Technical aspects of sperm DNA fragmentation testing, methods to select sperm with low DNA fragmentation, and usefulness of redox potential measurement in male infertility
We read with interest the thought provoking commentary by Dr. Henkel (1) in response to the practice recommendations by Agarwal et al. (2). Dr. Henkel appropriately pointed out the shortcomings of conventional semen analysis in predicting the outcome of assisted reproductive technologies (ART), to which we concur as discussed extensively elsewhere (3-7). The author also recognized the role of sperm DNA fragmentation (SDF) tests as complementary to semen analysis. Moreover, Dr. Henkel discussed important technical issues concerning SDF tests and argued that the guideline by Agarwal et al. (2) could have been more detailed with regards to the technical differences among the various tests. Lastly, Dr. Henkel suggested the use of tests to measure redox potential as an alternative to SDF testing. Here, in our response to his commentary, we wish to elaborate upon the (I) technical aspects of SDF testing, (II) methods to select sperm with low DNA fragmentation for intracytoplasmic sperm injection (ICSI), and (III) usefulness of redox potential measurement in male infertility.
Although clinicians would like to have a definitive diagnostic test for male infertility with precise cutoff level above or below which they could make a decision regarding diagnosis and management, it is unlikely that the perfect test will be available in the near future (8). The reasons stem for the complexity of semen as it consists of a heterogeneous population of spermatozoa produced over approximately 70–80 days. Furthermore, spermatozoa are mixed with and diluted by fluid secreted from accessory glands. As a result, semen quality is governed by (I) the state of testicular sperm production (II) epididymal transit, and (III) activity of accessory glands (9). To complicate matters, laboratory analysis of semen is subjected to variability due to operational and technical factors and ejaculatory abstinence period (4,10).
Despite recognizing the current limitations of SDF testing, we ponder that conventional semen analysis, i.e., sperm concentration, motility, and morphology, does not live up to the level of scrutiny or rigor that is often asked from SDF testing (8). In fact, routine semen analysis lacks the power to discriminate between fertile and infertile men, and rarely provides a clear path for management, unless the results are in extremely abnormal range (3,4,8). Given the overwhelming evidence of a negative association between SDF and male infertility and impaired reproductive outcomes, both natural and assisted, it seems sound to include SDF as an integral test of semen analysis to improve its predictive diagnostic value (6,8,11,12).
We agree with Dr. Henkel that SDF testing methods are not interchangeable. Indeed, SDF testing does measure different aspects of DNA, although these aspects are interrelated to a greater or lesser extent by the properties of the DNA molecule (13). The ideal method to measure SDF is still to be determined, so any decision to consider SDF testing should take into account the limitations of testing methods and the possible benefits for clinical outcomes, as highlighted in our recent publications (2,11,13-16). Therefore, suggesting the use of a specific assay to measure SDF is not scientifically correct until a gold standard method is established. Nevertheless, it is essential that a reliable SDF assay with a validated threshold is used (6,8).
Dr. Henkel ponders that more studies have to be conducted to widen and clarify the scope of sperm DNA testing. In fact, the number of studies published in this regard is increasing steadily; the reader will be astonished to learn that more than 2,000 articles about sperm DNA damage are indexed in PubMed, half of which was published in the last 5 years. As far as the tests to select viable sperm with reduced SDF for ART are concerned, the literature is also rich in studies comparing different methods. In a recent report, we examined some of these tests and concluded that they in general reduce the amount of sperm with SDF in the selected specimen, but none of them, alone or combined, can completely remove sperm with SDF (Table 1) (17). However, a recent report indicated that among motile sperm organelle morphology examination (IMSI), physiological ICSI (PICSI) using hyaluronic acid-selected spermatozoa, frequent ejaculation, and testicular sperm, the latter was more advantageous with regards to ICSI live birth rates (18).
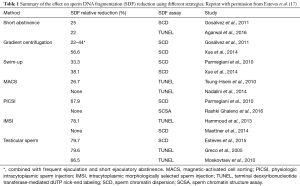
Full table
Lastly, Dr. Henkel pointed out that given oxidative stress is a major contributor to SDF, it would be beneficial and possibly easier to determine the redox potential either in semen or in the serum to determine that OS is causing SDF. Indeed, oxidation-reduction potential (ORP) has been advocated as a new measure of oxidative stress, as it reflects the balance between the total available oxidants and reductants in a given specimen (19). ORP seems to be a simple alternative to multiple individual markers of OS such as ROS (chemiluminescence), antioxidants (total antioxidant capacity and individual enzymatic and nonenzymatic antioxidants) and lipid peroxidation (MDA) (19-22). In addition to real-time measurement of redox capacity by ORP in semen specimens, other qualitative methods to measure OS have been investigated. In a recent multi-center study, we analyzed nitroblue tetrazolium reactivity in human semen as a potential marker of OS (23). Despite promising results, the potential clinical value of the newly aforementioned markers of OS warrants further validation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Henkel R. Clinical utility of sperm DNA fragmentation testing: A commentary. Transl Androl Urol 2017;6:S632-5.
- Agarwal A, Majzoub A, Esteves SC, et al. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol 2016;5:935-50. [Crossref] [PubMed]
- Esteves SC, Zini A, Aziz N, et al. Critical appraisal of World Health Organization's new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology 2012;79:16-22. [Crossref] [PubMed]
- Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol 2014;40:443-53. [Crossref] [PubMed]
- Esteves SC, Hamada A, Kondray V, et al. What every gynecologist should know about male infertility: an update. Arch Gynecol Obstet 2012;286:217-29. [Crossref] [PubMed]
- Esteves SC, Sharma RK, Gosálvez J, et al. A translational medicine appraisal of specialized andrology testing in unexplained male infertility. Int Urol Nephrol 2014;46:1037-52. [Crossref] [PubMed]
- Esteves SC. A clinical appraisal of the genetic basis in unexplained male infertility. J Hum Reprod Sci 2013;6:176-82. [Crossref] [PubMed]
- Gosálvez J, Lopez-Fernandez C, Fernandez JL, et al. Unpacking the mysteries of sperm DNA fragmentation: Ten frequently asked questions. J Reprod Biotechnol Fertil 2015;4:1-16. [Crossref]
- Agarwal A, Esteves SC, Gupta S, et al. Urology 2016;94:109-10. Author Reply. [Crossref] [PubMed]
- Agarwal A, Gupta S, Du Plessis S, et al. Abstinence time and its impact on basic and advanced semen parameters. Urology 2016;94:102-10. [Crossref] [PubMed]
- Agarwal A, Cho CL, Esteves SC. Should we evaluate and treat sperm DNA fragmentation? Curr Opin Obstet Gynecol 2016;28:164-71. [Crossref] [PubMed]
- Esteves SC. Novel concepts in male factor infertility: clinical and laboratory perspectives. J Assist Reprod Genet 2016;33:1319-35. [Crossref] [PubMed]
- Esteves SC, Roque M, Garrido N. Use of testicular sperm for intracytoplasmic sperm injection in men with high sperm DNA fragmentation: a SWOT analysis. Asian J Androl 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Esteves SC, Sánchez-Martín F, Sánchez-Martín P, et al. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril 2015;104:1398-405. [Crossref] [PubMed]
- Cho CL, Esteves SC, Agarwal A. Novel insights into the pathophysiology of varicocele and its association with reactive Oxygen species and sperm DNA fragmentation. Asian J Androl 2016;18:186-93. [Crossref] [PubMed]
- Majzoub A, Esteves SC, Gosálvez J, et al. Specialized sperm function tests in varicocele and the future of andrology laboratory. Asian J Androl 2016;18:205-12. [Crossref] [PubMed]
- Esteves SC, Agarwal A, Majzoub A. Comparison of strategies to reduce sperm DNA fragmentation in couples undergoing ICSI. Transl Androl Urol 2017;6:S570-3. [Epub ahead of print].
- Bradley CK, Mcarthur SJ, Gee AJ, et al. Intervention improves assisted conception intracytoplasmic sperm injection outcomes for patients with high levels of sperm DNA fragmentation: a retrospective analysis. Andrology 2016;4:903-10. [Crossref] [PubMed]
- Ahmad G, Agarwal A, Esteves SC, et al. Ascorbic acid reduces redox potential in human spermatozoa subjected to heat-induced oxidative stress. Andrologia 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Agarwal A, Roychoudhury S, Sharma RA, et al. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: clinical utility in male factor infertility. Reprod Biomed Online 2017;34:48-57. [Crossref] [PubMed]
- Agarwal A, Wang SM. Clinical Relevance of Oxidation-Reduction Potential in the Evaluation of Male Infertility. Urology 2017;104:84-9. [Crossref] [PubMed]
- Agarwal A, Sharma R, Roychoudhury S, et al. MiOXSYS: a novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertil Steril 2016;106:566-73.e10. [Crossref] [PubMed]
- Gosálvez J, Coppola L, Fernández JL, et al. Multi-centre assessment of nitroblue tetrazolium reactivity in human semen as a potential marker of oxidative stress. Reprod Biomed Online 2017;34:513-21. [Crossref] [PubMed]


