Etiologic classification, evaluation, and management of hematospermia
Introduction
Hematospermia (i.e., hemospermia or bloody ejaculate) is defined by the presence of blood in the semen. This medical condition has been documented for many centuries including in the writings of Hippocrates, Galen, Morgagni, etc. The etiology of hematospermia may be classified into inflammation, infection, ductal blockage, cyst formation, systemic conditions, tumors, vascular aberrations of accessory sex glands, and iatrogenic causes (1). Currently, the precise incidence of hematospermia is yet to be determined as most men do not frequently examine their semen, and there is a lack of reporting for consultation. Though the age range of those afflicted with hematospermia is commonly between 30 and 40 years, men over 40 have been observed to also present symptoms (2). We presently explore an up-to-date classification and catalog of etiology with associating manifestation of hematospermia. We also review the clinical evaluation and management options of hematospermia.
Anatomy of the ejaculatory apparatus
The ejaculatory apparatus is composed of the seminal vesicles, vas deferens, and ejaculatory ducts. The vas deferens rises from within the scrotal walls, crosses the inguinal canal, and inserts into the internal inguinal ring to enter the extraperitoneal space of the pelvis. From there, it inferomedially arches toward the bladder base where it ends as a tangled mass known as the ampulla of the vas deferens. The ampulla is medial to the seminal vesicles, causing them to merge at the base of the prostate to form the ejaculatory duct. The ejaculatory duct enters the glandular portion of the prostate, forming a prostatic capsule. The prostatic capsule traverses the verumontanum and continues to meet with the muscular fibers of Denonvilliers’ fascia, the structure responsible for peristalsis and sphincteric function of the ejaculatory apparatus. The ejaculatory duct is present within the central zone of the prostate and ends in the posterior urethra. The posterior urethra is lateral and proximal to the verumontanum. The seminal vesicles are glands usually exceeding 2.5 cm in length with anteroposterior dimensions between 0.7 and 1.5 cm, having the function of holding liquid to mix with sperm to form semen (3).
Physiology of emission and ejaculation
The stages of human sexual response are a series of phases that include desire, excitation, orgasm, and resolution. Emission of seminal fluid secretions from sex glands and testes is followed by expulsion, where a mixture of spermatozoa, enzymes, lipids, sugars, and oligo-elements are secreted into the posterior urethra by phasic contractions by the glands and ducts as well as pelvic-perineal striated muscle contractions. Orgasm is a neuropsychophysiological occurrence that concludes the ejaculatory response (4). During Phase I (i.e., erection), the urethra, which is usually a wide and curved tube, becomes a straight canal for fluid. The corpus spongiosum fills with blood, which constricts the perineal musculature and increases pressure within the tube. The periurethral Littré glands also begin secretion to ease the transport of semen. Phase II is defined as the orgasm and emission phase, which occurs almost simultaneously, as spermatozoa and plasma travel from production sites into the urethra. The smooth muscle contractions are initiated in the ductuli efferentes, which then affect the Cowper’s glands, prostate, ampulla, and seminal vesicles. In phase III, the pressure increases even further by the loading of semen into the posterior urethra. There is a small chamber near the distal tip of the urethra to collect semen. Then, it quickly transitions to the final stage, expulsion, where the contraction ejects the ejaculate out of the penis (5). Hematospermia often becomes an issue by creating anxiety in the affected person, or by signaling an internal problem that must be seriously evaluated by the evaluating physician.
Etiology
The etiology of hematospermia can be categorized into the following ten categories based on the pathophysiological mechanisms of hematospermia: (I) inflammatory; (II) infectious; (III) lithiasis; (IV) cystic; (V) obstructive; (VI) tumoral; (VII) vascular; (VIII) traumatic; (IX) iatrogenic; and (X) systemic origin (see Table 1). Alternatively, the etiology of hematospermia can also be subclassified based on anatomical origin, namely the prostate, bladder, spermatic cord, seminal vesicles, or epididymis.
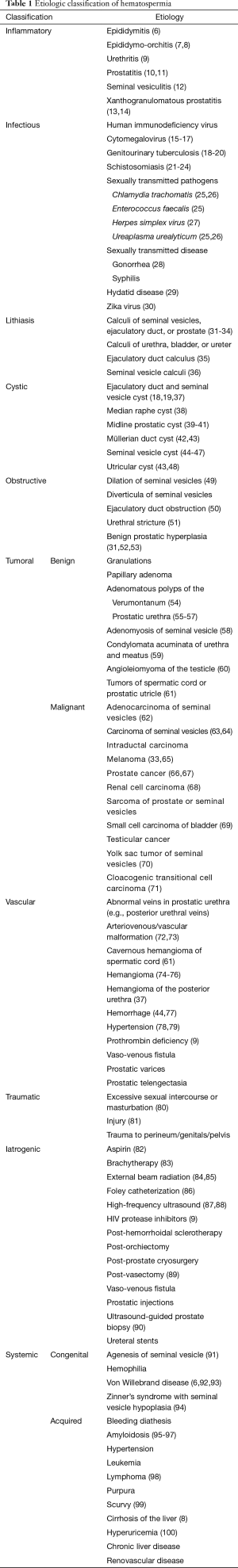
Full table
Prostate
Prostatitis
Enterococcus faecalis is a very commonly associated organism with chronic bacterial prostatitis. Prostatitis-associated hematospermia can be sub-classified into chronic bacterial prostatitis, chronic nonbacterial prostatitis, asymptomatic prostatitis, and prostatodynia. Patients having hematospermia with prostatitis have been associated with a poorer quality of life compared to prostatitis patients without hematospermia (10).
Xanthogranulomatous prostatitis
Xanthogranulomatous prostatitis is an unusual, nonspecific, yet benign inflammatory process of the prostate gland. It is a highly rare subtype of a common granulomatous prostatitis which is characterized by its classic histological feature: the presence of granuloma (13). Although the precise pathogenesis of this rare clinical entity is not yet known, it is believed to be caused by a blockage of prostatic ducts leading to stasis of prostate gland secretions, subsequently resulting in an inflammator response (13,14). Although hematospermia is reported as an accompanying symptom of xanthogranulomatous prostatitis, Pastore et al. concluded hematospermia as the presenting symptom in 40% of the cases (13). Thus, it is vital for physicians to be familiar with this rare clinical entity for a proper and timely diagnosis of the underlying condition and prompt treatment.
Prostate cancer
Han et al. found evidence that there is an increased risk of prostate cancer for people with hematospermia. However, hematospermia is exceedingly rare at an occurrence of 0.5% in patients with prostate cancer (101). In a separate study examining 302 individuals with prostate cancer, 45.3% of patients had hematospermia (66).
Bladder
Bladder tumor
Although rare, hematospermia has been recorded to be a symptom of a small cell bladder carcinoma. In a study, the average tumor size was 5.1 cm, rising toward the lateral bladder wall and fundus, and in some cases rising from the bladder diverticulum or urachal remnant (69).
Seminal vesicles
Adenocarcinoma
Adenocarcinoma is a rare and challenging diagnosis of hematospermia, as the adenocarcinoma frequently spreads to the surrounding areas such as the prostate. Tumor cells are present with papillary and glandular masses in the mass lesion filling the seminal vesicles (62). In a case study, a patient who had advanced testicular cancer presented with a severe antineutrophil cytoplasmic antibody vasculitis when he was diagnosed with metastatic cancer of the seminal vesicles. This demonstrates the possibility of developing autoimmune vasculitis in association with adenocarcinoma of the seminal vesicles (102).
Amyloidosis
Amyloidosis causes thickening of the seminal vesicle wall thereby narrowing the vesicular lumen (96). Recurrent or random episodes of hematospermia are known to occur simultaneously with seminal vesicle amyloidosis (96).
Epididymis
Epididymitis
Otherwise referred to as epididymo-orchitis or orchitis, inflammation of the epididymis can be caused by an infectious agent such as Chlamydia trachomatis and Neisseria gonorrhoeae. Dissemination of the organism of infection can spread through the bloodstream or by direct contact with a focus in the epididymis. The inflammation from epididymitis can cause hematospermia (6).
Tumors, lesions, and masses
Condylomata acuminata of urethra and meatus
Condylomata acuminata of urethra and meatus, also known as genital warts, are benign proliferative lesions produced by the human papillomavirus, usually of the types 6 and 11. About 20% of all genital warts occur in the urethra and external meatus of the urethra. Although it is not common, hematospermia can occur with condylomata (59).
Additionally, hematospermia can result due to dilation of the ejaculatory duct and seminal vesicle cysts (103,104). Transrectal ultrasonography (TRUS) can detect ejaculatory duct and seminal vesicle cysts, which may be congenital or secondary (22,23,103,104).
Hemangioma
Posterior urethral hemangiomas are benign vascular tumors that are believed to originate from unipotent angioblastic cells that do not successfully develop into normal blood vessels. Urethral hemangiomas are usually present between the verumontanum and the external urathral sphincter, causing pressure in the high venous area and thereby forming hemangioma. Hemangiomas are the spaces that contain blood and thrombi. Classifications of hemangioma such as cavernous, capillary, venous, and racemose can cause hematospermia (74).
Median raphe cyst
Median raphe cysts are benign lesions that are formed due to trapping of tissue during urethral fold development. These cysts are subdivided into four categories: urethral, epidermoid, glandular, and mixed. Although many patients with median raphe cysts are asymptomatic, voiding difficulties and hematospermia can result (38).
Melanoma
An isolated case of metastatic melanoma in the seminal vesicles showed hematospermia as the only symptom. A magnetic resonance imaging (MRI) scan was conducted instead of TRUS because infection with HIV made the patient susceptible to infection, and hematospermia had occurred for five months. Metastatic melanoma to the seminal vesicles is a rare occurrence, and is hypothesized to be due to complete regression of the primary melanoma or by malignant transformation of ectopic nevi cells (33).
Papillary urethritis
Papillary urethritis is a papillary lesion in the prostatic urethra representing proliferative reactive changes to chronic inflammation. Long-lasting hematospermia was noted in a case study of a patient that suffered from megacava associated with the circumaortic renal vein. Varicose plexus in the pelvic floor is an indicator of the presence of varicose plexuses in other areas of the lower extremity as well as for vulvar and pudendal varicosities, hemorrhoids, and varicoceles. For this reason, urine and blood tests, prostate-specific antigen (PSA) level tests, coagulation function, and cultures of urine and semen should be conducted (72).
Polypoid lesions
Polypoid lesions can develop in the prostatic urethra, which can contribute to hematospermia. In a case report, the polypoid lesions were identified as adenomatous polyps, appearing as a normal prostate, as well as intraductal carcinomas. After resection surgery, hematospermia can resolve itself, but the probability is not definite. Cystoscopy should be performed on patients that have hematospermia refractory to antibiotic therapy (55).
Seminal vesicle cyst
Congenital seminal vesicle cysts are usually associated with abnormal genitourinary structures, as well as ipsilateral renal agenesis or dysgenesis. About 44–60% of the patients with autosomal dominant polycystic kidney disease have bilateral seminal vesicle cysts (44).
Utricular cyst
Utricular cysts are endodermal in origin. Cystic dilation of the utricle can be a cause of the lesion for hematospermia. In these cases, seminal vesicle fluid is proven to be hemorrhagic in most patients, as the midline cyst communicates with the urethra or ejaculatory ducts. Midline cysts include the prostatic utricular, ejaculatory duct, müllerian duct, as well as prostatic and seminal vesicle cysts (48).
Iatrogenic
Brachytherapy
In a 2005 cross-sectional analysis, 16 patients experienced hematospermia after brachytherapy treatment (105). In 2001, hematospermia along with orgasmalgia occurred in 26% of the patients (83).
External beam radiation
Gold markers that are 5 mm in length, 1 mm in diameter, with a 0.3-mm diameter steel core are inserted into random positions within the prostate, which serve to improve visualization on MRI when testing for the inter-fraction motion of the prostate. Five hundred mg of prophylaxis antibiotic is taken twice daily for 3 days to prevent infection. These transrectal markers can cause complications of pain and fever, voiding difficulties, and hematospermia (84).
HIV protease inhibitors
Spontaneous bleeding has been known to occur in patients who were HIV-positive hemophiliacs taking HIV protease inhibitors (106). In a select few patients, bleeding appeared in the form of hematospermia upon taking HIV protease inhibitors (9).
TRUS
Although TRUS is a safe, rapid, and well-accepted procedure, the incidence of hematospermia following TRUS has been reported between 5.1% and 89%. The average time for spontaneous self-resolution was 3.5 weeks in a study by Manoharan et al. (107).
Vasectomy complication
Vaso-venous fistula occurring after vasectomy has been associated with hematospermia. In a case study, a patient who had a vasectomy had a testicle become swollen and tender. A significant amount of blood was voided upon ejaculation. Upon cystoscopy, the patient had blood originating from the ejaculatory duct. Surgical intervention showed a left scrotal vein traversing into the vas deferens as well as abnormal vascular structures, chronic inflammation, and nerve proliferation (89).
High-intensity focus ultrasonography
High-intensity focus ultrasonography causes tissue ablation by coagulative necrosis for patients undergoing benign prostatic hyperplasia treatment (52). An adverse side effect of this treatment includes hematospermia (53).
Systemic diseases
Cytomegalovirus
Cytomegalovirus is a common virus throughout the world that comes from the order Herpesvirales. Babies who are born with congenital cytomegalovirus by transmission through breastmilk or by birth show symptoms of jaundice, purple skin rashes, pneumonia, seizures, poor functioning livers, and low birth weight. This virus can manifest into an active form later in life in those who are immunocompromised (15,16). A man can contract cytomegalovirus, leading to hematospermia in various ways (17).
Genitourinary tuberculosis
Pulmonary tuberculosis mycobacterium may spread into the kidneys, bladder, or scrotum through blood. This may cause symptoms of irritated voiding, weight loss, dysuria, urgency, renal failure, flank pain, acute pyelonephritis, and fistulae. Hematospermia is reported in about 10% of the cases of genitourinary tuberculosis (18). In a case study, genitourinary tuberculosis was present 14% of the 35 cases studied (19). Genitourinary tuberculosis should be tested in patients with hematospermia by intradermal injection of tuberculin-purified protein derivatives, regardless of the absence of other genitourinary tuberculosis symptoms (20).
Hyperuricemia
In 2014, Kurkar et al. concluded that hyperuricemia is a possible cause of hematospermia. Out of 143 patients observed, the hyperuricemic and hematospermia patients were younger by an average of 13.5 years than those who did not have hematospermia (100).
Hypertension
Patients with hematospermia are known to have significantly higher blood pressures, as hematospermia is associated with hypertension. Men presenting with hematospermia should monitor their blood pressure levels to ensure no further complications develop and receive antihypertensive treatment if necessary (78).
Lymphoma
A lymphoma is a group of blood cancers that affects the immune system through the lymph nodes, spleen, thymus gland, and bone marrow. The two most common types of lymphoma are Hodgkin’s and non-Hodgkin’s lymphoma. Solid tumors in the immune system grow, as cancer affects lymphocytes, compromising the ability of the body to fight off infection. Geoghegan and Bonavia reported a case of hematospermia as a presenting symptom of lymphoma (98).
Prothrombin deficiency
Prothrombin deficiency is a bleeding disorder that is characterized by an extended amount of time for blood to clot. In severe cases, prothrombin deficiency causes heavy bleeding even after small injuries. The reason for the rarity of hematospermia with congenital bleeding disorders may be potentially due to sphincter muscles and perineal muscles having the ability to stop bleeding by compression (9).
Schistosomiasis
Schistosoma haematobium eggs can be found in the prostate and seminal vesicles, causing hematospermia as well as a decreased viscosity and yellow discoloration of semen. Hematospermia was noted primarily in patients with more severe cases of schismatic infection (21). An increased resistance to praziquantel, the treatment for S. haematobium, may develop due to the decreased immune response caused by these worms (22). Recently, Spanish male tourists developed hematospermia; S. intercalatum, S. haematobium, and S. mansoni eggs were found in their urine and feces as well as in the ejaculate (23).
Herpes simplex virus
Herpes simplex virus is widely known as one of the most common pathogens responsible for hematospermia. About 60% of patients who present with HSV-2 have abnormal variations of the disease that are usually unrecognizable. For example, patients with HSV-2 seropositivity had the virus without having genital ulcers or other symptoms, causing the patient to be unaware of their disease and corresponding hematospermia (27).
Von Willebrand disease
Von Willebrand disease is a congenital bleeding disorder that presents as mucocutaneous bleeding with easy bruising, menorrhagia, epistaxis, and in select few cases, hematospermia. Von Willebrand disease is caused by mutations at the von Willebrand factor locus on chromosome 12. Hematospermia occurs with this disease most commonly secondary to self-instrumentation, falls, straddle injuries, or catheterization (93).
Parasitic or bacterial infection
Echinococcosis
Echinococcosis, or hydatid disease, is a tapeworm infection that occurs when eggs or proglottid of Echinococcus granulosus or E. multilocularis are ingested. Although E. multilocularis usually affects the liver or the lung, it has been reported as a retrovesical pseudotumoral mass causing hematospermia (29).
Ureaplasma urealyticum
A possible pathogen responsible for hematospermia may be Ureaplasma urealyticum, which is a strain of bacterium causing urogenital or extragenital infections. In 2005, Golan et al. reported a correlation between U. urealyticum and hematospermia (25).
Miscellaneous
Congenital or drug-induced bleeding
Najafi and Noohi reported a case of a 32-year-old man admitted due to hematospermia which turned out to be due to acetylsalicylic acid use, as the aspirin may have contributed to damaging the congested mucosa or by changing the amount of platelet produced after the penis experienced trauma post-ejaculation (82).
Excessive sexual intercourse or masturbation
In most cases where hematospermia occurs due to excessive masturbation, the condition self-resolves in an average of 1 to 2 months. The most common reason for blood in the ejaculate is because of a ruptured blood vessel after continuous ejaculations, as the epididymal duct can become sensitive and unable to recover to its normal function (80). Thus, it is vital for the evaluating physician to take a detailed history including current medication use and surgical history. If a patient has a history of excessive coitus, the sexual history and ejaculation frequency of the patient should also be submitted for evaluation.
Hemorrhage
Hemorrhage in the seminal vesicles, vas deferens, or müllerian duct is usually present in patients with hematospermia. An axial T1-weighted MRI study can show a hemorrhage. Usually, hemorrhage can be shown along with renal agenesis or abnormal urogenital structures (44). Hemorrhage can occur due to a multitude of reasons, including post-transrectal prostate needle biopsy (77).
Vascular malformations
Arteriovenous malformation is defined as incorrect or inefficient communication between veins and arteries. Many causes of arteriovenous malformation exist, including gunshot and other trauma as well as erroneous surgical procedures. In a case study by Avargues et al., a patient had a circumaortic vein and presented a nonaneurysmal dilation of the inferior vena cava and iliac veins. The inferior vena cava experienced megacava, which caused reflux in the pelvic venous plexuses and created pelvic floor varicosities resulting in hematospermia (72).
Evaluation
History
Obtaining a thorough history is instrumental in pinpointing the etiology of hematospermia. Factors such as the amount of bleeding as well as type and duration of symptoms should also be determined. In addition to bleeding, patients may experience weight loss, fever, pain, voiding dysfunction, and sexual manifestations. Depending on etiology, hematospermia can clinically manifest as painful, painless, intermittent, and persistent. It is important to identify the treatable causes and avoid mistaking hematospermia from other conditions. Uterine or cervical carcinoma may cause bleeding following sexual intercourse and may imitate hematospermia. Urethral bleeding and melanospermia present with similar symptoms. Condom tests are recommended to distinguish the partner as a cause of hematospermia. If a patient presents with a history of dysuria, proper antibiotic treatment may be necessary if an infection is suspected. Travel history to places where the prevalence of schistosomiasis or tuberculosis is higher should also be investigated. For instance, there have been several cases reported in the literature where S. haematobium eggs were contained within the ejaculate in addition to hematospermia (21,108).
Physical examination
Similar to patient history, a thorough physical examination is essential for a proper diagnosis of hematospermia. Vital signs including blood pressure and temperature should be recorded, and the abdomen should be assessed for abnormal lumps to exclude liver and spleen enlargement or pelvic masses. The groin, perineum, as well as the external genitalia including the urethral meatus, testes, and spermatic cord, should be examined for dermal lesions and presence of hypospadias. A rectal examination should be performed to eliminate the possibility of rectal, prostate, and seminal vesicle cysts and masses. If masses are not identified in any area, a transperineal ultrasound or an MRI scan should be obtained to gain images of the genital glands and the respective drainage ducts (1). A condom test may be utilized to examine blood in the ejaculate (109). Figure 1 establishes an evaluation algorithm for hematospermia.
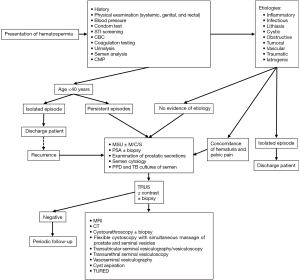
Laboratory studies
Laboratory tests such as urine cytology must be conducted to exclude any bladder-related pathologies (109). Further laboratory tests including semen culture, urethral swabs, mycobacterial cultures, and viral serology should be utilized to rule out an infectious etiology (109). Patients presenting with hematospermia and urethritis should also be tested for gonorrhea and chlamydia.
Basic metabolic panel (BMP) is a blood test that measures the amount of sodium, potassium, chloride, bicarbonate, blood urea nitrogen, creatinine, glucose, and calcium. BMP should be obtained to test the liver and kidney function.
PSA, a protein contained within the prostate that ensures sperm will survive when in the vagina after intercourse, should also be obtained to rule out possible prostate cancer as men with prostate cancer typically have increased PSA levels.
Furthermore, cystoscopy and seminal vesiculography should be utilized for direct visualization of an anatomical abnormality.
Imaging studies
There are various imaging modalities available for examining the ejaculatory apparatus. This includes cystourethroscopy, TRUS, and computed tomography (CT). TRUS is the most widely used, as it is safe, inexpensive, and has a high rate of detection. TRUS use is recommended if hematospermia persists longer than one month (110,111). TRUS is effective in visualizing the internal structures of the seminal vesicle, vas deferens, ejaculatory duct, and prostate (112).
If positive for a suspicious lesion or nodule, an MRI scan or a flexible cystoscopy should be obtained (113). If negative, the patient should have periodic follow-ups to ensure no complications are present (113). TRUS is useful in detecting communication between a midline cyst and the urethra by the utricular orifice, as it is the only modality able to identify this communication by the use of dye and contrast medium. One of the complications of TRUS is a failure to properly aspirate the lesion (48).
Transrectal MRI of the ejaculatory apparatus can also be performed to aid in the diagnosis. Since seminal vesicle amyloidosis appears hypointensive on a T2- and T1-weighted MRI scan (96).
Management
Hematospermia usually self-resolves in many cases, stopping in occurrence over time, especially for patients below the age of 40. In a prospective study, Furuya et al. concluded that in patients presenting with hematospermia without inflammation, infection, or malignancy, hematospermia resolved spontaneously in more than 88% of the patients with a mean disease duration of 1.5 months (114). Since hematospermia has a higher rate of persistence in patients with seminal vesical hemorrhage, midline cyst, seminal vesical dilation, and age older than 50, it is imperative that the underlying condition is properly diagnosed and treated to completely resolve hematospermia (114). Similarly, one of the most important aspects of managing hematospermia is patient reassurance. The physician must evaluate the patient, rule out life-threatening conditions such as cancer, and relieve the anxiety and stress meanwhile providing a proper follow-up and observation (114). Surgery should be performed and proper medication should be prescribed based on the pathophysiological nature of hematospermia. The primary care physician must be able to safely manage idiopathic conditions that often presents as hematospermia. Prompt consultation with a specialist should be obtained if a patient has recurring symptoms, elevated PSA, or unusual findings during the physical examinations. Patients over the age of 40 with high-risk factors such as recurrent symptoms, hematuria, or history of prostate cancer are required to seek urologist for a detailed investigation (115). If the seminal vesicles are dilated, and the patient has no resolution of hematospermia after conservative therapies, then the patient may opt to undergo bilateral seminal vesicle puncture and drug injection with ultrasound guidance to stop hematospermia (103).
Instrumentation
Transurethral seminal vesiculoscopy is an often-performed treatment in addition to the aforementioned diagnostic approach (116). Transutricular seminal vesiculoscopy can also be conducted with newer endoscopic equipment. Such methods are usual in targeting abnormal urethral or prostatic vessels (117). Additionally, duct obstruction can be managed by transurethral incision.
Pharmacotherapy
Certain drugs can also be taken to help alleviate hematospermia symptoms. For example, finasteride is used to control hematospermia due to benign prostatic hypertrophy, and even for patients with idiopathic refractory hematospermia after excluding other organic causes (118). To target bacterial infection or sexually transmitted infection-suspected cases of hematospermia, antibiotic therapy is utilized. The more frequent pathogens that cause hematospermia include chlamydia, gonorrhea, and herpes simplex. In these cases, antibiotic therapy should be considered; a course of doxycycline with 5-aminoquinolone or sulfamethoxazole may help absolve the infection (109).
Endoscopy
The endoscopic treatment utilizes a holmium laser to incise the obstructed ejaculatory duct, fragment stones, and coagulate hemorrhagic mucosa to treat diseases of the ejaculatory duct and seminal vesicle, which are common causes of hematospermia (119).
Conclusions
The occurrence of hematospermia can be quite alarming, especially for men who do not know the causes of blood in the ejaculate. As most causes of hematospermia are self-resolving, the physician should reassure the patient of his wellbeing and condition, and continue with follow up until hematospermia ceases. If the condition continues or worsens, different imaging modalities are available for observation and diagnosis of further complications. Case studies of the various etiologies of hematospermia are discussed but are not limited to the ones listed in this review. The etiology can be classified into inflammatory, infectious, lithiasis, cystic, obstructive, tumoral, vascular, traumatic, iatrogenic, and systemic origin or divided into subcategories based on anatomical origins such as prostate, bladder, spermatic cord, seminal vesicles, or epididymis. Evaluating the etiology is the best approach to the initial management of hematospermia.
Acknowledgements
The authors are thankful to Drs. Kelly Warren, Todd Miller, and Peter Brink for departmental support, as well as Mrs. Wendy Isser and Ms. Grace Garey for literature retrieval.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Munkel witz R, Krasnokutsky S, Lie J, et al. Current Perspectives on Hematospermia: A Review. J Androl 1997;18:6-14.
- Leary FJ, Aguilo JJ. Clinical significance of hematospermia. Mayo Clin Proc 1974;49:815-7. [PubMed]
- de Kretser DM, Temple-Smith PD, Kerr JB. Anatomical and Functional Aspects of the Male Reproductive Organs. In: Bandhauer K, Bartsch G, de Kretser DM et al., editors. Disturbances in Male Fertility. Berlin, Heidelberg: Springer Berlin Heidelberg, 1982:1-131.
- Clément P, Giuliano F. Physiology of Ejaculation. In: Mulhall PJ, Incrocci L, Goldstein I et al., editors. Cancer and Sexual Health. Totowa, NJ: Humana Press, 2011:77-89.
- Marberger H. The Mechanisms of Ejaculation. In: Coutinho EM, Fuchs F, editors. Physiology and Genetics of Reproduction: Part B. Boston, MA: Springer US, 1974:99-110.
- Ameur A, Touiti D, Jira H, et al. Hemospermia: diagnosis and therapeutic aspects. Seven case reports. Annales d’urologie 2002;36:74-80. [Crossref] [PubMed]
- Yanai T, Okazaki T, Yamataka A, et al. Cysts of the ejaculatory system: a report of two cases. Pediatr Surg Int 2005;21:939-42. [Crossref] [PubMed]
- Sampalmieri G, Giancola FL, Cabras A. Hemospermia: cause, clinical significance and our experience. Riv Eur Sci Med Farmacol 1992;14:135-7. [PubMed]
- Girolami A, Scarparo P, Candeo N, et al. Hemospermia in patients with congenital coagulation disorders: a study of three cases. Acta Haematol 2009;121:42-6. [Crossref] [PubMed]
- Lee G. Chronic Prostatitis: A Possible Cause of Hematospermia. World J Mens Health 2015;33:103-8. [Crossref] [PubMed]
- Sonnex C. Prostatitis, Chronic Pelvic Pain Syndrome, and Hematospermia. Sexual Health and Genital Medicine in Clinical Practice. Springer, 2015:93-7.
- Sue DE, Chicola C, Brant-Zawadzki MN, et al. MR imaging in seminal vesiculitis. J Comput Assist Tomogr 1989;13:662-4. [Crossref] [PubMed]
- Pastore AL, Palleschi G, Fuschi A, et al. Hematospermia and xanthogranulomatous prostatitis: An unusual onset of a rare diagnosis. Can Urol Assoc J 2013;7:E820-2. [Crossref] [PubMed]
- Sebök J, Füzesi L, Lóth I, et al. A contribution to the pathology and clinical features of granulomatous prostatitis. Int Urol Nephrol 1982;14:45-50. [Crossref] [PubMed]
- Neuberger P, Hamprecht K, Vochem M, et al. Case-control study of symptoms and neonatal outcome of human milk-transmitted cytomegalovirus infection in premature infants. J Pediatr 2006;148:326-31. [Crossref] [PubMed]
- Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002;34:1094-7. [Crossref] [PubMed]
- Koment RW, Poor PM. Infection by human cytomegalovirus associated with chronic hematospermia. Urology 1983;22:617-21. [Crossref] [PubMed]
- Kapoor R, Ansari MS, Mandhani A, et al. Clinical presentation and diagnostic approach in cases of genitourinary tuberculosis. Indian J Urol 2008;24:401. [Crossref] [PubMed]
- Pal DK. Haemospermia: an Indian experience. Trop Doct 2006;36:61-2. [Crossref] [PubMed]
- Çek M, Lenk S, Naber KG, et al. EAU Guidelines for the Management of Genitourinary Tuberculosis. Eur Urol 2005;48:353-62. [Crossref] [PubMed]
- Schwartz E, Pick N, Shazberg G, et al. Hematospermia due to schistosome infection in travelers: diagnostic and treatment challenges. Clin Infect Dis 2002;35:1420-4. [Crossref] [PubMed]
- William S, Sabra A, Ramzy F, et al. Stability and reproductive fitness of Schistosoma mansoni isolates with decreased sensitivity to praziquantel. Int J Parasitol 2001;31:1093-100. [Crossref] [PubMed]
- Corachan M, Valls ME, Gascon J, et al. Hematospermia: a new etiology of clinical interest. Am J Trop Med Hyg 1994;50:580-4. [Crossref] [PubMed]
- Pérignon A, Pelicot M, Consigny PH. Genital schistosomiasis in a traveler coming back from Mali. J Travel Med 2007;14:197-9. [Crossref] [PubMed]
- Golan S, Slomov E, Kra-Oz Z, et al. Detection of sexually transmitted pathogens in patients with hematospermia. Harefuah 2005;144:630-3, 76.
- Yao J. The investigation of relationship between hemospermia and infection with ureaplasma urealyticum and chlamydia trachomatis. Medicine and Pharmacy of Yunnan 2009;4:11.
- Bamberger E, Madeb R, Steinberg J, et al. Detection of sexually transmitted pathogens in patients with hematospermia. Isr Med Assoc J 2005;7:224-7. [PubMed]
- Wang X, Gao Y. Gonorrhea. Radiology of Infectious Diseases: Volume 2. Springer; 2015:103-12.
- Whyman MR, Morris DL. Retrovesical hydatid causing haemospermia. Br J Urol 1991;68:100-1. [Crossref] [PubMed]
- Musso D, Roche C, Robin E, et al. Potential sexual transmission of Zika virus. Emerging infectious diseases 2015;21:359. [Crossref] [PubMed]
- Yagci C, Kupeli S, Tok C, et al. Efficacy of transrectal ultrasonography in the evaluation of hematospermia. Clinical Imaging 2004;28:286-90. [Crossref] [PubMed]
- Papp GK, Kopa Z, Szabo F, et al. Aetiology of haemospermia. Andrologia 2003;35:317-20. [Crossref] [PubMed]
- Meng MV, Werboff LH. Hematospermia as the presenting symptom of metastatic malignant melanoma of unknown primary origin. Urology 2000;56:330. [Crossref] [PubMed]
- Gupta E, Torigian DA. MR Imaging of the Prostate Gland. PET Clinics 2009;4:139-54. [Crossref] [PubMed]
- Singh I, Sharma N, Singh N, et al. Hematospermia (ejaculatory duct calculus)–an unusual cause. Int Urol Nephrol 2003;35:517-8. [Crossref] [PubMed]
- Yun SJ, Kim TH, Kwon WA, et al. A large stone in the dilated left seminal vesicle: laparoscopic removal and partial seminal vesiculectomy. Korean J Urol 2008;49:656-8. [Crossref]
- Han H, Zhou XG, Fan DD, et al. An Unusual Etiology for Hematospermia and Treatments That Were Successful. Urology 2015;86:740-3. [Crossref] [PubMed]
- Shao IH, Chen TD, Shao HT, et al. Male median raphe cysts: serial retrospective analysis and histopathological classification. Diagn Pathol 2012;7:121. [Crossref] [PubMed]
- Moukaddam HA, Haddad MC, El-Sayyed K, et al. Diagnosis and treatment of midline prostatic cysts. Clinical imaging 2003;27:44-6. [Crossref] [PubMed]
- Ishikawa M, Okabe H, Oya T, et al. Midline prostatic cysts in healthy men: incidence and transabdominal sonographic findings. AJR Am J Roentgenol 2003;181:1669-72. [Crossref] [PubMed]
- Schwartz JM, Bosniak MA, Hulnick DH, et al. Computed tomography of midline cysts of the prostate. J Comput Assist Tomogr 1988;12:215-8. [Crossref] [PubMed]
- Furuya S, Ogura H, Shimamura S, et al. Transurethral endoscopic treatment for chronic hematospermia caused by mullerian duct cyst and ejaculatory duct obstruction. Hinyokika kiyo Acta urologica Japonica 2001;47:839-42. [PubMed]
- Coppens L, Bonnet P, Andrianne R, et al. Adult müllerian duct or utricle cyst: clinical significance and therapeutic management of 65 cases. J Urol 2002;167:1740-4. [Crossref] [PubMed]
- Kim B, Kawashima A, Ryu J-A, et al. Imaging of the Seminal Vesicle and Vas Deferens. RadioGraphics 2009;29:1105-21. [Crossref] [PubMed]
- Livingston L, Larsen CR. Seminal Vesicle Cyst with Ipsilateral Renal Agenesis. AJR Am J Roentgenol 2000;175:177-80. [Crossref] [PubMed]
- Wang TM, Chuang CK, Lai MK. Seminal vesicle cyst: an unusual cause of hematospermia--a case report. Changgeng Yi Xue Za Zhi 1993;16:275-8. [PubMed]
- Mayersak JS, Viviano CJ. Unilateral seminal vesicle cyst presenting as hematospermia; diagnosis established by transrectal prostatic ultrasound. Wisconsin medical journal 1992;91:629-31. [PubMed]
- Furuya S, Kato H. A clinical entity of cystic dilatation of the utricle associated with hemospermia. J Urol 2005;174:1039-42. [Crossref] [PubMed]
- Kang PM, Seo WI, Yoon JH, et al. Transutricular seminal vesiculoscopy in the management of symptomatic midline cyst of the prostate. World J Urol 2016. [Epub ahead of print]. [PubMed]
- Manohar T, Ganpule A, Desai M. Transrectal ultrasound- and fluoroscopic-assisted transurethral incision of ejaculatory ducts: a problem-solving approach to nonmalignant hematospermia due to ejaculatory duct obstruction. J Endourol 2008;22:1531-5. [Crossref] [PubMed]
- Ponce Campuzano A, Gonzalez Satue C, Rodriguez Tolra J, et al. Treatment of urethral stenosis with thermo-expandable prosthesis “Memotherm”. Our experience. Arch Esp Urol 2000;53:253-8. [PubMed]
- Georgiades F, Demosthenous S, Antoniades G, et al. Giant benign prostatic hyperplasia in a young adult male. Urology 2014;84:e4-5. [Crossref] [PubMed]
- Madersbacher S, Kratzik C, Szabo N, et al. Tissue ablation in benign prostatic hyperplasia with high-intensity focused ultrasound. Eur Urol 1993;23 Suppl 1:39-43. [Crossref] [PubMed]
- Fan K, Schaefer RF, Venable M. Urethral verumontanal polyp: evidence of prostatic origin. Urology 1984;24:499-501. [Crossref] [PubMed]
- Stein AJ, Prioleau PG, Catalona WJ. Adenomatous polyps of the prostatic urethra: a cause of hematospermia. J Urol 1980;124:298-9. [Crossref] [PubMed]
- Furuya S, Ogura H, Shimamura S, et al. Clinical manifestations of 25 patients with prostatic-type polyps in the prostatic urethra. Hinyokika kiyo Acta urologica Japonica 2002;48:337-42. [PubMed]
- Chou K, Lo KY, Lin WC, et al. Adenomatous polyps of the prostatic urethra. A report of four cases. Zhonghua Yi Xue Za Zhi (Taipei) 1989;43:355-8. [PubMed]
- Fujisawa M, Ishigami J, Kamidono S, et al. Adenomyosis of the seminal vesicle with hematospermia. Hinyokika Kiyo 1993;39:73-6. [PubMed]
- Blokker RS, Lock TM, de Boorder T. Comparing thulium laser and Nd: YAG laser in the treatment of genital and urethral condylomata acuminata in male patients. Lasers Surg Med 2013;45:582-8. [Crossref] [PubMed]
- Stimac G, Demirovic A, Kruslin B, et al. Testicular angioleiomyoma presenting with haematospermia. Asian J Androl 2013;15:573-4. [Crossref] [PubMed]
- Harada M, Tokuda N, Tsubaki H, et al. Cavernous hemangioma of the spermatic cord: a case report. Hinyokika Kiyo 1992;38:591-4. [PubMed]
- Eken A, Izol V, Aridogan IA, et al. An unusual cause of hematospermia: Primary adenocarcinoma of the seminal vesicle. Can Urol Assoc J 2012;6:E259-E62. [Crossref] [PubMed]
- Egevad L, Ehrnström R, Håkansson U, et al. Primary seminal vesicle carcinoma detected at transurethral resection of prostate. Urology 2007;69:778.e11-3. [Crossref] [PubMed]
- Sollini M, Silvotti M, Casali M, et al. The role of imaging in the diagnosis of recurrence of primary seminal vesicle adenocarcinoma. World J Mens Health 2014;32:61-5. [Crossref] [PubMed]
- Foahom Kamwa AD, Mateus C, Thanigasalam R, et al. Seminal vesicle metastasis of cutaneous malignant melanoma: An unusual and challenging presentation. Can Urol Assoc J 2015;9:E220-3. [Crossref] [PubMed]
- Rietbergen JBW, Kruger AEB, Kranse R, et al. Complications of transrectal ultrasound-guided systematic sextant biopsies of the prostate: evaluation of complication rates and risk factors within a population-based screening program. Urology 1997;49:875-80. [Crossref] [PubMed]
- Yang L, Deng J, Zhong H, et al. Transrectal ultrasound-guided systematic 13-core prostate biopsy to diagnose prostate carcinoma. Chinese J Clin Oncol 2005;2:849-51. [Crossref]
- Yamamoto S, Mamiya Y, Noda K, et al. A case of metastasis to the seminal vesicle of renal cell carcinoma. Nihon Hinyokika Gakkai Zasshi 1998;89:563-6. [Crossref] [PubMed]
- Choong NW, Quevedo JF, Kaur JS. Small cell carcinoma of the urinary bladder. Cancer 2005;103:1172-8. [Crossref] [PubMed]
- Gill JD, Bhattarai S, Patel CN, et al. Yolk sac tumor of the seminal vesicles: A rare malignant cause of hematospermia. Urol Ann 2015;7:107. [Crossref] [PubMed]
- Hennessey AM, Clement JM, Forouhar F, et al. Hematospermia and Cloacogenic Transitional Cell Carcinoma: A Twist on Significance and Meaning. Case Rep Urol 2016;2016:8050459. [Crossref] [PubMed]
- Avargues A, Rogel R, Sanchez-Nevarez I, et al. Long-standing hemospermia in a patient with megacava associated to a circumaortic renal vein. Urol Ann 2015;7:405-7. [PubMed]
- Cattolica EV. Massive hemospermia: a new etiology and simplified treatment. J Urol 1982;128:151-2. [Crossref] [PubMed]
- Saito S. Posterior Urethral Hemangioma: One of the Unknown Causes of Hematuria and/or Hematospermia. Urology 2008;71:168.e11-e4. [Crossref] [PubMed]
- Furuya S, Ogura H, Tanaka Y, et al. Hemangioma of the prostatic urethra: hematospermia and massive postejaculation hematuria with clot retention. Int J Urol 1997;4:524-6. [Crossref] [PubMed]
- Serizawa RR, Nørgaard N, Horn T, et al. Hemangioma of the prostate-an unusual cause of lower urinary tract symptoms: Case report. BMC urology 2011;11:4. [Crossref] [PubMed]
- Petroski RA, Griewe GL, Schenkman NS. Delayed life-threatening hemorrhage after transrectal prostate needle biopsy. Prostate cancer and prostatic diseases 2003;6:190-2. [Crossref] [PubMed]
- Close CF, Yeo WW, Ramsay LE. The association between haemospermia and severe hypertension. Postgrad Med J 1991;67:157-8. [Crossref] [PubMed]
- Scheier RB. Report of a case of hematospermia due to high blood-pressure, associated with chronic interstitial nephritis. JAMA 1913;61:198-9. [Crossref]
- Correa-Pérez JR. Short Communication: Occurrence of Nonpersistent Hematospermia After a Prolonged Period of Daily Ejaculatory Intensity Longer Than 3 Weeks. J Assist Reprod Genet 2004;21:341-2. [Crossref] [PubMed]
- Cheng YS, Lin JS, Lin YM. Isolated posterior urethral injury: an unusual complication and presentation following male coital trauma. Asian J Androl 2006;8:379-81. [Crossref] [PubMed]
- Najafi L, Noohi AH. Recurrent hematospermia due to aspirin. Indian J Med Sci 2009;63:259-60. [Crossref] [PubMed]
- Merrick GS, Wallner K, Butler WM, et al. Short-term sexual function after prostate brachytherapy. Int J Cancer 2001;96:313-9. [Crossref] [PubMed]
- Moman MR, van der Heide UA, Kotte ANTJ, et al. Long-term experience with transrectal and transperineal implantations of fiducial gold markers in the prostate for position verification in external beam radiotherapy; feasibility, toxicity and quality of life. Radiother Oncol 2010;96:38-42. [Crossref] [PubMed]
- Langenhuijsen JF, van Lin EN, Kiemeney LA, et al. Ultrasound-guided transrectal implantation of gold markers for prostate localization during external beam radiotherapy: complication rate and risk factors. Int J Radiat Oncol Biol Phys 2007;69:671-6. [Crossref] [PubMed]
- Kilciler M, Erdemir F, Demir E, et al. The Effect of Rectal Foley Catheterization on Rectal Bleeding Rates after Transrectal Ultrasound-guided Prostate Biopsy. J Vasc Interv Radiol 2008;19:1344-6. [Crossref] [PubMed]
- Pushkar’ DI, Govorov AV. Complications of transrectal biopsy of the prostate. Urologiia 2005.40-2. [PubMed]
- Gattoni F, Avogadro A, Sacrini A, et al. Transrectal prostatic echography in the study of hemospermia. An assessment of an 85-patient case load. Radiol Med 1996;91:424-8. [PubMed]
- Rajan RR, Cuesta KH, Squadrito J Jr. Vasovenous fistula after vasectomy. J Urol 1997;158:2243. [Crossref] [PubMed]
- Kakehi Y, Naito S. Complication rates of ultrasound‐guided prostate biopsy: A nation‐wide survey in Japan. Int J Urol 2008;15:319-21. [Crossref] [PubMed]
- Wu HF, Qiao D, Qian LX, et al. Congenital agenesis of seminal vesicle. Asian J Androl 2005;7:449-52. [Crossref] [PubMed]
- Lemesh RA. Case Report: Recurrent Hematuria and Hematospermia Due to Prostatic Telangiectasiain Classic von Willebrand’s Disease. Am J Med Sci 1993;306:35-6. [Crossref] [PubMed]
- Minardi D, Scortechini AR, Milanese G, et al. Spontaneous recurrent hematuria and hematospermia: Unique manifestations of von Willebrand disease type I. Case report. Arch Ital Urol Androl 2016;88:62-3. [Crossref] [PubMed]
- Kardoust Parizi M, Shakhssalim N. Management of Zinner’s syndrome associated with contralateral seminal vesicle hypoplasia: a case report. Case Rep Urol 2013;2013:494215. [PubMed]
- Vandwalle J, Dugardin F, Petit T, et al. Haemospermia due to seminal vesicle amyloidosis. Treatment by laparoscopic vesiculectomy. A case report. Prog Urol 2007;17:1382-4. [Crossref] [PubMed]
- Furuya S, Masumori N, Furuya R, et al. Characterization of localized seminal vesicle amyloidosis causing hemospermia: an analysis using immunohistochemistry and magnetic resonance imaging. J Urol 2005;173:1273-7. [Crossref] [PubMed]
- Botash RJ, Poster RB, Abraham JL, et al. Senile seminal vesicle amyloidosis associated with hematospermia: demonstration by endorectal MRI. J Comput Assist Tomogr 1997;21:748-9. [Crossref] [PubMed]
- Geoghegan JG, Bonavia I. Haemospermia as a presenting symptom of lymphoma. Br J Urol 1990;66:658. [Crossref] [PubMed]
- Ross JC. Haemospermia. Practitioner 1969;202:59-62. [PubMed]
- Kurkar A, Elderwy AA, Awad SM, et al. Hyperuricemia: a possible cause of hemospermia. Urology 2014;84:609-12. [Crossref] [PubMed]
- Han M, Brannigan RE, Antenor JA, et al. Association of hemospermia with prostate cancer. J Urol 2004;172:2189-92. [Crossref] [PubMed]
- Lote H, Mannion E, Cook T, et al. Adenocarcinoma of the seminal vesicles complicated by antineutrophil cytoplasmic antibody vasculitis: a case report and review of the literature. J Med Case Rep 2013;7:59. [Crossref] [PubMed]
- Fuse H, Sumiya H, Ishii H, et al. Treatment of hemospermia caused by dilated seminal vesicles by direct drug injection guided by ultrasonography. J Urol 1988;140:991-2. [Crossref] [PubMed]
- Worischeck JH, Parra RO. Chronic hematospermia: assessment by transrectal ultrasound. Urology 1994;43:515-20. [Crossref] [PubMed]
- Finney G, Haynes AM, Cross P, et al. Cross-sectional analysis of sexual function after prostate brachytherapy. Urology 2005;66:377-81. [Crossref] [PubMed]
- Racoosin JA, Kessler CM. Bleeding episodes in HIV-positive patients taking HIV protease inhibitors: a case series. Haemophilia 1999;5:266-9. [Crossref] [PubMed]
- Manoharan M, Ayyathurai R, Nieder AM, et al. Hemospermia following transrectal ultrasound-guided prostate biopsy: a prospective study. Prostate Cancer Prostatic Dis 2007;10:283-7. [Crossref] [PubMed]
- van Delft F, Visser L, Polderman A, et al. Cough and alterations in semen after a tropical swim. Neth J Med 2007;65:304-6. [PubMed]
- Kumar P, Kapoor S, Nargund V. Haematospermia - a systematic review. Ann R Coll Surg Engl 2006;88:339-42. [Crossref] [PubMed]
- Raviv G, Laufer M, Miki H. Hematospermia--the added value of transrectal ultrasound to clinical evaluation: is transrectal ultrasound necessary for evaluation of hematospermia? Clin Imaging 2013;37:913-6. [Crossref] [PubMed]
- Zhao H, Luo J, Wang D, et al. The value of transrectal ultrasound in the diagnosis of hematospermia in a large cohort of patients. J Androl 2012;33:897-903. [Crossref] [PubMed]
- Lu CH, Chen WC, Wu HC, et al. Transrectal ultrasonographic findings in patients with hemospermia. Zhonghua Yi Xue Za Zhi (Taipei) 2000;63:558-62. [PubMed]
- Szlauer R, Jungwirth A. Haematospermia: diagnosis and treatment. Andrologia 2008;40:120-4. [Crossref] [PubMed]
- Furuya S, Masumori N, Takayanagi A. Natural history of hematospermia in 189 Japanese men. Int J Urol 2016;23:934-40. [Crossref] [PubMed]
- Stefanovic KB, Gregg PC, Soung M. Evaluation and treatment of hematospermia. Am Fam Physician 2009;80:1421-7. [PubMed]
- Liu ZY, Sun YH, Xu CL, et al. Transurethral seminal vesiculoscopy in the diagnosis and treatment of persistent or recurrent hemospermia: a single-institution experience. Asian J Androl 2009;11:566-70. [Crossref] [PubMed]
- Byon SK, Rha KH, Yang SC. Transutricular seminal-vesiculoscopy in the management of hematospermia. Korean J Urol 2001;42:329-33.
- Badawy AA, Abdelhafez AA, Abuzeid AM. Finasteride for treatment of refractory hemospermia: prospective placebo-controlled study. Int Urol Nephrol 2012;44:371-5. [Crossref] [PubMed]
- Oh TH, Seo IY. Endoscopic Treatment for Persistent Hematospermia: A Novel Technique Using a Holmium Laser. Scand J Surg 2016;105:174-7. [Crossref] [PubMed]


