does not rule out the possibility of infection, a positive result can help further tailor antibiotic selection.
With regards to pain control and management, a 2–4 weeks trial of anti-inflammatories is also a logical consideration for first choice therapy. Typically, we recommend a regiment of ibuprofen (PO 600–800 mg q 4–6 hours), celocoxib (PO, 200 mg daily) (19) or naproxen (500 mg PO BID). The latter of these is typically the most cost-effective. In men with pain refractory to NSAID therapy, other oral agents including both gabapentin and amitriptyline have been shown to possess some efficacy in mitigating the symptoms of CSCP (Figure 1). Sinclair et al. found that out of 19 patients with chronic testicular pain, 61.5% of those initiated on gabapentin and 66.6% those initiated on nortriptyline reported 50% or greater improvement of their pain (20). Since TCA therapy may take 2–3 weeks of treatment prior to achieving therapeutic effect, we recommend 10–20 mg of amitriptyline nightly for at least one month (21). If this is unsuccessful, gabapentin 300 mg three times daily can be added in replacement of, or in addition to, TCA therapy. A further neuromodulator, pregabalin/lyrica, can be given at 75–150 mg daily; however, insurance coverage typically necessitates failure with gabapentin prior to coverage.
Several adjunctive treatment options are also available. Clinical experience and limited literature has shown that lifestyle modifications can improve symptoms for certain CP/CPPS patients (22). These measures include alteration of fluid intake and avoidance of factors that may trigger symptoms such as caffeine, citrus products, or chocolate (23). For men with pain that is aggravated by prolonged sitting, scheduled breaks during the workday may provide some relief. Although results have historically been variable, Chen et al. recently showed in a placebo-controlled, prospective phase III study of 100 Chinese men that tamsulosin can significantly improve CP/CPPS symptoms as measured by NIH-CPSI (24). This is contrast to previous studies that examined older, second generation alpha-blockers such as alfuzosin (25).
Physical therapy is another adjunctive option that has long been a mainstay of treatment for CP/CPPS. A recent retrospective study of 30 patients by Farrell et al. has confirmed that its benefits appear to extend to members of the CSCP population who demonstrate CP/CPPS symptoms on digital rectal exam (26,27).
Narcotics simply mask the symptoms of CSCP without treating the underlying condition and carry significant risks to overall patient well-being. These medications are hardly ever helpful in the long-term and should be strictly limited. Local cord blocks can also provide short-term benefit and are much preferred. When medical management and lifestyle adjustments are found to be unsuccessful, the next step is typically surgery.
Microscopic vasovasostomy is an accepted option for men suffering post-vasectomy pain syndrome, but this obviates the goal of the patient’s initial surgery and is clearly limited to this specific patient population (28-30). For patients with a clinically apparent varicocele, it is prudent to discuss varicocele repair but if their pain is actually neuropathic rather than vascular, their pain may still persist post-operatively (31). Indeed, in cases where a varicocele is present in the setting of scrotal pain, we tend to offer a more aggressive varicocele repair consisting of aggressive dissection of the cremasteric fibers as well as spermatic cord vasculature. Varicocele repair for chronic pain is best reserved for non-vasectomized men with a strong desire for future paternity.
Although both orchiectomy and epididymectomy have been historically described as options for addressing CSCP, their success is variable with rates ranging from 20–70% (2,32,33). As such, most consider these to be treatments of last resort (Figure 1).
For all others, microsurgical spermatic cord denervation (MSCD) offers a minimally invasive option for treatment of CSCP with minimal morbidity, durable results, and success rates in excess of 70–80% (4,18). As such, MSCD has become a primary surgical intervention for this patient population.
Patient selection and technique for MSCD
The first critical step for successful MSCD is patient selection. When no medically reversible cause can be identified for a patient’s scrotal pain and MSCD is being considered, patients should first be screened with an in-office spermatic cord block to assess success (18,34). The primary goal of the spermatic block is develop a temporary resolution of the pain over the next 6–8 hours over which the anesthetic will be effective. If successful, this would strongly suggest that neural stimulation is the result of the pain and discomfort.
Although a variety of agents can be used, we prefer to perform blocks with a generous injection of 10–20 cc of a 50:50 mixture consisting of 2% lidocaine with 0.25% bupivacaine at the level of the pubic tubercle. Bupivacaine blocks the fast voltage-gated Na channels essential for neuronal transmission and has a longer duration of action (4–8 hours) compared to that of lidocaine (~2 hours) (35). The spermatic cord can be readily palpated at this level after exiting the external inguinal ring, allowing for easy targeting of the injection. For patients with challenging anatomy secondary to obesity or prior surgery, ultrasound guidance is easily utilized to ensure good positioning of the block (36). After performing the block, the patient’s response is assessed with a rating on the numerical pain scale, which is then compared to a pre-block value.
Although some authors endorse the use of ‘sham blocks’ with normal saline as part of the screening process, we do not recommend this for routine clinical practice given obvious ethical concerns (4,37). Occasionally, patients may report relief of their pain lasting much longer than the half-life of the anesthetic used. Although some authors may postulate that this indicates a non-anatomic cause of the patient’s scrotal pain, this positive outcome can often be the result of breaking the previously mentioned ‘cycle of pain’. Some patients, upon finding even temporary relief to their pain, become less sensitized to subsequent painful stimuli. In some cases, these patients can be followed and managed conservatively, either with medical therapy or interval blocks. True responders are defined as patients who experience a transient relief of their pain in excess of 90%. These patients are then considered as candidates for MSCD.
Multiple techniques for MSCD have been described over the years. The procedure was first reported in 1978 (38). In regards to incision site selection, there are essentially two approaches: the traditional inguinal approach or a sub-inguinal approach. Both options have been described with good success and each has their relative strengths (39-41). The inguinal approach, as described by Devine et al. in their original 1978 paper, allows the surgeon to bury the proximal segment of the ilioinguinal nerve after being severed to reduce the risk of neuroma formation while the subinguinal approach may be more comfortable for some surgeons as this approach is similar to that for microscopic varicocelectomy. The non-muscle splitting nature of the subinguinal incision has led to this approach being the modality of choice in this operation. Both have been described with good results (4,18).
After incision, the cord is isolated circumferentially. At our institution, we elect to use Army/Navy retractors to dissect through the subcutaneous tissues down to the level of the cord. The Babcock grasping forceps are then utilized to grasp the cord atraumatically. Any remaining attachments are brushed away bluntly with a Kittner dissecting instrument. Once free, the cord is elevated and supported with the assistance of a knife handle to tent the cord up into the incision. The external spermatic fascia is then opened anteriorly, exposing the cord contents. An operating microscope is then brought into the field.
Initially, all cremasteric fibers are identified and transected with care being taken to avoid damage to the cremasteric artery. Given the potential for sympathetic innervation of these structures and the possible contribution of cremasteric spasm to CSCP as a whole, this extra step can serve to make a large impact in resolution of a patient’s scrotal pain.
Once the cremasteric fibers have been removed, with the operating microscope and intraoperative Doppler ultrasound, each of the cord structures are systematically identified including the testicular artery, testicular veins, vas deferens, and lymphatics. Great care must be taken to identify and preserve the testicular artery while the testicular veins are ligated between 2-0 or 3-0 silk ties in a sequential fashion (Figure 2).
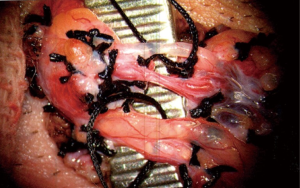
In men who have not undergone vasectomy, we elect to preserve the vas deferens as backpressure with subsequent epididymal congestion resulting from ligation have been theorized to be contributing factors towards post-vasectomy pain syndrome (14). In men who have not had a vasectomy and do not have any interest in fertility, the outer vasal sheath is stripped for approximately 2 cm as studies have shown extensive innervation of this structure (10). The vas is divided once more and an approximate1 cm segment is excised. The lymphatics are preserved as they do not possess any inherent innervation and are necessary to leave in situ to reduce the risk of postoperative hydrocele.
With the testicular artery and lymphatics preserved, flow in the artery in confirmed one last time before returning the cord to its original position. The wound is injected with a generous amount of 2% lidocaine and 0.25% bupivacaine mixture and closed in layers. A scrotal support with fluffy dressings is then applied.
It is important to educate patients that post-operative pain is normal and any true pain relief from the surgery itself may not be appreciated for several weeks. If pain is unchanged several months post-operatively, it is only then that more drastic measures, such as orchiectomy, are considered.
Outcomes and complications
Several studies have examined the efficacy of MSCD over the years. In 2008, Strom and Levine published their cumulative experience to date with a total of 95 testicular units from 79 men (18). Each of these men had failed conservative management and had undergone an extensive workup to rule-out underlying pathology including a positive response to cord block with local anesthetic. With a mean follow-up of 20.3 months following MSCD, complete relief was reported in 61 (71%) testicular units while greater than 50% relief was reported in 17 (17%). Complications were minimal and included 2 cases of testicular atrophy, 2 hydroceles, 1 hematocele, and 1 superficial wound infection.
Similar success rates have been reported in other series. In 2014, Oomen et al. retrospectively presented their results of 189 patients who had presented to their clinic complaining of CSCP (37). Each patient was subjected to a series of injections including local anesthetic cord blocks and a normal saline ‘sham block.’ A total of 69 patients reported a positive response to the block series and underwent MSCD. With a mean follow-up of 42.8 months, 26 (49%) reported complete pain relief and 20 (38%) reported a greater than 50% improvement in pain levels. Complications included a single hematocele and a single hydrocele that were both managed successfully with secondary procedures. In 2015, Marconi et al. reported their results of 50 CSCP patients undergoing MSCD in a prospective multicenter open label trial (4). Each of these patients underwent a similar workup including a positive response to a series of injections including a normal saline ‘sham block’. Six months following surgery, 40 (80%) patients reported completed resolution of their pain and 6 (12%) reported persistent intermittent discomfort that was able to be managed with NSAIDs. Complications included a single hematocele and a single hydrocele that were both managed with secondary procedures.
Conclusions
CSCP is a challenging but a not uncommon dilemma that every urologist will encounter. Although treatment for it has historically been unsatisfying, there are now a number of proven interventions available to practitioners. Lifestyle modifications and judicious anti-inflammatory use may reduce patients’ reliance on prescription medication while physical therapy empowers patients with coping exercises and allows them to take control of their own disease process. For those patients that require further assistance, various non-narcotic and non-habit forming medical regimens are available. Even when all these measures fail, MSCD offers an efficacious solution with 80–88% of patients reporting significant or total pain relief (4,18,37). The systematic approach presented in this current manuscript offers a reasonable framework that trained microsurgeons can refer to throughout the management of CSCP.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Strebel RT, Leippold T, Luginbuehl T, et al. Chronic scrotal pain syndrome: management among urologists in Switzerland. Eur Urol 2005;47:812-6. [Crossref] [PubMed]
- Davis BE, Noble MJ, Weigel JW, et al. Analysis and management of chronic testicular pain. J Urol 1990;143:936-9. [PubMed]
- Levine LA, Hoeh MP. Evaluation and management of chronic scrotal content pain. Curr Urol Rep 2015;16:36. [Crossref] [PubMed]
- Marconi M, Palma C, Troncoso P, et al. Microsurgical Spermatic Cord Denervation as a Treatment for Chronic Scrotal Content Pain: A Multicenter Open Label Trial. J Urol 2015;194:1323-7. [Crossref] [PubMed]
- Wesselmann U, Burnett AL, Heinberg LJ. The urogenital and rectal pain syndromes. Pain 1997;73:269-94. [Crossref] [PubMed]
- Heidenreich A, Olbert P, Engelmann UH. Management of chronic testalgia by microsurgical testicular denervation. Eur Urol 2002;41:392-7. [Crossref] [PubMed]
- Masarani M, Cox R. The aetiology, pathophysiology and management of chronic orchialgia. BJU Int 2003;91:435-7. [Crossref] [PubMed]
- Levine L. Chronic orchialgia: evaluation and discussion of treatment options. Therapeutic Advances in Urology 2010;2:209-14. [Crossref] [PubMed]
- Oka S, Shiraishi K, Matsuyama H. Microsurgical Anatomy of the Spermatic Cord and Spermatic Fascia: Distribution of Lymphatics, and Sensory and Autonomic Nerves. J Urol 2016;195:1841-7. [Crossref] [PubMed]
- Parekattil SJ, Gudeloglu A, Brahmbhatt JV, et al. Trifecta nerve complex: potential anatomical basis for microsurgical denervation of the spermatic cord for chronic orchialgia. J Urol 2013;190:265-70. [Crossref] [PubMed]
- Voscopoulos C, Lema M. When does acute pain become chronic? Br J Anaesth 2010;105 Suppl 1:i69-85. [Crossref] [PubMed]
- Al-Kandari AM, Kehinde EO, Khudair S, et al. Intermittent Testicular Torsion in Adults: An Overlooked Clinical Condition. Med Princ Pract 2017;26:30-4. [Crossref] [PubMed]
- Christiansen CG, Sandlow JI. Testicular pain following vasectomy: a review of postvasectomy pain syndrome. J Androl 2003;24:293-8. [Crossref] [PubMed]
- McMahon AJ, Buckley J, Taylor A, et al. Chronic testicular pain following vasectomy. Br J Urol 1992;69:188-91. [Crossref] [PubMed]
- Sinaki M, Merritt JL, Stillwell GK. Tension myalgia of the pelvic floor. Mayo Clin Proc 1977;52:717-22. [PubMed]
- Chen R, Nickel JC. Acupuncture ameliorates symptoms in men with chronic prostatitis/chronic pelvic pain syndrome. Urology 2003;61:1156-9; discussion 1159. [Crossref] [PubMed]
- Wagenlehner FM, van Till JW, Magri V, et al. National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) symptom evaluation in multinational cohorts of patients with chronic prostatitis/chronic pelvic pain syndrome. Eur Urol 2013;63:953-9. [Crossref] [PubMed]
- Strom KH, Levine LA. Microsurgical denervation of the spermatic cord for chronic orchialgia: long-term results from a single center. J Urol 2008;180:949-53. [Crossref] [PubMed]
- Tan WP, Levine LA. An overview of the management of post-vasectomy pain syndrome. Asian J Androl 2016;18:332-7. [Crossref] [PubMed]
- Sinclair AM, Miller B, Lee LK. Chronic orchialgia: consider gabapentin or nortriptyline before considering surgery. Int J Urol 2007;14:622-5. [Crossref] [PubMed]
- Dworkin RH, O'Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc 2010;85:S3-14. [Crossref] [PubMed]
- Rovner E, Propert KJ, Brensinger C, et al. Treatments used in women with interstitial cystitis: the interstitial cystitis data base (ICDB) study experience. The Interstitial Cystitis Data Base Study Group. Urology 2000;56:940-5. [Crossref] [PubMed]
- Bharucha AE, Lee TH. Anorectal and Pelvic Pain. Mayo Clin Proc 2016;91:1471-86. [Crossref] [PubMed]
- Chen Y, Wu X, Liu J, et al. Effects of a 6-month course of tamsulosin for chronic prostatitis/chronic pelvic pain syndrome: a multicenter, randomized trial. World J Urol 2011;29:381-5. [Crossref] [PubMed]
- Nickel JC, Krieger JN, McNaughton-Collins M, et al. Alfuzosin and symptoms of chronic prostatitis-chronic pelvic pain syndrome. N Engl J Med 2008;359:2663-73. [Crossref] [PubMed]
- Cornel EB, van Haarst EP, Schaarsberg RW, et al. The effect of biofeedback physical therapy in men with Chronic Pelvic Pain Syndrome Type III. Eur Urol 2005;47:607-11. [Crossref] [PubMed]
- Farrell MR, Dugan SA, Levine LA. Physical therapy for chronic scrotal content pain with associated pelvic floor pain on digital rectal exam. Can J Urol 2016;23:8546-50. [PubMed]
- Tu XA, Zhao L, Zhao LY, et al. Microsurgical vasovasostomy for the treatment of intractable chronic scrotal pain after vasectomy. Asian J Androl 2013;15:850-1. [Crossref] [PubMed]
- Nangia AK, Myles JL, Thomas AJ. Vasectomy reversal for the post-vasectomy pain syndrome: a clinical and histological evaluation. J Urol 2000;164:1939-42. [Crossref] [PubMed]
- Morley C, Rogers A, Zaslau S. Post-vasectomy pain syndrome: clinical features and treatment options. Can J Urol 2012;19:6160-4. [PubMed]
- Abrol N, Panda A, Kekre NS. Painful varicoceles: Role of varicocelectomy. Indian J Urol 2014;30:369-73. [Crossref] [PubMed]
- Costabile RA, Hahn M, McLeod DG. Chronic orchialgia in the pain prone patient: the clinical perspective. J Urol 1991;146:1571-4. [PubMed]
- Ronka K, Vironen J, Kokki H, et al. Role of orchiectomy in severe testicular pain after inguinal hernia surgery: audit of the Finnish Patient Insurance Centre. Hernia 2015;19:53-9. [Crossref] [PubMed]
- Benson JS, Abern MR, Larsen S, et al. Does a positive response to spermatic cord block predict response to microdenervation of the spermatic cord for chronic scrotal content pain? J Sex Med 2013;10:876-82. [Crossref] [PubMed]
- Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med 1987;16:752-7. [Crossref] [PubMed]
- Gordon J, Rifenburg RP. Spermatic Cord Anesthesia Block: An Old Technique Re-imaged. West J Emerg Med 2016;17:811-3. [Crossref] [PubMed]
- Oomen RJ, Witjens AC, van Wijck AJ, et al. Prospective double-blind preoperative pain clinic screening before microsurgical denervation of the spermatic cord in patients with testicular pain syndrome. Pain 2014;155:1720-6. [Crossref] [PubMed]
- Devine CJ Jr, Schellhammer PF. The use of microsurgical denervation of the spermatic cord for orchialgia. Trans Am Assoc Genitourin Surg 1978;70:149-51. [PubMed]
- Levine LA, Matkov TG, Lubenow TR. Microsurgical denervation of the spermatic cord: a surgical alternative in the treatment of chronic orchialgia. J Urol 1996;155:1005-7. [Crossref] [PubMed]
- Cassidy DJ. Early experience with microsurgical spermatic cord denervation for chronic orchialgia at a Canadian centre. Can Urol Assoc J 2015;9:e72-4. [Crossref] [PubMed]
- Levine LA. Microsurgical denervation of the spermatic cord. J Sex Med 2008;5:526-9. [Crossref] [PubMed]


