Surveillance strategies in the management of penile cancer
Introduction
Penile cancer is uncommon, accounting for an estimated 2,120 new cases and 360 deaths in 2017 (1). Because of the rarity of the disease, there is limited level I evidence on management approaches. Although surgery is the mainstay of treatment of both the primary and regional nodal metastatic disease, conservative treatment approaches, such as laser ablation or local excision, are often employed for low risk disease (Tis, T1, Grades 1 and 2) and occasionally for high risk disease (T2) (2-4). Thus, the surveillance strategy largely depends on the disease severity and treatment administered. Given that recurrences may be curable if detected early, close follow up is recommended (5). Herein, we present a review of general surveillance principles and strategies for the penis, the inguinal and pelvic group of lymph nodes, and potential distant recurrence sites. We also examine the evidence for utilizing imaging modalities as adjuncts to clinical evaluation. Lastly, we present a summary surveillance schedule for penile cancer.
General principles of surveillance
Penis
The risk of recurrence after primary therapy for penile cancer is grade and stage dependent. Therefore, a grade and stage appropriate surveillance strategy is imperative in all patients including those treated with organ-preservation, such as phallus-sparing surgeries (e.g., local excision, glansectomy and distal corporectomy), laser ablation, topical therapies and radiation therapy. The incidence of local recurrence after organ-preserving treatment of the primary tumor is as high as 50% within 5 years depending on the stage of the disease (2,3). In a large series of patients with invasive (pT1–T4) disease, the 5-year cumulative incidence of local recurrence was 27% for those treated with penile preservation versus 3.8% for those treated with partial penectomy; despite this though, there was no difference in cancer-specific survival (4). Other studies, however, reported that local recurrence after partial penectomy portends poor prognosis (4,6). The incidence of local recurrence with partial or total penectomy is generally low (0–7%) but may approach 50% in patients treated with more conservative approaches (7-11). However, if local recurrence is detected and treated early, the cancer specific survival may be unaffected (12). Thus, clinical evaluation—history and physical examination—aimed at early detection of recurrent lesions at the site of the treated primary tumor or elsewhere on the penis should be performed. Palpation of the penis provides an important initial assessment of the extent of the disease (13). Imaging modalities such as magnetic resonance imaging (MRI) and ultrasound (US) may be used as adjuncts to the physical exam to determine the extent of disease and assist with surgical planning. Evidence for their routine utilization is inconclusive and should be used at the discretion of the treating physician (14-18). Any suspicious penile lesion or abnormality detected during surveillance warrants further evaluation with an incisional biopsy for adequate histopathological evaluation (17).
Inguinal lymph node
As with the primary tumor, the surveillance protocol for the inguinal lymph nodes depends on the findings and management at the time of initial diagnosis. The approach to the inguinal nodes is dictated by the pathological stage and grade of the primary penile tumor (19-22). A detailed discussion of measures proposed to manage the inguinal nodes is beyond the scope of this article. Briefly, patients with low risk of lymph node metastasis or recurrence, such as those with in situ or Ta disease, can be managed conservatively. Although some patients have clinically negative inguinal lymph nodes at diagnosis (i.e., impalpable and negative imaging), they may harbor micro-metastatic disease and risk development of a clinically evident recurrence in the inguinal lymph nodes. In a review by Busby and Pettaway, 6% of patients with T1, grades 1–2 primary tumors had lymph node metastasis at the time of bilateral inguinal lymph node dissection (ILND) (23). Hence, while this group of patients may be observed without upfront ILND, early detection of nodal recurrence is critical to avoid a poor outcome (24).
Conversely, patients with ≥ T2 disease have a 59% risk of inguinal lymph node metastasis (23). Although dynamic sentinel node biopsy (DSNB) is being performed at some institutions for high risk patients, bilateral ILND (open or endoscopic) remains the treatment of choice at most North American tertiary care centers due in large part based on the paucity of expertise using this diagnostic modality (20,25-32). A rigorous follow up schedule, especially in the first 2 years, should be implemented in patients with negative DSNB given the relatively high false negative rates (33). In addition, patients with non-visualization following DSNB but with high risk disease (≥ T1G2) should be considered for repeat DNSB given the risk of metastatic disease (34). While a modified template ILND is recommended as a reasonable alternative to standard lymph node dissection in high risk patients, some may choose observation (35). Additionally, patients who underwent a modified template ILND with negative results still harbor the risk of inguinal recurrences; up to 15% within the first 2 years of follow up (36,37). Patients with pathologically node positive (pN+) disease may have poorer cancer specific survival compared to men with pathologically node negative (pN0) disease (35). Thus, continued long term surveillance is recommended in all patients given the high risk of recurrence—a predictor of poor overall survival (38).
Advanced and systemic disease
Locally advanced or widely metastatic primary penile cancer is fairly uncommonly seen within the United States but is unfortunately associated with a high cancer specific mortality rate. Neoadjuvant chemotherapy followed by surgery may render some patients disease-free, though, with a 5-year survival up to 50% (39-42). Regardless of the treatment offered, a more intense surveillance schedule is warranted when compared to low-risk disease, adding periodic cross-sectional imaging as an adjunct to the physical examination.
Surveillance strategies
History and physical examination
A history and physical examination should be performed on patients treated for penile cancer during surveillance. In addition to evaluations by a physician, it is crucial to teach patients how to perform monthly self-exams of the penis (if a penile conserving treatment was performed) and of the inguinal region. Patients should alert their provider if they identify any concerning findings. Recurrence has been reported in distant and uncommon sites such as the brain, cervical lymph nodes, lungs, prostate, and perineum even in patients with pN0 disease, highlighting the importance of a comprehensive clinical evaluation (43,44).
Imaging modalities
Enhanced imaging techniques (e.g., MRI and US) serve as adjuncts to clinical assessment of the penis. The role of MRI and US was evaluated in the staging of primary penile cancer with conflicting results (14-16,18). Although cross-sectional imaging may be indicated in the primary tumor setting, physical examination alone performs just as well or even superior to MRI in a head-to-head comparison (45). Inducing an artificial erection, however, may improve the performance of MRI in evaluating the depth of invasion of penile tumors (46,47). Regardless of the MRI or US finding, any suspicious penile lesion or abnormality detected during surveillance warrants further evaluation with an incisional biopsy for adequate histopathological evaluation (17).
Ultrasound may be useful in evaluating the groin for recurrence. Though with limited sensitivity and specificity, US may detect evidence of metastasis in inguinal lymph nodes and may guide fine-needle aspiration (FNA) for cytological or histological diagnosis (48-50). Especially in the obese or in patients with a history of prior inguinal surgery who are at risk metastasis, CT or MRI scans of the abdomen and pelvis should be performed periodically to detect recurrence in inguinal and pelvic nodes. Patients with positive inguinal lymph nodes at diagnosis are at higher risk of pelvic lymph node involvement and thus warrant close surveillance (51). Furthermore, PET-CT may improve detection of nodal recurrence (52). The role of lymphotropic nano-particle-enhanced MRI using ferumoxtran-10 is promising but requires further evaluation (53). Other sites, such as the chest/lungs, bones and brain, may be imaged depending on findings from the clinical assessment or other imaging studies. Chest X-ray may be used to evaluate the lungs, and any abnormality can be evaluated further with a chest CT.
Molecular diagnostics and biomarkers
Currently, there is no available penile cancer biomarker to detect disease recurrence or response to treatment. However, certain markers have been associated with recurrence or poor prognosis. For example, HPV expression; lack of p16 expression; nuclear accumulation of p53; EGFR, MYC and CCND1 amplifications were found to be associated with poorer overall survival (54-57). The evidence for association of Ki67 expression with poor outcome is inconclusive (57-59). Testing of these markers in the primary tumor may be considered in patients at higher risk of recurrence to guide surveillance strategies or perhaps inform decision-making for prophylactic lymphadenectomy and/or adjuvant treatment.
Intensity and duration of surveillance
In general, surveillance after a diagnosis of penile cancer is life-long as these patients have the potential to develop recurrent disease or a new primary tumor at any point along their course. The intensity of follow-up, however, is dependent on the pathological stage and grade of disease and the treatment performed (60). Sanchez-Ortiz and Pettaway proposed a risk-adapted strategy for follow-up (61). They proposed three target groups for surveillance, namely: (I) patients treated with phallus-sparing strategies; (II) patients with high-risk primary tumors (pT2–3, grade 3, vascular invasion) and clinically negative inguinal lymph nodes without lymphadenectomy performed; and (III) patients with positive lymph nodes at lymphadenectomy. Two follow up schedules were recommended, a rigorous follow up for patients at high risk of local or regional recurrence and a less rigorous schedule for patients with a low risk of recurrence (Tables 1,2).

Full table
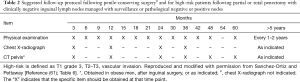
Full table
Most guidelines, including the European Association of Urology© and the National Comprehensive Cancer Network© guidelines recommend follow-up visits every 3 months in the first 2 years, every 6 months for years 3 to 5, and annually thereafter (20,22,62). These recommendations are based on an observational study that showed that 92% of the recurrences occurred within the first 5 years after primary treatment (63). These recommendations, though, do not take disease stage and grade into account. Life-long follow-up is recommended because recurrence can occur beyond 5 years (64). Most surveillance strategies proposed, though, end after year 10 from primary treatment. It is reasonable to refer patients to their primary care provider with instructions for yearly evaluations moving forward after year 10.
Conclusions
Penile cancer is a rare disease, with certain patients having a high risk of recurrence in the inguinal and pelvic lymph nodes. Because of the morbidity and mortality associated with recurrences, a risk-adjusted surveillance strategy geared towards early detection and treatment is recommended. Physical examination is the single most important component of surveillance, but imaging modalities may be used as adjuncts.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Windahl T, Andersson SO. Combined laser treatment for penile carcinoma: results after long-term followup. J Urol 2003;169:2118-21. [Crossref] [PubMed]
- Veeratterapillay R, Teo L, Asterling S, et al. Oncologic Outcomes of Penile Cancer Treatment at a UK Supraregional Center. Urology 2015;85:1097-101. [Crossref] [PubMed]
- Djajadiningrat RS, van Werkhoven E, Meinhardt W, et al. Penile sparing surgery for penile cancer-does it affect survival? J Urol 2014;192:120-5. [Crossref] [PubMed]
- Montie JE. Follow-up after penectomy for penile cancer. Urol Clin North Am 1994;21:725-7. [PubMed]
- Lont AP, Gallee MP, Meinhardt W, et al. Penis conserving treatment for T1 and T2 penile carcinoma: clinical implications of a local recurrence. J Urol 2006;176:575-80; discussion 580. [Crossref] [PubMed]
- Horenblas S, van Tinteren H, Delemarre JF, et al. Squamous cell carcinoma of the penis. II. Treatment of the primary tumor. J Urol 1992;147:1533-8. [Crossref] [PubMed]
- McLean M, Akl AM, Warde P, et al. The results of primary radiation therapy in the management of squamous cell carcinoma of the penis. Int J Radiat Oncol Biol Phys 1993;25:623-8. [Crossref] [PubMed]
- Windahl T, Hellsten S. Laser treatment of localized squamous cell carcinoma of the penis. J Urol 1995;154:1020-3. [Crossref] [PubMed]
- Bandieramonte G, Santoro O, Boracchi P, et al. Total resection of glans penis surface by CO2 laser microsurgery. Acta Oncol 1988;27:575-8. [Crossref] [PubMed]
- Kroon BK, Horenblas S, Nieweg OE. Contemporary management of penile squamous cell carcinoma. J Surg Oncol 2005;89:43-50. [Crossref] [PubMed]
- Lerner SE, Jones JG, Fleischmann J. Management of recurrent penile cancer following partial or total penectomy. Urol Clin North Am 1994;21:729-37. [PubMed]
- Horenblas S, van Tinteren H, Delemarre JF, et al. Squamous cell carcinoma of the penis: accuracy of tumor, nodes and metastasis classification system, and role of lymphangiography, computerized tomography scan and fine needle aspiration cytology. J Urol 1991;146:1279-83. [Crossref] [PubMed]
- Horenblas S, Kröger R, Gallee MP, et al. Ultrasound in squamous cell carcinoma of the penis; a useful addition to clinical staging? A comparison of ultrasound with histopathology. Urology 1994;43:702-7. [Crossref] [PubMed]
- Agrawal A, Pai D, Ananthakrishnan N, et al. Clinical and sonographic findings in carcinoma of the penis. J Clin Ultrasound 2000;28:399-406. [Crossref] [PubMed]
- Yamashita T, Ogawa A. Ultrasound in penile cancer. Urol Radiol 1989;11:174-7. [Crossref] [PubMed]
- Letendre J, Saad F, Lattouf JB. Penile cancer: what’s new? Curr Opin Support Palliat Care 2011;5:185-91. [Crossref] [PubMed]
- de Kerviler E, Ollier P, Desgrandchamps F, et al. Magnetic resonance imaging in patients with penile carcinoma. Br J Radiol 1995;68:704-11. [Crossref] [PubMed]
- Moses KA, Winer A, Sfakianos JP, et al. Contemporary management of penile cancer: greater than 15 year MSKCC experience. Can J Urol 2014;21:7201-6. [PubMed]
- Pizzocaro G, Algaba F, Horenblas S, et al. EAU penile cancer guidelines 2009. Eur Urol 2010;57:1002-12. [Crossref] [PubMed]
- Thuret R, Sun M, Lughezzani G, et al. A contemporary population-based assessment of the rate of lymph node dissection for penile carcinoma. Ann Surg Oncol 2011;18:439-46. [Crossref] [PubMed]
- Spiess PE. National Comprehensive Cancer Network. New treatment guidelines for penile cancer. J Natl Compr Canc Netw 2013;11:659-62. [Crossref] [PubMed]
- Busby JE, Pettaway CA. What’s new in the management of penile cancer? Curr Opin Urol 2005;15:350-7. [Crossref] [PubMed]
- Mistry T, Jones RW, Dannatt E, et al. A 10-year retrospective audit of penile cancer management in the UK. BJU Int 2007;100:1277-81. [Crossref] [PubMed]
- Tanis PJ, Lont AP, Meinhardt W, et al. Dynamic sentinel node biopsy for penile cancer: reliability of a staging technique. J Urol 2002;168:76-80. [Crossref] [PubMed]
- Kroon BK, Horenblas S, Estourgie SH, et al. How to avoid false-negative dynamic sentinel node procedures in penile carcinoma. J Urol 2004;171:2191-4. [Crossref] [PubMed]
- Spiess PE, Izawa JI, Bassett R, et al. Preoperative lymphoscintigraphy and dynamic sentinel node biopsy for staging penile cancer: results with pathological correlation. J Urol 2007;177:2157-61. [Crossref] [PubMed]
- Leijte JA, Kroon BK, Valdés Olmos RA, et al. Reliability and safety of current dynamic sentinel node biopsy for penile carcinoma. Eur Urol 2007;52:170-7. [Crossref] [PubMed]
- Ficarra V, Galfano A. Should the dynamic sentinel node biopsy (DSNB) be considered the gold standard in the evaluation of lymph node status in patients with penile carcinoma? Eur Urol 2007;52:17-9; discussion 20-1. [Crossref] [PubMed]
- Protzel C, Alcaraz A, Horenblas S, et al. Lymphadenectomy in the surgical management of penile cancer. Eur Urol 2009;55:1075-88. [Crossref] [PubMed]
- Josephson DY, Jacobsohn KM, Link BA, et al. Robotic-assisted endoscopic inguinal lymphadenectomy. Urology 2009;73:167-70; discussion 170-1. [Crossref] [PubMed]
- Marconnet L, Rigaud J, Bouchot O. Long-term followup of penile carcinoma with high risk for lymph node invasion treated with inguinal lymphadenectomy. J Urol 2010;183:2227-32. [Crossref] [PubMed]
- Lont AP, Horenblas S, Tanis PJ, et al. Management of clinically node negative penile carcinoma: improved survival after the introduction of dynamic sentinel node biopsy. J Urol 2003;170:783-6. [Crossref] [PubMed]
- Sahdev V, Albersen M, Christodoulidou M, et al. The management of non-visualisation following dynamic sentinel lymph node biopsy for squamous cell carcinoma of the penis. BJU Int 2017;119:573-8. [Crossref] [PubMed]
- Djajadiningrat RS, Graafland NM, van Werkhoven E, et al. Contemporary management of regional nodes in penile cancer-improvement of survival? J Urol 2014;191:68-73. [Crossref] [PubMed]
- d’Ancona CA, de Lucena RG, Querne FA, et al. Long-term followup of penile carcinoma treated with penectomy and bilateral modified inguinal lymphadenectomy. J Urol 2004;172:498-501; discussion 501. [Crossref] [PubMed]
- Lopes A, Rossi BM, Fonseca FP, et al. Unreliability of modified inguinal lymphadenectomy for clinical staging of penile carcinoma. Cancer 1996;77:2099-102. [Crossref] [PubMed]
- Rieken M, Djajadiningrat RS, Kluth LA, et al. Predictors of cancer-specific mortality after disease recurrence in patients with squamous cell carcinoma of the penis. Eur Urol 2014;66:811-4. [Crossref] [PubMed]
- Dickstein RJ, Munsell MF, Pagliaro LC, et al. Prognostic factors influencing survival from regionally advanced squamous cell carcinoma of the penis after preoperative chemotherapy. BJU Int 2016;117:118-25. [Crossref] [PubMed]
- Spiess PE, Horenblas S, Pagliaro LC, et al. Current concepts in penile cancer. J Natl Compr Canc Netw 2013;11:617-24. [Crossref] [PubMed]
- Wang J, Pettaway CA, Pagliaro LC. Treatment for Metastatic Penile Cancer After First-line Chemotherapy Failure: Analysis of Response and Survival Outcomes. Urology 2015;85:1104-10. [Crossref] [PubMed]
- Zou B, Han Z, Wang Z, et al. Neoadjuvant therapy combined with a BMP regimen for treating penile cancer patients with lymph node metastasis: a retrospective study in China. J Cancer Res Clin Oncol 2014;140:1733-8. [Crossref] [PubMed]
- Aita G, da Costa WH, de Cassio Zequi S, et al. Pattern of invasion is the most important prognostic factor in patients with penile cancer submitted to lymph node dissection and pathological absence of lymph node metastasis. BJU Int 2015;116:584-9. [Crossref] [PubMed]
- Pow-Sang MR, Ferreira U, Pow-Sang JM, et al. Epidemiology and natural history of penile cancer. Urology 2010;76:S2-6. [Crossref] [PubMed]
- Lont AP, Besnard AP, Gallee MP, et al. A comparison of physical examination and imaging in determining the extent of primary penile carcinoma. BJU Int 2003;91:493-5. [Crossref] [PubMed]
- Scardino E, Villa G, Bonomo G, et al. Magnetic resonance imaging combined with artificial erection for local staging of penile cancer. Urology 2004;63:1158-62. [Crossref] [PubMed]
- Petralia G, Villa G, Scardino E, et al. Local staging of penile cancer using magnetic resonance imaging with pharmacologically induced penile erection. Radiol Med 2008;113:517-28. [Crossref] [PubMed]
- Crawshaw JW, Hadway P, Hoffland D, et al. Sentinel lymph node biopsy using dynamic lymphoscintigraphy combined with ultrasound-guided fine needle aspiration in penile carcinoma. Br J Radiol 2009;82:41-8. [Crossref] [PubMed]
- Kroon BK, Horenblas S, Deurloo EE, et al. Ultrasonography-guided fine-needle aspiration cytology before sentinel node biopsy in patients with penile carcinoma. BJU Int 2005;95:517-21. [Crossref] [PubMed]
- Saisorn I, Lawrentschuk N, Leewansangtong S, et al. Fine-needle aspiration cytology predicts inguinal lymph node metastasis without antibiotic pretreatment in penile carcinoma. BJU Int 2006;97:1225-8. [Crossref] [PubMed]
- Lont AP, Kroon BK, Gallee MP, et al. Pelvic lymph node dissection for penile carcinoma: extent of inguinal lymph node involvement as an indicator for pelvic lymph node involvement and survival. J Urol 2007;177:947-52; discussion 952. [Crossref] [PubMed]
- Graafland NM, Leijte JA, Valdés Olmos RA, et al. Scanning with 18F-FDG-PET/CT for detection of pelvic nodal involvement in inguinal node-positive penile carcinoma. Eur Urol 2009;56:339-45. [Crossref] [PubMed]
- Tabatabaei S, Harisinghani M, McDougal WS. Regional lymph node staging using lymphotropic nanoparticle enhanced magnetic resonance imaging with ferumoxtran-10 in patients with penile cancer. J Urol 2005;174:923-7; discussion 927. [Crossref] [PubMed]
- Muneer A, Kayes O, Ahmed HU, et al. Molecular prognostic factors in penile cancer. World J Urol 2009;27:161-7. [Crossref] [PubMed]
- Lopes A, Bezerra AL, Pinto CA, et al. p53 as a new prognostic factor for lymph node metastasis in penile carcinoma: analysis of 82 patients treated with amputation and bilateral lymphadenectomy. J Urol 2002;168:81-6. [Crossref] [PubMed]
- McDaniel AS, Hovelson DH, Cani AK, et al. Genomic Profiling of Penile Squamous Cell Carcinoma Reveals New Opportunities for Targeted Therapy. Cancer Res 2015;75:5219-27. [Crossref] [PubMed]
- Zhu Y, Zhou XY, Yao XD, et al. The prognostic significance of p53, Ki-67, epithelial cadherin and matrix metalloproteinase-9 in penile squamous cell carcinoma treated with surgery. BJU Int 2007;100:204-8. [Crossref] [PubMed]
- Protzel C, Knoedel J, Zimmermann U, et al. Expression of proliferation marker Ki67 correlates to occurrence of metastasis and prognosis, histological subtypes and HPV DNA detection in penile carcinomas. Histol Histopathol 2007;22:1197-204. [PubMed]
- Stankiewicz E, Ng M, Cuzick J, et al. The prognostic value of Ki-67 expression in penile squamous cell carcinoma. J Clin Pathol 2012;65:534-7. [Crossref] [PubMed]
- Molina Escudero R, Herranz Amo F, Jara Rascón J, et al. Predictive factors for recurrence in clinically localized squamous cell carcinoma of the penis. Analisys of our case series. Arch Esp Urol 2011;64:525-32. [PubMed]
- Sánchez-Ortiz RF, Pettaway CA. Natural history, management, and surveillance of recurrent squamous cell penile carcinoma: a risk-based approach. Urol Clin North Am 2003;30:853-67. [Crossref] [PubMed]
- Van Poppel H, Watkin NA, Osanto S, et al. Penile cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi115-24. [Crossref] [PubMed]
- Leijte JA, Kirrander P, Antonini N, et al. Recurrence patterns of squamous cell carcinoma of the penis: recommendations for follow-up based on a two-centre analysis of 700 patients. Eur Urol 2008;54:161-8. [Crossref] [PubMed]
- Horenblas S, Newling DW. Local recurrent tumour after penis-conserving therapy. A plea for long-term follow-up. Br J Urol 1993;72:976. [Crossref] [PubMed]


