Relationship between human papillomavirus and penile cancer—implications for prevention and treatment
Introduction
Penile cancer is a rare disease with significant morbidity and mortality when present in the advanced stages of the disease. Its prevalence is highest in the developing countries of Africa, Asia, and South America, and its most common histology is squamous cell carcinoma (SCC) (1,2). The overall incidence in the United States is approximately 0.69 per 100,000 men with incidence associated with increasing age at diagnosis (3). Traditionally, radical penile and inguinal surgery has been considered the mainstay treatment even though it can carry substantial physical and psychosexual morbidity for those treated. Recently, however, organ-sparing has become a widely accepted approach due to established equivalent oncologic control while achieving satisfactory somatic and sexual health outcomes (4,5).
The etiology of penile cancer is multifactorial with many risk factors identified including phimosis, poor hygiene, smoking, and chronic inflammatory states such as balanitis xerotica obliterans (BXO) (2). Other risk factors for penile cancer include an increasing number of sexual partners, or history of genital warts or other sexually transmitted diseases. In particular, infection with human papillomavirus (HPV) has been linked to penile cancer carcinogenesis (6), although exact pathways have not been fully elucidated to date. Nonetheless, the pathogenesis of HPV infection provides an actionable target for newer therapeutic agents to treat this rare and disfiguring disease. In this review, we provide a thorough update on the epidemiology of HPV infection, mechanisms of pathogenesis, as well as current and future therapeutic strategies against HPV-mediated penile carcinogenesis.
HPV pathogenesis
Population infection rates
HPV infection has been implicated in many cancers, including cervical (7), penile (1) and oropharyngeal malignancies (8). However, most HPV infections do not develop into pathogenic external lesions, and the majority are immunologically cleared by 12 months (9). Yet, HPV DNA is detected in more than 90% of cervical tumor cells (10), 68% of tonsillar tumor cells (11) and an estimated more than 20% of penile tumor cells (although this value varies depending on the literature) (12). The variations in prevalence of HPV-positive penile cancer may be due to differences in sampling methods, genital sites sampled (e.g., glans, shaft, or scrotum), molecular testing, and populations studied. For instance, a higher estimate of HPV prevalence can be found when samples are collected from a larger number of anatomic sites (13,14). Higher estimates can also result from the use of more sensitive sampling techniques, such as a pre-wetted Dacron swab versus a cytobrush or urine sample (14).
It appears that there may be gender disparities in HPV infections. The prevalence of HPV infection in women has been estimated to be around 11.7% with specifically higher prevalence seen in sub-Saharan Africa, Eastern Europe, and Latin America (15). The prevalence of HPV infection in men is more variable, ranging from 1.3% to 72.9%. However, most studies report estimates greater than 20% in men, with higher prevalence in uncircumcised versus circumcised men (12,16).
Both genital (17-19) and oral (20) HPV prevalence have been shown to be higher in men than in women. Additionally, there does not seem to be an association between age and HPV prevalence in men. In contrast, HPV prevalence in women is highest among 14 to 24 years old, which then decreases until middle age (18). These differences indicate that HPV infection and clearance may differ by gender. In fact, it has been shown that men take significantly longer to clear an oral HPV infection than women (5.3 vs. 3.0 months; P<.001) (21). Additionally, in serologic studies women have been shown to have a higher prevalence of HPV antibodies in comparison to men, regardless of age (22,23). In another study, women were shown to have a higher prevalence of antibodies for one or more of HPV types 6, 11, 16 and 18 than men (32.5% vs. 12.2%) (22). Thus, evidence suggests that men do not exhibit as robust an immune response to HPV infection as women do. In the general population, the penile epithelium of asymptomatic men has consistently higher prevalence of HPV infection than the cervix of women with normal cytological testing (24). It is possible that these gender differences occur because HPV infection of keratinized epithelium does not induce an immune response as effectively as that of mucosal epithelium, the tissue type most commonly infected in women.
Penile cancer has been considered a relatively rare malignancy in the western world. In fact, between 1973 and 2002, the overall incidence of primary malignant penile cancer had decreased in the United States (3). Additionally, between 1943 and 1990 there was a statistically significant decline in the overall rate of penile cancer in Denmark (25). However, recent reports indicate an increase in incidence rates in various countries. For example, the Netherlands had an increase from 1.4 to 1.6 per 10,000 person-years from 1989 to 2006 (26). In Denmark, despite older studies showing a decline in penile cancer incidence as noted above, there was actually an increase from 1.0 to 1.3 per 100,000 men-years more recently from 1978 to 2008 (27). More recently, England had an increase from 1.10 to 1.33 per 100,000 men from 1979 to 2009 (28).
This increase in incidence of SCC penile cancer observed in England, Denmark and the Netherlands mirror increases in incidence of HPV-positive oropharynx cancer rates seen in the U.S., Sweden and Finland. HPV infection is a risk factor for both oropharyngeal cancer and penile cancer. From 1988 to 2004 in the U.S., the prevalence of HPV-positive SCC oropharynx cancer (SCCOP) has increased 225%, while HPV-negative SCCOP fell by 50% from 1988 to 2004 (8). The drop in HPV-negative SCCOP incidence correlates with decreased smoking, while the rise in HPV-positive SCCOP suggests increased oral HPV exposure (29). From 1970 to 2002 in Sweden, there has been a 2.8 fold increase in the incidence of tonsillar cancer and a significant increase (2.9-fold; P<0.001) in the proportion of HPV-positive tonsillar cancer (11). From 1956 to 2000 in Finland, the age standardized incidence rate of tonsillar cancer doubled (30).
Interestingly, treatment with radiation of HPV-positive SCCOP had more pronounced improvements in 2-year survival rates than HPV-negative SCCOP (31). In another study investigating the viral load of HPV-16 in tonsillar cancer, six patients with tumors with greater than or equal to the median value of 190 HPV-16 copies per B-actin had significantly better survival than those with lesser than or equal to 60 HPV-16 copies/B-actin (P=0.039, log rank test) with the higher viral load group tumor free 3 years after diagnosis versus 2/5 tumor free in the lower viral load group (P=0.026, ×2 test) (32). These results seem to indicate that HPV-presence may influence disease outcome however, more research is needed in this line of inquiry.
HPV serotypes
HPV is a DNA virus with more than 100 different known genotypes which can affect both cutaneous and mucosal sites. There are 20 types which are known to infect the genital tract (33). These types are generally classified as “low-risk” or “high-risk” depending on their association with cervical malignancy. HPV types 16, 18, 31, 33, 45, 56, and 65 are considered high-risk and are associated with penile malignancies, whereas types 6 and 11 are found in benign lesions (e.g., condyloma acuminata, or genital warts) and are considered low risk for malignant transformation. In patients with HPV, coinfection with more than one HPV type is common (34).
High-risk HPV-16 and HPV-18 are particularly implicated in genital malignancies. In cervical cancer, HPV-16 was present in more than 50% of cases and HPV-18 in another 11% (10). In penile cancer, HPV-16 or HPV-18 were implicated in approximately 31% of penile cancers, with HPV-16 being the predominant subtype (35). HPV-16 DNA is also a risk factor for developing tonsillar cancer and causes the majority of oropharyngeal SCC in the United States (8,36,37).
HPV type influences the rate of progression from genital HPV infection to disease (38). In one study, 16% of genital HPV-6 infections in men developed into HPV-6-positive condyloma and 22% of genital HPV-11 infections in men developed into HPV-11-positive condyloma. Both types of infections had rapid rates of progression to disease after initial genital infection with a median of 7.7 months. However, only 2% of genital HPV-16 developed into penile intraepithelial neoplasia (PeIN) within a 24-month period. HPV-16 infection had a relatively slow rate of progression to disease, with 50% taking more than 19 months for PeIN to be detected (39). PeIN is considered to be a precursor lesion of invasive SCC and is defined as a change of the penile squamous epithelium indicated by dysplastic changes with an intact basement membrane (34).
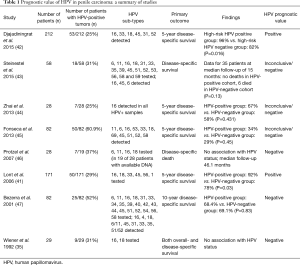
The role of HPV as a prognostic factor in penile cancer remains unclear. It is uncertain whether cancers involving HPV infection have better survival profiles than cancers without HPV infection. In a study with 82 penile cancer patients, 30.5% of tumors had HPV DNA, with HPV-16 being the most prevalent. This study demonstrated no association between HPV negative and positive patients when considering lymph node metastasis (P=0.386) and 10-year survival rate (68.4% vs. 69.1%; P=0.83) (40). In another study with 29 patients with invasive penile SCC, 31% of tumors had either HPV-16 or HPV-18 DNA. This study found no difference between HPV negative and positive patients in terms of nodal metastasis or survival even after adjustment controlling for tumor stage (35). However, these results differ from another study which examined HPV status as a prognostic indicator in 171 penile cancer patients. In this study, high-risk HPV DNA was found in 29% of tumors, with 76% containing HPV-16; high-risk HPV was associated with improved 5-year disease-specific survival (78% vs. 93%; P=0.03). Additionally, high-risk HPV was an independent predictor of disease specific mortality in multivariate analysis [hazard ratio (HR), 0.14; 95% CI, 0.03–0.63; P=0.01] (41). It is possible that these conflicting results may be due to differing study designs, sample sizes and sampling methods for HPV DNA, especially since there has yet to be a universal protocol for HPV DNA testing of penile SCC tumors. The prognostic role of HPV in the literature remains unclear. A summary of the available studies is shown in Table 1.
Full table
HPV pathogenesis/carcinogenesis
HPV-mediated pathogenesis of human epithelial cells is a multistep process. Although the HPV-mediated pathogenesis of cervical squamous cell cancer is well understood, it is not as well understood in penile cancer. Penile cancers are predominantly squamous cell in origin. However, there are several subtypes, including basaloid, warty, keratinizing, and verrucous. HPV infection has been associated with basaloid and warty subtypes and less so with other subtypes. A study found HPV DNA in 42% of penile carcinomas, 90% of dysplasias and 100% of condylomas. Additionally, basaloid and warty SCC subtypes had 80% and 100% of HPV DNA, respectively, whereas keratinizing and verrucous subtypes had the lowest percentage of HPV DNA, at 34.9% and 33.3%, respectively (48).
HPV affects the squamous epithelium in two ways, either as a viral infection or as a viral-associated precancerous lesion. A HPV viral infection is largely transient and occurs when the squamous epithelium supports virion production and develops into a morphologic low-grade lesion (e.g., condyloma and mild dysplasia). In contrast, an HPV viral-associated precancerous lesion (e.g., HPV-associated PeIN) occurs when the viral genome integrates into the host genome, leading to virally-induced overexpression of oncogenes that drive cell proliferation, which can develop into malignant transformation (34). In HPV-16 mediated penile cancer, integration occurs at the chromosomal 8q21.3 locus (FAM92A1 gene) and at the 16p13.3 locus (TRAP1 gene). This HPV integration marks an end point in the clonal selection of cells, resulting in functional genes that are altered. This allows for uncontrolled growth and dedifferentiation which can progress into invasive cancer (24).
HPV is a double-stranded DNA virus that has a circular genome which encodes eight genes, including E5, E6 and E7 oncogenes. However, only the E6 and E7 oncogenes are necessary for malignant transformation and maintenance of malignant phenotype of the host cells (34). The activation of viral E5 oncogene is not necessary for malignant transformation. However, it may contribute to carcinogenesis through manipulating viral uptake of host target cells, leading to progression of dysplasia related to premalignant lesions. The E5 gene product is a transmembrane protein that regulates activation of epidermal growth factor receptor (EGFR); EGFR upregulation leads to a decrease in E-cadherin expression. This decrease, combined with an associated increase in MMP-9, results in a reduction of cell-to-cell adhesion (49).
Viral oncogenes E6 and E7 are actively transcribed in HPV-infected cells. E6 and E7 contribute carcinogenesis by disrupting centrosome synthesis, which is central to mitosis. Thus, a hallmark feature of both HPV-mediated premalignant lesions and malignant tumors is the development of multipolar mitosis. Additionally, viral E6 oncoprotein targets p53 tumor suppressor protein whereas viral E7 oncoprotein primarily targets retinoblastoma-1 (RB1) tumor suppressor protein (50). These oncoproteins inactivate their respective target tumor suppressor proteins through binding. Since the p53 and RB1 are negative regulators of cellular proliferation, the inactivation leads to uncontrolled cellular growth. In HPV-negative penile, head and neck squamous cell carcinomas (HNSCC), p53 is mutated and may be associated with lymph node metastases (51,52). However, wild-type p53 function is crucial to the development of cervical tumors, but p53 mutations frequently occur later in the metastatic progression of HPV-positive primary cervical cancers (53). In HPV-positive tonsillar cancer, HPV-16 oncogenes E6 and E7 oncogenes are generally expressed (36,37). In the matter of HPV-mediated cervical cancer pathogenesis, it is known that HPV and chromosomal recombination is frequent and required for progression to cancer; DNA hypermethylation of the L1 gene is established as a biomarker for cancer progression. Some studies have shown that there are high rates of HPV-16 methylation in penile cancer similar to those seen in cervical cancers (54).
In high-risk HPVs, viral E7 oncoprotein binds with Rb tumor-suppressor protein with much higher affinity than E7 produced by low-risk HPVs, such as HPV-6 and HPV-11. One of the major functions of Rb is to bind and inhibit transcription factors of the E2F-family, which leads to the repression of transcription of genes with products involved in DNA and chromosomal replication (55). Viral E7 oncoprotein interferes with the Rb and E2F interaction, leading to the release of E2F factors in their transcriptionally active forms, resulting in autonomous cell proliferation without G1 cell-cycle stops (56). This allows for cyclin-dependent kinase inhibitor p16ink4a to accumulating in the nucleus, which inhibits G1 cyclin-dependent kinase 4 (CDKN4), CDKN6, and cyclin-D dependent kinases, thus initiating phosphorylation (inactivation) of the Rb tumor-suppressor protein. Hence, in high-risk HPV transcriptionally-active infection, p16(INK4a) overexpression can serve as a surrogate immunohistochemical marker (57,58). However, this inactivation of RB1 and enhancement of p16 expression can occur both in HPV-associated tumors and in HPV-negative tumors that have mutations leading to RB1 loss (58).
HPV and pre-neoplastic lesions
HPV infection can result in two types of external genital lesions depending on how the virus affects the squamous epithelium. One type is condyloma acuminata (genital warts), which are low grade lesions resulting from the production of virus in the squamous epithelium due to a transient HPV viral infection. The other type of external genital lesions are undifferentiated PeIN, which are HPV viral-associated precancerous lesions, a result of viral genome integration into the host genome leading to overexpression of oncogene, driving cell proliferation leading to malignant transformation (34). PeIN lesions have a broad range of morphology and can be classified as differentiated and undifferentiated (59). Differentiated PeIN is usually associated with nonviral factors (e.g., chronic inflammation, lichen sclerosis, phimosis) and is anticipated to progress to well-differentiated and keratinized SCC (60). Undifferentiated PeIN is usually associated with HPV infection and is considered to be a precancerous lesion that progresses to basaloid and warty subtypes of SCC (61). Alemany et al. executed a large international study from 25 countries to explore HPV prevalence in penile cancer tumors and precancerous lesions. This study diagnosed 85 precancerous lesions and 1,010 penile cancers in which HPV DNA was found in 87.1% of precancerous lesions and 33.1% of penile tumors (62).
Aynaud et al. found HPV DNA in approximately 75% of Grade I PeIN, 93% of Grade II PeIN, and 100% of Grade III PeIN (63). Sudenga et al. studied 1,788 HPV-positive men and found that 92 acquired external genital lesions, with 9 developing PeIN and 86 developing condyloma (39). In both of these studies, HPV-16 was found in a vast majority of PeIN lesions. In a study of males from varying age groups, the prevalence of HPV in prepuce samples absent of external lesions were examined for high-risk HPV subtypes. High-risk HPV subtypes were found in 45.5% of children (0–10 years old), 60.6% of adolescents (11–20 years old), and 58.3% in adults (>20 years old). The highest prevalence (59.8%) was shown in age groups that had estimated higher rates of sexual activity (>14 years old) (64). These results indicate that although PeIN transformation is a rare event, high-risk HPV infection is common in males and supports vaccination protocols in boys and young adults.
Current therapies against HPV
Preventative lifestyle factors
Several factors predispose to genital HPV infection, yielding a series of preventative measures that can be undertaken to minimize this risk. Inconsistent or lack of condom use has been linked to higher rates of HPV infection (65-69). Hence, their regular use is an important point on which to counsel patients. Alcohol (70) and tobacco use (71-73) also have a correlation with increased HPV infection independent of the number of sexual partners, so avoidance of these substances is advisable. A study of South African women showed high rates of co-infection with sexually-transmitted infections and HPV, most commonly herpes simplex type 2 and chlamydia trachomatis (74). Likewise, higher number of sexual partners has been repeatedly demonstrated as a risk factor for genital HPV infection (29,66,75-77). Another widely-investigated intervention for the reduction of HPV infection is circumcision (16,78-83). In fact, a 2017 systematic review and meta-analysis of 24,401 men over 30 articles found significantly reduced odds of HPV prevalence in circumcised versus uncircumcised men [odds ratio (OR): 0.68; 95% CI: 0.56–0.82] (83). In summary, genital HPV infection risk can be reduced by circumcision and safe sex practices, including consistent condom use, reducing sexual partners and avoidance of sexually-transmitted infections, alcohol and tobacco.
HPV vaccination
The first HPV vaccine (Gardasil, Merck & Co., Inc., Whitehouse Station, NJ, USA) was approved by the U.S. Food and Drug Administration (FDA) on June 8, 2006. It consisted of the quadrivalent strain, covering HPV types 6, 11, 16 and 18. This was later followed by the bivalent vaccine (Cervarix, GlaxoSmithKline Biologicals, Rixensart, Belgium) in 2009 for types 16 and 18 (84). Most recent to be FDA-approved in 2014 was the 9-valent version (Gardasil 9, Merck & Co., Inc.), protecting against HPV types 6, 11, 16, 18, 31, 33, 45, 52 and 58 (85).
The most robust available data regarding HPV vaccines regards cervical intraepithelial neoplasia and cervical cancer. A recent 10-year review and meta-analysis of several randomized controlled trials (RCT) cited efficacy from 89.8–100% at follow-up of 34.9 months to 9.4 years (86). The most common adverse event was pain at the injection site and any serious adverse events were determined not to be vaccine-related (86). Another 10-year review found similar efficacy, specifically of the bivalent and 9-valent vaccines, again, in CIN2+ lesions (87).
However, regarding male HPV cases, a significant concern is the low rate of seroconversion after natural infection (88). In addition, it has been suggested that HPV antibody seropositivity does not provide significant immunity to future infections like it does in women (89). Fortunately, the quadrivalent HPV vaccine has been shown to be highly immunogenic in men age 16 to 26, with seroconversion by month 7, remaining elevated even at 36 months, with titers comparable to those in women (90). A 2011 randomized, placebo-controlled double-blind trial of 4,065 boys age 16 to 26 across 18 countries showed promising efficacy of the quadrivalent vaccine in preventing external genital lesions. Observed efficacy in the intention-to-treat population was 60.2% (95% CI: 45.8–78.6); efficacy in the per-protocol group was 83.8% (95% CI: 61.2–94.4). PeIN was observed only in the placebo group. The trial showed similar adverse events data and time to seroconversion as previous reports (91). Immunogenicity of the quadrivalent vaccine has also been demonstrated in a Phase II clinical trial in men aged 27–45 in the U.S. and Mexico, comparable to levels seen in younger men (92).
Current recommendations from the Centers for Disease Control’s Advisory Committee on Immunization Practices include routine HPV vaccination with either the bi- or quadrivalent vaccine in boys and girls age 11–12 years. Girls 13–26 who have not been previously vaccinated should receive the vaccine, as well as boys 13–21. Males up to age 26 who have sex with men or are immunocompromised should also be vaccinated. Vaccine schedule includes a single dose, followed by a second 1–2 months later and the final dose 6 months after that (93). The 9-valent vaccine was added to the recommendations in 2015 (94). Dosing schedules were also recently updated in late 2016. If vaccinated before age 15, two doses of the HPV vaccine are appropriate, the second dose 6–12 months following the first. After 15 years of age, the standard 3-dose regimen still applies (95).
Increasing evidence and awareness of the link between HPV and various cancers, and the availability of a vaccine, have made international vaccination programs more ubiquitous, including 18 countries (96). In the United States specifically, the most recent data available show that in 2015, 28.1% of males received a full series of the HPV vaccine, compared to 41.9% of females and 34.9% of adolescents, overall (97). The trend has been steadily improving with each year (98) but more work is needed to augment these numbers and work toward eradicating HPV. This certainly serves to emphasize the importance of appropriate patient counseling and advocacy for ongoing research.
Current therapies against HPV
Targeted therapies
While well-established treatment recommendations exist for penile cancer and its metastatic manifestations, HPV-specific targeted therapies remain a promising area of active investigation. Unfortunately, therapeutic HPV vaccine research is lacking, but investigation of nanoparticles might possibly yield an effective drug delivery system to make this a reality (99,100). Otherwise, a majority of available research has been done in head and neck SCC. For example, HPV-positive HNSCCs have demonstrated improved prognosis over HPV-negative tumors (101) and even appear differently on CT imaging, based on texture (102). They are more radiosensitive (101), leading to the development of a multicenter phase II/III RCT investigating outcomes of lower radiation dose in HPV-positive tumors (103).
Additionally, HPV-positive HNSCCs show markedly increased intratumoral immune cell infiltrate, as well as decreased Cox-2 and increased programmed cell death protein-1 (PD-1) mRNA expression (104). In fact, a study on mice suggested that the use of HPV vaccine to upregulate PD-1 expression acted in synergism with PD-1 blockade to enhance antitumor efficacy (105). Furthermore, PD-1 and its ligand, programmed cell death ligand 1 (PD-L1), are elevated in high risk-associated HPV CIN lesions and are associated with impaired cell-mediated immunity (106). Likewise, favorable response in metastatic cervical cancer has been demonstrated in early studies with use of HPV tumor-infiltrating T-cells, with persistence of clinical benefit at 1 month (107). Nivolumab, an anti-PD-1 monoclonal antibody has shown improved outcomes in HNSCC treatment after platinum-based chemotherapy (108), and ipilimumab, a CTLA-4 inhibitor, is currently in clinical trials, given its success in melanoma. Another study showed downregulation of antigen presentation of HPV E7 peptides in HNSCC, suggesting a target for immunotherapy, as well as highlighting the goal of stimulating antigen presentation (109). Another study showed that HPV-16 E6 protein could be targeted by engineered T cells, another potential target for therapeutics (110).
Research specifically in penile cancer is limited, but expression of PD-L1 has been found to be higher in non-high-risk HPV penile tumors, with low levels associated with absent lymph node metastases and better prognosis (111). A study of 148 penile SCCs showed increased HER3 expression in HPV-positive tumors and higher phosphorylated EGFR in HPV-negative tumors (112). Interestingly, another study found low levels of miRNA-146a, a downregulator of EGFR, in HPV-positive tumors, suggesting a mechanism of EGFR upregulation in HPV-positive patients (113). Other studies have demonstrated aberrant DNA methylation (114), and differential miRNA and mRNA (115) levels based on whether penile tumors were HPV-negative or positive, yielding more potential targets.
While there are several biomarkers under investigation, the majority of research is still in HPV-related cancers other than penile SCC. However, the potential is evident, and with time and more translational research, diagnostic and therapeutic targets will likely become available in the near future.
Clinical trials
There are currently several clinical trials investigating targeted therapies for HPV-related cancers. These include anti-HPV T-cells (NCT02280811, NCT02379520, NCT02858310), a novel therapeutic vaccine in combination with platinum-based chemotherapy (NCT02526316), nivolumab versus combination therapy (NCT02488759) and an investigational drug (NCT01807546).
Summary
With increasing rates in our population, burden of HPV-related pre-malignant lesions will continue to affect a great proportion of our population. Increased patient education along with prevention strategies such us condom use, hygienic measures, smoking cessation, and avoidance of chronic inflammatory states can have considerable impact on pathogenesis of pre-cancerous lesions of the penis. Although, the adoption of HPV vaccination has led to some success in female HPV-related cancers, the results are yet to be elucidated in the male population. With vaccination programs aimed at adolescents and young adults, a decrease in the incidence of pre-malignant lesions and subsequent progression to penile cancer should be evident in the coming years. Longer prospective follow-up will be required to fully assess the benefit of widespread vaccine adoption in the male population. In the meantime, more translational research and collaboration is encouraged aiming to provide increased therapeutic options for a significantly morbid and rare disease with potentially preventive and curative pathways.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol 2009;27:141-50. [Crossref] [PubMed]
- Hakenberg OW, Comperat EM, Minhas S, et al. EAU guidelines on penile cancer: 2014 update. Eur Urol 2015;67:142-50. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol 2007;25:361-7. [Crossref] [PubMed]
- Djajadiningrat RS, van Werkhoven E, Meinhardt W, et al. Penile sparing surgery for penile cancer-does it affect survival? J Urol 2014;192:120-5. [Crossref] [PubMed]
- Mahesan T, Hegarty PK, Watkin NA. Advances in Penile-Preserving Surgical Approaches in the Management of Penile Tumors. Urol Clin North Am 2016;43:427-34. [Crossref] [PubMed]
- Diorio GJ, Giuliano AR. The Role of Human Papilloma Virus in Penile Carcinogenesis and Preneoplastic Lesions: A Potential Target for Vaccination and Treatment Strategies. Urol Clin North Am 2016;43:419-25. [Crossref] [PubMed]
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12-9. [Crossref] [PubMed]
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294-301. [Crossref] [PubMed]
- Lu B, Wu Y, Nielson CM, et al. Factors associated with acquisition and clearance of human papillomavirus infection in a cohort of US men: a prospective study. J Infect Dis 2009;199:362-71. [Crossref] [PubMed]
- Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003;348:518-27. [Crossref] [PubMed]
- Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer 2006;119:2620-3. [Crossref] [PubMed]
- Dunne EF, Nielson CM, Stone KM, et al. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis 2006;194:1044-57. [Crossref] [PubMed]
- Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine 2012;30 Suppl 5:F12-23. [Crossref] [PubMed]
- Weaver BA, Feng Q, Holmes KK, et al. Evaluation of genital sites and sampling techniques for detection of human papillomavirus DNA in men. J Infect Dis 2004;189:677-85. [Crossref] [PubMed]
- Bruni L, Diaz M, Castellsague X, et al. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 2010;202:1789-99. [Crossref] [PubMed]
- Castellsague X, Bosch FX, Munoz N, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med 2002;346:1105-12. [Crossref] [PubMed]
- Bleeker MC, Berkhof J, Hogewoning CJ, et al. HPV type concordance in sexual couples determines the effect of condoms on regression of flat penile lesions. Br J Cancer 2005;92:1388-92. [Crossref] [PubMed]
- Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA 2007;297:813-9. [Crossref] [PubMed]
- Giuliano AR, Tortolero-Luna G, Ferrer E, et al. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine 2008;26 Suppl 10:K17-28. [Crossref] [PubMed]
- Chaturvedi AK, Graubard BI, Broutian T, et al. NHANES 2009-2012 Findings: Association of Sexual Behaviors with Higher Prevalence of Oral Oncogenic Human Papillomavirus Infections in U.S. Men. Cancer Res 2015;75:2468-77. [Crossref] [PubMed]
- D’Souza G, Westra WH, Wang SJ, et al. Differences in the Prevalence of Human Papillomavirus (HPV) in Head and Neck Squamous Cell Cancers by Sex, Race, Anatomic Tumor Site, and HPV Detection Method. JAMA Oncol 2016. [Epub ahead of print]. [PubMed]
- Markowitz LE, Sternberg M, Dunne EF, et al. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003-2004. J Infect Dis 2009;200:1059-67. [Crossref] [PubMed]
- Svare EI, Kjaer SK, Poll P, et al. Determinants for contraceptive use in young, single, Danish women from the general population. Contraception 1997;55:287-94. [Crossref] [PubMed]
- Annunziata C, Buonaguro L, Buonaguro FM, et al. Characterization of the human papillomavirus (HPV) integration sites into genital cancers. Pathol Oncol Res 2012;18:803-8. [Crossref] [PubMed]
- Frisch M, Friis S, Kjaer SK, et al. Falling incidence of penis cancer in an uncircumcised population (Denmark 1943-90). BMJ 1995;311:1471. [Crossref] [PubMed]
- Graafland NM, Verhoeven RH, Coebergh JW, et al. Incidence trends and survival of penile squamous cell carcinoma in the Netherlands. Int J Cancer 2011;128:426-32. [Crossref] [PubMed]
- Baldur-Felskov B, Hannibal CG, Munk C, et al. Increased incidence of penile cancer and high-grade penile intraepithelial neoplasia in Denmark 1978-2008: a nationwide population-based study. Cancer Causes Control 2012;23:273-80. [Crossref] [PubMed]
- Arya M, Li R, Pegler K, et al. Long-term trends in incidence, survival and mortality of primary penile cancer in England. Cancer Causes Control 2013;24:2169-76. [Crossref] [PubMed]
- Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults--United States, 2006. MMWR Morb Mortal Wkly Rep 2007;56:1157-61. [PubMed]
- Syrjanen S. HPV infections and tonsillar carcinoma. J Clin Pathol 2004;57:449-55. [Crossref] [PubMed]
- Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 2008;26:612-9. [Crossref] [PubMed]
- Mellin H, Dahlgren L, Munck-Wikland E, et al. Human papillomavirus type 16 is episomal and a high viral load may be correlated to better prognosis in tonsillar cancer. Int J Cancer 2002;102:152-8. [Crossref] [PubMed]
- Leto M, Santos GF Junior, Porro AM, et al. Human papillomavirus infection: etiopathogenesis, molecular biology and clinical manifestations. An Bras Dermatol 2011;86:306-17. [Crossref] [PubMed]
- Spiess PE, Dhillon J, Baumgarten AS, et al. Pathophysiological basis of human papillomavirus in penile cancer: Key to prevention and delivery of more effective therapies. CA Cancer J Clin 2016. [Crossref] [PubMed]
- Wiener JS, Effert PJ, Humphrey PA, et al. Prevalence of human papillomavirus types 16 and 18 in squamous-cell carcinoma of the penis: a retrospective analysis of primary and metastatic lesions by differential polymerase chain reaction. Int J Cancer 1992;50:694-701. [Crossref] [PubMed]
- Snijders PJ, Cromme FV, van den Brule AJ, et al. Prevalence and expression of human papillomavirus in tonsillar carcinomas, indicating a possible viral etiology. Int J Cancer 1992;51:845-50. [Crossref] [PubMed]
- Wilczynski SP, Lin BT, Xie Y, et al. Detection of human papillomavirus DNA and oncoprotein overexpression are associated with distinct morphological patterns of tonsillar squamous cell carcinoma. Am J Pathol 1998;152:145-56. [PubMed]
- Ingles DJ, Pierce Campbell CM, Messina JA, et al. Human papillomavirus virus (HPV) genotype- and age-specific analyses of external genital lesions among men in the HPV Infection in Men (HIM) Study. J Infect Dis 2015;211:1060-7. [Crossref] [PubMed]
- Sudenga SL, Ingles DJ, Pierce Campbell CM, et al. Genital Human Papillomavirus Infection Progression to External Genital Lesions: The HIM Study. Eur Urol 2016;69:166-73. [Crossref] [PubMed]
- Bezerra AL, Lopes A, Landman G, et al. Clinicopathologic features and human papillomavirus dna prevalence of warty and squamous cell carcinoma of the penis. Am J Surg Pathol 2001;25:673-8. [Crossref] [PubMed]
- Lont AP, Kroon BK, Horenblas S, et al. Presence of high-risk human papillomavirus DNA in penile carcinoma predicts favorable outcome in survival. Int J Cancer 2006;119:1078-81. [Crossref] [PubMed]
- Djajadiningrat RS, Jordanova ES, Kroon BK, et al. Human papillomavirus prevalence in invasive penile cancer and association with clinical outcome. J Urol 2015;193:526-31. [Crossref] [PubMed]
- Steinestel J, Al Ghazal A, Arndt A, et al. The role of histologic subtype, p16(INK4a) expression, and presence of human papillomavirus DNA in penile squamous cell carcinoma. BMC Cancer 2015;15:220. [Crossref] [PubMed]
- Zhai JP, Wang QY, Wei D, et al. Association between HPV DNA and disease specific survival in patients with penile cancer. Zhonghua Yi Xue Za Zhi 2013;93:2719-22. [PubMed]
- Fonseca AG, Soares FA, Burbano RR, et al. Human Papilloma Virus: Prevalence, distribution and predictive value to lymphatic metastasis in penile carcinoma. Int Braz J Urol 2013;39:542-50. [Crossref] [PubMed]
- Protzel C, Knoedel J, Zimmermann U, et al. Expression of proliferation marker Ki67 correlates to occurrence of metastasis and prognosis, histological subtypes and HPV DNA detection in penile carcinomas. Histol Histopathol 2007;22:1197-204. [PubMed]
- Bezerra AL, Lopes A, Santiago GH, et al. Human papillomavirus as a prognostic factor in carcinoma of the penis: analysis of 82 patients treated with amputation and bilateral lymphadenectomy. Cancer 2001;91:2315-21. [Crossref] [PubMed]
- Gross G, Pfister H. Role of human papillomavirus in penile cancer, penile intraepithelial squamous cell neoplasias and in genital warts. Med Microbiol Immunol 2004;193:35-44. [Crossref] [PubMed]
- Agarwal G, Gupta S, Spiess PE. Novel targeted therapies for the treatment of penile cancer. Expert Opin Drug Discov 2014;9:959-68. [Crossref] [PubMed]
- Flaherty A, Kim T, Giuliano A, et al. Implications for human papillomavirus in penile cancer. Urol Oncol 2014;32:53 e1-8.
- Protzel C, Spiess PE. Molecular research in penile cancer-lessons learned from the past and bright horizons of the future? Int J Mol Sci 2013;14:19494-505. [Crossref] [PubMed]
- Wichmann G, Rosolowski M, Krohn K, et al. The role of HPV RNA transcription, immune response-related gene expression and disruptive TP53 mutations in diagnostic and prognostic profiling of head and neck cancer. Int J Cancer 2015;137:2846-57. [Crossref] [PubMed]
- Crook T, Vousden KH. Properties of p53 mutations detected in primary and secondary cervical cancers suggest mechanisms of metastasis and involvement of environmental carcinogens. EMBO J 1992;11:3935-40. [PubMed]
- Kalantari M, Villa LL, Calleja-Macias IE, et al. Human papillomavirus-16 and -18 in penile carcinomas: DNA methylation, chromosomal recombination and genomic variation. Int J Cancer 2008;123:1832-40. [Crossref] [PubMed]
- Lipinski MM, Jacks T. The retinoblastoma gene family in differentiation and development. Oncogene 1999;18:7873-82. [Crossref] [PubMed]
- Chellappan S, Kraus VB, Kroger B, et al. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci U S A 1992;89:4549-53. [Crossref] [PubMed]
- Deng Z, Hasegawa M, Aoki K, et al. A comprehensive evaluation of human papillomavirus positive status and p16INK4a overexpression as a prognostic biomarker in head and neck squamous cell carcinoma. Int J Oncol 2014;45:67-76. [Crossref] [PubMed]
- Reuschenbach M, Seiz M, von Knebel Doeberitz C, et al. Evaluation of cervical cone biopsies for coexpression of p16INK4a and Ki-67 in epithelial cells. Int J Cancer 2012;130:388-94. [Crossref] [PubMed]
- Velazquez EF, Chaux A, Cubilla AL. Histologic classification of penile intraepithelial neoplasia. Semin Diagn Pathol 2012;29:96-102. [Crossref] [PubMed]
- Chaux A, Velazquez EF, Algaba F, et al. Developments in the pathology of penile squamous cell carcinomas. Urology 2010;76:S7-S14. [Crossref] [PubMed]
- Gregoire L, Cubilla AL, Reuter VE, et al. Preferential association of human papillomavirus with high-grade histologic variants of penile-invasive squamous cell carcinoma. J Natl Cancer Inst 1995;87:1705-9. [Crossref] [PubMed]
- Alemany L, Cubilla A, Halec G, et al. Role of Human Papillomavirus in Penile Carcinomas Worldwide. Eur Urol 2016;69:953-61. [Crossref] [PubMed]
- Aynaud O, Ionesco M, Barrasso R. Penile intraepithelial neoplasia. Specific clinical features correlate with histologic and virologic findings. Cancer 1994;74:1762-7. [Crossref] [PubMed]
- Klinglmair G, Pichler R, Zelger B, et al. Prevalence of the human papillomavirus (HPV) expression of the inner prepuce in asymptomatic boys and men. World J Urol 2013;31:1389-94. [Crossref] [PubMed]
- Beder Ribeiro CM, Ferrer I, Santos de Farias AB, et al. Oral and genital HPV genotypic concordance between sexual partners. Clin Oral Investig 2014;18:261-8. [Crossref] [PubMed]
- Deshmukh AA, Tanner RJ, Luetke MC, et al. Prevalence and Risk of Penile Human Papillomavirus Infection: Evidence From The National Health and Nutrition Examination Survey 2013-2014. Clin Infect Dis 2017;64:1360-6. [Crossref] [PubMed]
- Walczak L, Dutkiewicz S, Marszalek A. Incidence and prevalence of multiple types of genital human papillomavirus (HPV) infection in men: a study in Poland. Ginekol Pol 2013;84:112-5. [PubMed]
- Pierce Campbell CM, Lin HY, Fulp W, et al. Consistent condom use reduces the genital human papillomavirus burden among high-risk men: the HPV infection in men study. J Infect Dis 2013;208:373-84. [Crossref] [PubMed]
- Repp KK, Nielson CM, Fu R, et al. Male human papillomavirus prevalence and association with condom use in Brazil, Mexico, and the United States. J Infect Dis 2012;205:1287-93. [Crossref] [PubMed]
- Schabath MB, Thompson ZJ, Egan KM, et al. Alcohol consumption and prevalence of human papillomavirus (HPV) infection among US men in the HPV in Men (HIM) study. Sex Transm Infect 2015;91:61-7. [Crossref] [PubMed]
- Schabath MB, Villa LL, Lazcano-Ponce E, et al. Smoking and human papillomavirus (HPV) infection in the HPV in Men (HIM) study. Cancer Epidemiol Biomarkers Prev 2012;21:102-10. [Crossref] [PubMed]
- Schabath MB, Villa LL, Lin HY, et al. A prospective analysis of smoking and human papillomavirus infection among men in the HPV in Men Study. Int J Cancer 2014;134:2448-57. [Crossref] [PubMed]
- Wiley DJ, Elashoff D, Masongsong EV, et al. Smoking enhances risk for new external genital warts in men. Int J Environ Res Public Health 2009;6:1215-34. [Crossref] [PubMed]
- Menezes LJ, Pokharel U, Sudenga SL, et al. Patterns of prevalent HPV and STI co-infections and associated factors among HIV-negative young Western Cape, South African women: the EVRI trial. Sex Transm Infect 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Anic GM, Lee JH, Villa LL, et al. Risk factors for incident condyloma in a multinational cohort of men: the HIM study. J Infect Dis 2012;205:789-93. [Crossref] [PubMed]
- Taylor S, Bunge E, Bakker M, et al. The incidence, clearance and persistence of non-cervical human papillomavirus infections: a systematic review of the literature. BMC Infect Dis 2016;16:293. [Crossref] [PubMed]
- Vardas E, Giuliano AR, Goldstone S, et al. External genital human papillomavirus prevalence and associated factors among heterosexual men on 5 continents. J Infect Dis 2011;203:58-65. [Crossref] [PubMed]
- Albero G, Villa LL, Lazcano-Ponce E, et al. Male circumcision and prevalence of genital human papillomavirus infection in men: a multinational study. BMC Infect Dis 2013;13:18. [Crossref] [PubMed]
- Giuliano AR, Lazcano E, Villa LL, et al. Circumcision and sexual behavior: factors independently associated with human papillomavirus detection among men in the HIM study. Int J Cancer 2009;124:1251-7. [Crossref] [PubMed]
- Gray RH, Serwadda D, Kong X, et al. Male circumcision decreases acquisition and increases clearance of high-risk human papillomavirus in HIV-negative men: a randomized trial in Rakai, Uganda. J Infect Dis 2010;201:1455-62. [Crossref] [PubMed]
- Tobian AA, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med 2009;360:1298-309. [Crossref] [PubMed]
- Wilson LE, Gravitt P, Tobian AA, et al. Male circumcision reduces penile high-risk human papillomavirus viral load in a randomised clinical trial in Rakai, Uganda. Sex Transm Infect 2013;89:262-6. [Crossref] [PubMed]
- Zhu YP, Jia ZW, Dai B, et al. Relationship between circumcision and human papillomavirus infection: a systematic review and meta-analysis. Asian J Androl 2017;19:125-31. [PubMed]
- Schiller JT, Castellsague X, Villa LL, et al. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine 2008;26 Suppl 10:K53-61. [Crossref] [PubMed]
- Gardasil 9. U. S. Food & Drug Administration. 2016. Available online: https://www.fda.gov/biologicsbloodvaccines/vaccines/approvedproducts/ucm426445.htm
- Angioli R, Lopez S, Aloisi A, et al. Ten years of HPV vaccines: State of art and controversies. Crit Rev Oncol Hematol 2016;102:65-72. [Crossref] [PubMed]
- Harper DM, DeMars LR. HPV vaccines—A review of the first decade. Gynecol Oncol 2017. [Epub ahead of print]. [Crossref]
- Giuliano AR, Viscidi R, Torres BN, et al. Seroconversion Following Anal and Genital HPV Infection in Men: The HIM Study. Papillomavirus Res 2015;1:109-15. [Crossref] [PubMed]
- Lu B, Viscidi RP, Wu Y, et al. Prevalent serum antibody is not a marker of immune protection against acquisition of oncogenic HPV16 in men. Cancer Res 2012;72:676-85. [Crossref] [PubMed]
- Hillman RJ, Giuliano AR, Palefsky JM, et al. Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old. Clin Vaccine Immunol 2012;19:261-7. [Crossref] [PubMed]
- Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011;364:401-11. [Crossref] [PubMed]
- Giuliano AR, Isaacs-Soriano K, Torres BN, et al. Immunogenicity and safety of Gardasil among mid-adult aged men (27-45 years)--The MAM Study. Vaccine 2015;33:5640-6. [Crossref] [PubMed]
- Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014;63:1-30. [PubMed]
- Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015;64:300-4. [PubMed]
- Meites E, Kempe A, Markowitz LE. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination-Updated Recommendations of the Advisory Committee on Immunization Practices. Am J Transplant 2017;17:834-7. [Crossref] [PubMed]
- Bailey HH, Chuang LT, duPont NC, et al. American Society of Clinical Oncology Statement: Human Papillomavirus Vaccination for Cancer Prevention. J Clin Oncol 2016;34:1803-12. [Crossref] [PubMed]
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13-17 Years - United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65:850-8. [Crossref] [PubMed]
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13-17 Years--United States, 2014. MMWR Morb Mortal Wkly Rep 2015;64:784-92. [Crossref] [PubMed]
- Bolhassani A, Ghasemi N, Servis C, et al. The efficiency of a novel delivery system (PEI600-Tat) in development of potent DNA vaccine using HPV16 E7 as a model antigen. Drug Deliv 2009;16:196-204. [Crossref] [PubMed]
- Saleh T, Bolhassani A, Shojaosadati SA, et al. MPG-based nanoparticle: An efficient delivery system for enhancing the potency of DNA vaccine expressing HPV16E7. Vaccine 2015;33:3164-70. [Crossref] [PubMed]
- Mirghani H, Amen F, Tao Y, et al. Increased radiosensitivity of HPV-positive head and neck cancers: Molecular basis and therapeutic perspectives. Cancer Treat Rev 2015;41:844-52. [Crossref] [PubMed]
- Fujita A, Buch K, Li B, et al. Difference Between HPV-Positive and HPV-Negative Non-Oropharyngeal Head and Neck Cancer: Texture Analysis Features on CT. J Comput Assist Tomogr 2016;40:43-7. [Crossref] [PubMed]
- Owadally W, Hurt C, Timmins H, et al. PATHOS: a phase II/III trial of risk-stratified, reduced intensity adjuvant treatment in patients undergoing transoral surgery for Human papillomavirus (HPV) positive oropharyngeal cancer. BMC Cancer 2015;15:602. [Crossref] [PubMed]
- Partlova S, Boucek J, Kloudova K, et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 2015;4:e965570. [Crossref] [PubMed]
- Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 2013;73:128-38. [Crossref] [PubMed]
- Yang W, Song Y, Lu YL, et al. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology 2013;139:513-22. [Crossref] [PubMed]
- Stevanovic S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol 2015;33:1543-50. [Crossref] [PubMed]
- Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375:1856-67. [Crossref] [PubMed]
- Albers A, Abe K, Hunt J, et al. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res 2005;65:11146-55. [Crossref] [PubMed]
- Draper LM, Kwong ML, Gros A, et al. Targeting of HPV-16+ Epithelial Cancer Cells by TCR Gene Engineered T Cells Directed against E6. Clin Cancer Res 2015;21:4431-9. [Crossref] [PubMed]
- Ottenhof SR, Djajadiningrat RS, de Jong J, et al. Expression of Programmed Death Ligand 1 in Penile Cancer is of Prognostic Value and Associated with HPV Status. J Urol 2017;197:690-7. [Crossref] [PubMed]
- Stankiewicz E, Prowse DM, Ng M, et al. Alternative HER/PTEN/Akt pathway activation in HPV positive and negative penile carcinomas. PLoS One 2011;6:e17517. [Crossref] [PubMed]
- Peta E, Cappellesso R, Masi G, et al. Down-regulation of microRNA-146a is associated with high-risk human papillomavirus infection and epidermal growth factor receptor overexpression in penile squamous cell carcinoma. Hum Pathol 2017;61:33-40. [Crossref] [PubMed]
- Kuasne H, Colus IM, Busso AF, et al. Genome-wide methylation and transcriptome analysis in penile carcinoma: uncovering new molecular markers. Clin Epigenetics 2015;7:46. [Crossref] [PubMed]
- Kuasne H, Barros-Filho MC, Busso-Lopes A, et al. Integrative miRNA and mRNA analysis in penile carcinomas reveals markers and pathways with potential clinical impact. Oncotarget 2017;8:15294-306. [PubMed]


