Office-based andrology and male infertility procedures—a cost-effective alternative
Introduction
Andrologic and male infertility procedures represent a distinct class of urologic surgeries. In contrast to other urologic procedures, which may involve intra-abdominal organs and thus require general anesthesia, andrologic and male infertility procedures are performed in the penis and scrotum, where pain can be fully controlled with local anesthesia. Office-based surgical procedures under local anesthesia offer several potential advantages over those performed with general or monitored anesthesia, including absence of post-op extended recovery, elimination of risk of pulmonary or cardiac complications, ability to communicate with the patient, avoidance of side effects from general anesthetic medications, and improved convenience for patients and surgeons.
Despite these known benefits, relatively little has been published on the topic of office-based andrologic and male infertility procedures (1). Authors have previously described performing several andrologic and male infertility procedures under local anesthesia including hydrocelectomy, malleable penile prosthesis, microepididymal and testicular sperm aspiration (MESA)/TESE, orchiectomy, spermatocelectomy, and varicocelectomy (1-15). However, many of these reports include the use of IV sedation, monitored anesthesia care, or are limited to 3rd world countries, with relatively few described in contemporary practices. Additionally, to our knowledge, no studies have reported on the complex procedure of vasoepididymostomy (VE) under local anesthesia alone.
Similarly, very limited data exist on the comparative cost-effectiveness of andrologic and male infertility office-based procedures over those performed under general anesthesia in the OR. Cost efficiency has become an important consideration in clinical practice, as insurance reimbursements continue to decline and value-based care is increasingly utilized as a metric of overall quality. This is particularly the case with andrologic and male infertility surgery, where the decision to proceed is often based on deductibles, copayments, and insurance coverage, rather than medical necessity alone. As many male infertility procedures are cash pay, some couples may even elect to pursue less effective options or to avoid treatment altogether to limit costs.
As just one example, cost considerations have led many to recommend PESA/TESA over the arguably superior MESA/TESE procedures due to lower costs, easier technique, ready availability, and minimally-invasive nature (16,17). Similarly, numerous publications and debates have argued the role for VR versus in-vitro fertilization (IVF), with cost-effectiveness often used as a key differentiator (18-22). Men seeking VR may also face a dilemma of choosing a less expensive office-based procedure (where a VE is not available) or paying significantly more for the ability to perform the more complex surgery in the OR (13). Investigators have even attempted to mitigate this limitation by defining pre-operative predictors for VE in an attempt to identify patients who would be appropriate candidates for office-based procedures (23).
Given the limited published data, we sought to report our experience in performing office-based andrology and male-infertility procedures. The objective of the current manuscript is therefore to assist providers who wish to introduce office-based procedures by providing practical tips and tricks as well as what to avoid in the hopes of reducing the overall learning curve. Additionally, clinical and financial-analysis comparisons are presented for male infertility procedures to highlight potential cost savings without compromising outcomes.
Methods
Comparison of outcomes for male infertility procedures
Although a wider variety of andrologic and male infertility procedures have been performed in our office from 2014–2016, in the current manuscript, direct comparisons are limited to male infertility procedures. These procedures were chosen specifically, as sufficient numbers were available in the OR as well as clinic to perform reasonable cost analyses and comparisons of outcomes. Many of the other andrologic procedures had either only been performed in the clinic by our surgical service (hydrocelectomy, spermatocelectomy, circumcision), or had too small of numbers to review meaningful outcomes (orchiectomies, testicular prostheses, penile plication, varicocelectomy, malleable penile prosthesis). This limited the ability to expand the comparative analyses to all office-based procedures. Limited results are also presented for office hydrocelectomies, as these procedures were performed using a novel, minimally-invasive approach.
Male infertility cases—patient cohort
A retrospective analysis was performed of a prospective registry maintained of all men undergoing TESE, MESA, and VR at our institution from January 2014 through December 2016. Relevant clinicopathologic variables were abstracted and analyzed. Due to the descriptive nature of the current report, statistical comparisons were not required. Data were also obtained from a prospective registry of all office hydrocelectomy procedures to report post-operative recurrence rates and complications. All protocols were reviewed and approved by the Institutional Review Board at the Mayo Clinic in Rochester, MN and conform to the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000). All patients with data abstracted had previously provided informed consent to permit use of their records for research purposes.
Male infertility cases—financial assessment
To determine potential cost savings associated with office-based procedures, a comparison was performed of five cases each in the office and OR for TESE, MESA, and VR. As noted previously, infertility cases were specifically selected as they often represent procedures that are partially or not covered by insurance and sufficient numbers of cases were available for review. All cases were performed by the same surgeon (LT) to limit variability with surgical time, technique, and supplies. For the financial assessments, only cases with a single procedure were included (i.e., MESA alone or TESE alone), to assure consistency and avoid confounding results with combined cases (i.e. MESA/TESE).
Costs were evaluated to include both fixed and variable expenses and included surgeon, anesthesia, and facility fees, as well as the cost of supplies and medications. To better represent true cost savings, analyses did not include data on amounts billed to insurance or patients, but rather the specific costs to an institution to perform these procedures.
Comparative results from each of the procedures and settings (office vs. OR) were averaged among the cases for TESE, MESA, and VR. Due to requests by our institution to maintain financial confidentiality, all results were reported as a percent relative reduction.
Clinical setup
To accommodate the needs for an office-based practice, a surgical suite was equipped with an operating microscope and a standard OR table. Two nurses were available during the majority of the procedure, and no anesthesiologists were involved. All other instruments, sutures, and equipment were identical between the office-based procedure room and the OR for all cases. With the patient’s consent, partners of the patient were allowed to be present in the room and observe.
Pain control
Systemic therapy
Prior to beginning the procedure, patients were administered an oral antibiotic (ciprofloxacin 500 mg). In the case of longer procedures such as hydrocelectomy, malleable/testicular prostheses, penile plication, spermatocelectomy, varicocelectomy, and VR, additional medications were offered including an oral pain medication (oxycodone 5 mg and acetaminophen 500 mg) and midazolam (5–15 mg depending on age and body habitus: >50 years =10 mg, >65 years =5 mg). Patients presenting without a driver were not offered oxycodone or midazolam, and many patients elected to not receive the supplementary medications. In the case of VR, patients were occasionally given an additional 5–10 mg of midazolam 90 minutes into the procedure if the effect of the first dose had resolved and if desired. Intravenous midazolam was not offered, as this requires oversight by anesthesiology at our institution and would require additional expenses for the patient. It is notable that these generalized medical therapies did not appear to enhance pain control, but did help to alleviate anxiety and minimize bother associated with lying flat for extended periods.
Local therapy
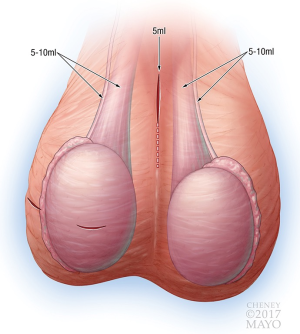
Local anesthesia was administered at the beginning of the case using liposomal bupivacaine. To expand the available volume, liposomal bupivacaine was diluted with normal saline to achieve a final volume of 80 mL. The location and volumes of anesthetic administered are demonstrated in Figure 1. In cases of hydrocelectomy, MESA, scrotal orchiectomy, spermatocelectomy, TESE, or testicular prosthesis, local anesthesia was applied to the incision (5–10 mL) and testicular cord (10 mL) on one or both sides. Varicoceles and inguinal orchiectomies received an additional 5–10 ml in the cord and external inguinal canal directly. For VR, 5 mL is used on the incision, 5–10 mL on each vas, and 5–10 mL on each testicular cord. Circumcisions, penile mass excisions, penile plications, and malleable penile prosthesis placement were performed using a penile block with 20–30 mL.
Technical aspects of procedures performed
As all procedures in the current text have been extensively described in the surgical literature, only select modifications relevant to minimally-invasive, office-based approaches are described below. No specific changes to technique were performed in regards to circumcisions, malleable penile prostheses, orchiectomies, penile plications, spermatocelectomies, testicular implants, or varicocelectomies. Similarly, although changes to technique are not required for hydrocelectomy, MESA/TESE, VR, we began utilizing a less-invasive approach to further minimize recovery and potential complications. See Figure 2 for graphical depiction of suggested location of incisions with infertility-specific procedures.
Hydrocelectomy
Multiple modifications were introduced for the office hydrocelectomy in an attempt to reduce the rate of recurrence and hematomas. This technique will be described in greater detail in a forthcoming publication, including outcomes and comparisons to historical outcomes with the standard procedure at our institution. Although the standard technique for hydrocelectomy could be performed under local anesthesia alone, in our practice we incorporated both the minimally-invasive approach and use of local anesthesia concomitantly, and therefore we have not performed the surgery in the standard fashion where the hydrocele sac is fully delivered before draining.
After local anesthetic, a transverse, paramedian, high-scrotal incision is made on the ipsilateral side of the hydrocele over a 2 cm length. This is carried down to the level of the hydrocele sac, which is entered. At this point, a cord block is performed, as most often, the hydrocele is too large to preclude an adequate block previously. The hydrocele sac is then pulled through the wound and incised longitudinally in 4–6 separate regions. This is done to facilitate full delivery of the sac. This process is continued until the majority of the sac has been pulled through the wound and the testicle is directly compressed against the wound. At this point, all excess sac is excised, and the wound edges are oversew to include the dartos muscle as well as the hydrocele sac. This prevents any dissection of dartos off of the sac without directly oversewing afterwards and likely accounts for the very low rate of hematomas encountered.
We then place a 1-inch Penrose drain from the incision to a region approximately 4–5 cm caudal to the incision and bring the drain out through this location. This creates a through-and-through drain that is then secured to itself. The intent of the drain is to permit the testicle and remaining hydrocele sac to scar together in a decompressed state. The drain is left in place for 2 weeks and then subsequently removed.
MESA
Although a MESA can be performed in a standard fashion where the testicle is completely delivered, we have elected to utilize a more minimally-invasive approach without needing to deliver the testicle through the wound (15).
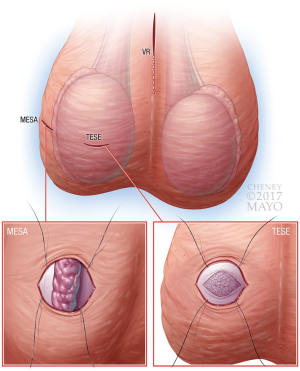
A transverse scrotal incision is made on the lateral aspect of the scrotum. This is preferable to the anterior approach, as it places the epididymis directly over the incision. If an anterior approach is used, the testicle can be rotated to place the epididymis in the center of the incision. Once the epididymis is visualized, parallel stay sutures are placed on each side of the epididymis (Figure 2). To improve visibility, an eyelid retractor can be placed into the wound. Alternatively, the pre-placed sutures can be secured to the drape to pull the testicle up to the scrotal wound and prevent migration during the case. The remainder of the MESA procedure is performed in the usual fashion.
TESE
Either a midline or transverse scrotal incision is made and extended for 1–2 cm. The testicle is grasped, with the epididymis positioned posteriorly, to avoid injury during initial dissection. This incision is carried through the tunica vaginalis with stay sutures placed on each side of the incision to provide retraction. Next, two horizontal, parallel stay sutures are placed into the tunica albuginea of the testicle, and a 1 cm incision is made into the testicle. See Figure 2 for graphical depiction of pre-placed stay sutures resulting in elevation of the testicle to the wound surface. The tunica is undermined using sharp scissors for 1 cm beyond the incision in all directions, and seminiferous tubules are expressed and excised. Hemostasis is achieved, and the tunica albuginea and vaginalis are sequentially closed using the pre-placed sutures. These minor modifications are preferred over the standard technique where the testicle is delivered to avoid testicular pressure during replacement of the testicle into its normal anatomic position within the scrotum.
Comment on microTESE
We have attempted to perform microTESE procedures on two occasions with difficulty in obtaining complete pain control. It has been our experience that while incisions and cautery can be completely blocked with local numbing medications, the sensation of testicular pressure cannot be controlled, even with direct application of anesthetic. Given the amount of manipulation required to perform an adequate microTESE, including the need for ongoing pressure on the tunica, we stopped attempting to perform this in the office. It is our belief that with additional general anesthetic (IV midazolam), this would be a feasible and cost-effective procedure. Although other providers may have more success, in our experience, oral midazolam dosing is not sufficiently precise to use as a pain-control agent.
Vasectomy reversal (VR)
Limited modifications are required to optimize pain control with an office-based VR procedure. A midline high scrotal incision is made (2–3 cm) after which the vasa are brought through the wound. Care should be taken throughout the procedure to minimize traction on the abdominal portion of the vas to limit sensations of flank, lower back, or inguinal pain pressure from this maneuver. No modifications are required during the dissection of the proximal and distal segments of the vasa, with the exception of granulomas, which often require additional direct administration of local numbing medication.
Once the vasa are dissected, the testicular end of the vas is sampled to assess for sperm. Fluid is also instilled using a 24-F angiocatheter into the abdominal portion of the vas to assess for patency. The volume of fluid instilled should be limited, as this may result in a painful sensation with volumes >1 mL.
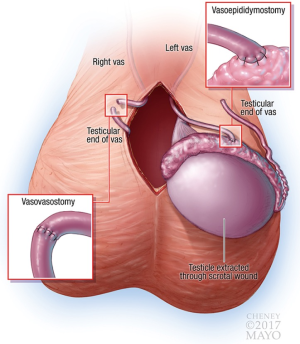
If the decision is made to proceed with a VV, this is performed in the usual fashion (in our practice, double-layer with 8-0 and 10-0 interrupted sutures) without specific modifications. If a VE is required, the scrotal incision is extended and the testicle delivered. If a VE is required on both sides, it has been our preference to only deliver one testicle at a time. This limits the extent of incision required, amount of traction placed on the abdominal portion of the vas, time spent with the testicle external to the body, and likelihood for venous congestion (facilitates pain control). Once the testicle is delivered, it should be moistened frequently to avoid drying out and thereby reducing sensitivity to pressure. As it is not possible to fully numb the testicle to sensations of pressure (in our experience), care should be taken to avoid compressing the testicle during microsurgery, and gentle manipulation should be used to return it to the scrotum. The VE is otherwise performed in the usual fashion using an intussuscepted technique (parallel 10-0 sutures with 8-0 second layer). See Figure 3 for graphical depiction of a right VV and left VE.
Results
Outcomes
From 2014–2016, a total of 113 in-office procedures were performed, including 32 VRs, 24 hydrocelectomies, 24 TESEs only, 10 circumcisions, 9 MESA +/− TESEs, 4 spermatocelectomies, 3 orchiectomies (1 inguinal), 2 microTESEs, 2 testicular prostheses and 1 each of malleable penile prosthesis, penile plication, and varicocelectomy. All surgeries were successfully completed, with no cases aborted prematurely. It is notable that not all procedures were immediately available in 2014 as our practice evolved from performing simple office-based procedures (TESE) to increasingly complex ones (VR beginning March 2016). Only two microTESE procedures were attempted, however, this was discontinued due to difficulty in obtaining complete anesthesia of the tunica, which we felt compromised our ability to comfortably perform an adequate dissection.
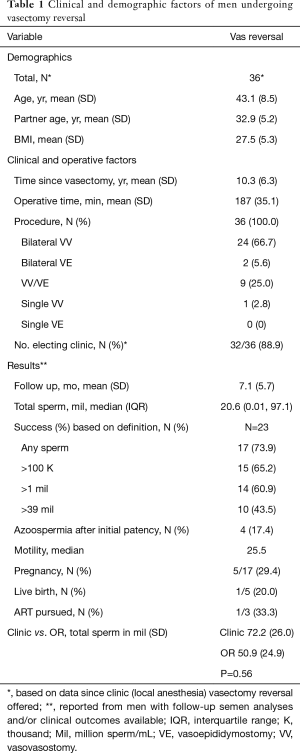
Tables 1 and 2 report the demographics, clinicopathologic information, and results of procedures for VR, MESA/TESE, and TESE cases. Note that numbers reflect the total amount of procedures performed once both office (local anesthesia) and OR (general anesthesia) offerings were available and are not reflective of the total number performed since the beginning of 2014.
Full table
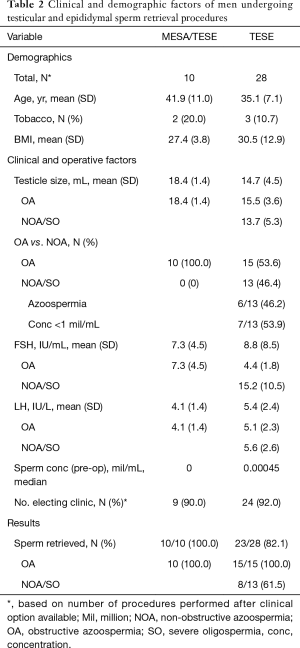
Full table
Overall, VR patients were an average of 10.3 years post vasectomy, and mean operative time was similar between settings (office 181 vs. OR 190 minutes, P=0.34). The rate of performing a VE on one or both sides was also similar, with 23% of cases requiring a VE in the office compared to 32% in the OR, P=0.56. At a mean follow-up of 7.1 months, no differences in total sperm counts were observed between cases in the office (72.2 million; SD 26.0) versus OR (50.9 million; SD 24.9), P=0.56.
For sperm retrieval procedures, testicular size was smaller, FSH higher, and sperm retrieval rates lower for microTESE and solitary TESE cases compared to MESA/TESE (P<0.05 for all variables). When comparing office versus OR, sperm retrieval rates were similar (MESA/TESE 100% vs. 100%, P=1.00; TESE 80% vs. 100%, P=0.36; microTESE 50% vs. 29%, P=0.58).
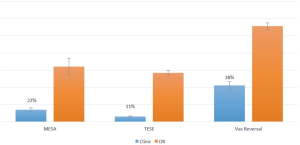
Analysis of direct and indirect costs demonstrated significant savings when performing surgery in an office setting without anesthesiology involvement compared to the OR. TESE procedures were the most cost-effective, at 11% of the costs of the OR, while MESA/TESE (22%), and VR (38%) also demonstrated significant cost reductions (see Figure 4).
Hydrocelectomy
Direct comparisons were not performed between office-based hydrocelectomies and those in the OR as all cases have been performed as a minimally-invasive modification in the office. Overall, mean hydrocele size was 332 mL, and 50% of patients were on some form of antiplatelet or blood thinner at the time of surgery (9 aspirin, 1 rivaroxaban, 1 warfarin, 1 ibuprofen). One patient (4%) experienced a delayed post-operative hematoma (warfarin patient, INR 5.1 at time of hematoma), and no patients developed post-operative infections (although we provided antibiotics until the wound edges fully closed by secondary intention). At a mean follow-up of 4.2 months, one patient (4%) experienced a small (estimated 30 mL) recurrence of hydrocele, likely relating to inadequate sac excision at the time of the procedure.
Learning curve
There is a clear learning curve in performing office-based surgical cases without monitored or general anesthesia, much of which is centered on appropriate tissue handling and targeted application of local anesthetic. Providers desiring to transition their practice to an office-based setting may elect to initially transition from an OR with general anesthesia to monitored care and finally to oral medications alone with anesthesia on standby if needed. In this setting, if deeper anesthesia is required, it can be done without rescheduling or disrupting the procedure. Similarly, providers may wish to step-wise transition from simpler procedures (vasectomy), to progressively more complex ones: MESA/TESE, circumcisions, varicocelectomy, orchiectomy, penile mass excisions, penile plication, and ultimately VR.
The following represents a listing of several key factors that have been identified during our learning curve in the office setting. Please also refer to Table 3 for a list of common issues with numbing and trouble-shooting measures.
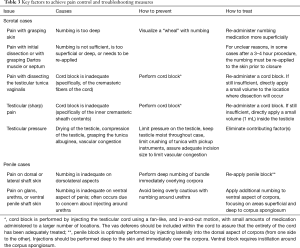
Full table
Anesthetic application
- No period of delay is required after local anesthetic application and surgical dissection. This is significant as there is near immediate feedback available from patients if numbing is inadequate or if additional numbing has been applied to the appropriate location;
- Liposomal bupivacaine is recommended. Although lidocaine provides adequate anesthesia, its duration of action is insufficient for VR and often wears off too rapidly for patients to achieve adequate pain control from oral agents. Other medium-term agents such as marcaine do not provide complete anesthesia in our experience unless combined with lidocaine (although the duration is longer);
- Despite manufacturer claims, liposomal bupivacaine cannot be diluted much beyond 60–80 mL without losing efficacy in the scrotum and penis. Some dilution is often required though, as the 20 mL volume is often inadequate to perform more complex scrotal surgery;
- Judicious use of local anesthetic is critical. It is preferred to have ≥20 mL remaining after all initial blocks are performed in case additional application is required. Given the toxicity limits of local anesthetics, if insufficient medication remains, it may require procedure cancellation;
- Numbing of any region is better accomplished utilizing a smaller volume spread out over a larger area rather than inserting the needle into one location and depositing 10 mL. For structures like the testicular cord, instillation is performed using a fan-like distribution while repeatedly advancing and withdrawing the needle. This results in repeated entry into the testicular cord, however, given the known wide distribution of nerves throughout the cord, it is necessary;
- Application of anesthetic to the vas requires direct instillation immediately adjacent to the vas. This can be practiced with vasectomy procedures by assuring that the patient experiences no discomfort;
- The sensation and pain with excessive testicular pressure is not fully controlled with local anesthetic. If the patient does not have pain with dissection of the tunica vaginalis, vas deferens, and epididymis, the local block is functioning appropriately.
Patient selection and consent
- Patients with baseline addictions to high-dose narcotics and poorly controlled anxiety are likely better suited for procedures under general anesthesia. In our experience with two patients meeting these criteria, although pain control was achieved and the procedures successfully completed, the difficulty in achieving anxiety control (difficult to dose midazolam with such tolerance) was particularly stressful on the surgical team;
- Informing the patient as to each person’s role and responsibility is essential, as many patients often do not fully understand how surgery is often a team-effort, which requires the help of one or more assistants in the operating suite.
Patient comfort and communication
- Patient comfort is an important aspect of satisfaction with office-based procedures. In our practice, patients are given an iPad with movies and games available and may have their partners present in the room;
- The procedure may be an interactive experience, where the partners may view the sperm under the microscope or periodically observe how the case is progressing;
- Prior to any significant portions of the case, patients are adequately warned that something notable will be upcoming. Examples include prior to applying numbing medication, grasping the scrotum for the first time, pulling on the abdominal portion of the vas to achieve greater length, replacing the testicles within the scrotum;
- Communication as a team must be worked out so that conversations and word choices are appropriately considered. This is particularly the case when a resident or other trainee is participating and when instruction or correction are required for surgical technique, needle placement, or other similar points of feedback;
- Patients are also able to directly provide feedback on desired outcomes. In the case of our penile plication, the patient and partner were able to view the anticipated correction of curvature prior to tying the sutures, which allowed them to report satisfaction before finalizing the surgery.
Discussion
The current manuscript demonstrates the viability of an office-based approach to the majority of surgical andrology procedures, including nearly all male infertility cases. There are several potential advantages to creating such a practice, including reduced costs, increased convenience for surgeons and patients (no NPO requirements, less waiting time before and after procedures), increased availability of procedures, reduced turnover times, and ability to verify complete pain control (cannot verify success of a cord block in the OR). There are clearly several financial benefits to patients and the overall healthcare system, as pre- and post-operative preparation and recovery are minimal, fewer teams (anesthesia) are required, rooms can be turned over more rapidly (no anesthesia required), and more rooms are often available (full OR suite not required). Patients and partners are also able to more directly participate in their own care, including providing feedback during a case (reporting satisfaction with penile curvature correction) or viewing outcomes as they occur (visualizing sperm under the microscope). Further advantages include reduced risks with anesthesia (ability to perform in traditionally poor surgical candidates) and improved convenience for the surgical team.
In our practice, the introduction of office-based procedures has permitted same day surgeries, reduced costs significantly for patients (office-based VR offered for $4,550, even if bilateral VE required), and most importantly, has not altered our surgical technique or outcomes. The issue of costs is particularly relevant in the field of infertility, as many therapies are not covered by insurance and couples are often left to ration decisions based on comparative costs. As such, any effort to reduce costs is welcomed and may increase access for a subset of patients who may have otherwise been excluded.
Regarding infertility cases, the combination of improved costs and availability along with the use of minimally-invasive techniques may also increase the utilization of gold-standard MESA/TESE procedures over lesser-specialized options such as PESA/TESA. Although the advantages/disadvantages of one approach over another (MESA vs. PESA and TESE vs. TESA) are beyond the scope of this manuscript, factors including cost, convenience, and invasiveness are likely no longer viable arguments against MESA/TESE.
Similarly, multiple publications have addressed the comparative cost-effectiveness of VR versus IVF (18-22). The concept of which option is preferred in each clinical scenario is complex and includes factors such as indirect vs. direct costs, age of female partner, duration since vasectomy, success rates of the specific VR surgeon and IVF clinic, duration of follow-up used to define success, rates of multiple gestation, potential for increased chromosomal abnormalities and subsequent care required, issues of diminished ovarian reserve, and couple preferences, among others. Although the current manuscript does not detail any financial aspects of IVF, the significant reduction in costs with an office-based VR (62% reduction) likely re-opens the debate and provides further support for the cost-effectiveness of VR over IVF in many scenarios.
It is important to highlight that improved cost-efficiency and reductions in invasiveness are ultimately secondary considerations to achieving optimal outcomes. Office-based VR have been available for many years at reduced costs (13). However, to our knowledge, these have all been limited to VV procedures, with no reports of office VE published to date. Similarly, office-based TESE and MESA have thus-far been minimally or not reported in contemporary practice, with IV sedation typically employed (15). The current series therefore bridges the divide between the benefits of office-based (cost, convenience) and OR procedures (efficacy, ability to do more complex reconstructions). Of note, a separate article in this special edition also offers an excellent detailed example of an office based MESA using monitored-anesthesia care.
The current description is limited by several factors. First, financial and outcomes analyses were limited to male infertility cases. This was done intentionally, as there were relatively limited numbers of select non-infertility cases available (circumcision, malleable penile prosthesis, orchiectomy, penile plication, spermatocelectomy, testicular prostheses, and varicocele), and some cases were uniquely performed in the office (hydrocelectomy). In the case of hydrocelectomies, we also began performing a different technique in the office at the same time, which precluded our ability to compare outcomes directly with those in the OR.
A second limitation with the current manuscript is the relative small numbers of isolated MESA cases. As with the hydrocelectomies, we rapidly converted our MESA practice to the clinic, which reduced the number of comparative OR cases to evaluate financial outcomes. It is unlikely that this impacted the reported cost analyses significantly, however, as the percentage reduction among all procedures was relatively similar. A third limitation is a lack of objective measures on comfort such as patient pain scores. As it was not our intent to publish outcomes initially, these were not routinely obtained.
It is also relevant that the current office-based techniques are likely not appropriate for all scrotal procedures, with microTESE being a notable example. Arguably, these are less common in the general male infertility practice than TESE procedures, however, this introduces new challenges in selecting cost-effective, step-wise algorithms for the appropriate treatment of men with non-obstructive azoospermia. It is likely that microTESE procedures could feasibly be performed in the office if IV midazolam were employed, although in many institutions this is not permitted without anesthesia oversight, thus reducing the cost-efficiency.
Despite the limitations, the current report has several strengths. First, these data represent a relatively large series of novel concepts and techniques over a three-year period. Second, the techniques have altered our practice patterns where cases in the OR are increasingly rare. In contrast to many procedures where patients are ‘selected’ for a given procedure, the current cohort represents a consecutive series, with 86% electing to proceed with surgery in the office. This is perhaps a better measure of long-term viability of a procedure, as often, new techniques may be described but not continued. Third, all cost analyses demonstrated clear, significant benefits with the office-based cases when considering both direct and indirect costs. Fourth, it is noteworthy that no cases were aborted or required upgrading to monitored or general anesthesia. This is an important factor, as many providers may not have ready access to convert the procedure if performed in an office-based setting.
Conclusions
Office-based surgical procedures performed under local anesthesia are a viable and cost-effective option for men seeking many andrologic or male-infertility procedures. In contrast to those performed in the OR, the current data demonstrate cost savings ranging from 62–89% with office-based procedures, while successfully completing all procedures and without compromising technique or outcomes. The ability to perform procedures in the office offers several advantages beyond cost, including improved patient and surgeon convenience, reduced risks with general/monitored anesthesia, and increased availability, among others. The improved cost-effectiveness of office-based procedures also re-opens debates on VR versus IVF as well as MESA/TESE vs. PESA/TESA and suggests a possible need for modification of current cost-based treatment algorithms. External validation is required to assess the translatability of these techniques to other practices.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All protocols were reviewed and approved by the Institutional Review Board at the Mayo Clinic in Rochester, MN and conform to the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000). All patients with data abstracted had previously provided informed consent to permit use of their records for research purposes.
References
- Leach GE. Local anesthesia for urologic procedures. Urology 1996;48:284-8. [Crossref] [PubMed]
- Magoha GA. Local infiltration and spermatic cord block for inguinal, scrotal and testicular surgery. East Afr Med J 1998;75:579-81. [PubMed]
- Agbakwuru EA, Salako AA, Olajide AO, et al. Hydrocelectomy under local anaesthesia in a Nigerian adult population. Afr Health Sci 2008;8:160-2. [PubMed]
- Kadihasanoglu M, Karaguzel E, Kacar CK, et al. Local or spinal anesthesia in subinguinal varicocelectomy: a prospective randomized trial. Urology 2012;80:9-14. [Crossref] [PubMed]
- Gontero P, Pretti G, Fontana F, et al. Inguinal versus subinguinal varicocele vein ligation using magnifying loupe under local anesthesia: which technique is preferable in clinical practice? Urology 2005;66:1075-9. [Crossref] [PubMed]
- Hsu GL, Ling PY, Hsieh CH, et al. Outpatient varicocelectomy performed under local anesthesia. Asian J Androl 2005;7:439-44. [Crossref] [PubMed]
- Klevmark B, Andersen M, Schultz A, et al. Congenital and acquired curvature of the penis treated surgically by plication of the tunica albuginea. Br J Urol 1994;74:501-6. [Crossref] [PubMed]
- Donatucci CF, Lue TF. Correction of penile deformity assisted by intracavernous injection of papaverine. J Urol 1992;147:1108-10. [Crossref] [PubMed]
- Ghanem H, Fouad G. Penile prosthesis surgery under local penile block anaesthesia via the infrapubic space. Int J Androl 2000;23:357-9. [Crossref] [PubMed]
- Benson RC Jr, Barrett DM, Patterson DE. The Jonas prosthesis--technical considerations and results. J Urol 1983;130:920-2. [Crossref] [PubMed]
- Harrison GS. A local anaesthetic technique for orchiectomy in advanced prostatic cancer. Br J Urol 1983;55:246. [Crossref] [PubMed]
- Kaye KW, Clayman RV, Lange PH. Outpatient hydrocele and spermatocele repair under local anesthesia. J Urol 1983;130:269-71. [Crossref] [PubMed]
- Kaye KW, Gonzalez R, Fraley EE. Microsurgical vasovasostomy: an outpatient procedure under local anesthesia. J Urol 1983;129:992-4. [Crossref] [PubMed]
- Ezeh UI, Shepherd S, Moore HD, et al. Morbidity and cost-effectiveness analysis of outpatient analgesia versus general anaesthesia for testicular sperm extraction in men with azoospermia due to defects in spermatogenesis. Hum Reprod 1999;14:321-8. [Crossref] [PubMed]
- Nudell DM, Conaghan J, Pedersen RA, et al. The mini-micro-epididymal sperm aspiration for sperm retrieval: a study of urological outcomes. Hum Reprod 1998;13:1260-5. [Crossref] [PubMed]
- Belker AM, Sherins RJ, Dennison-Lagos L, et al. Percutaneous testicular sperm aspiration: a convenient and effective office procedure to retrieve sperm for in vitro fertilization with intracytoplasmic sperm injection. J Urol 1998;160:2058-62. [Crossref] [PubMed]
- Levine LA, Lisek EW. Successful sperm retrieval by percutaneous epididymal and testicular sperm aspiration. J Urol 1998;159:437-40. [Crossref] [PubMed]
- Kolettis PN, Thomas AJ Jr. Vasoepididymostomy for vasectomy reversal: a critical assessment in the era of intracytoplasmic sperm injection. J Urol 1997;158:467-70. [Crossref] [PubMed]
- Pavlovich CP, Schlegel PN. Fertility options after vasectomy: a cost-effectiveness analysis. Fertil Steril 1997;67:133-41. [Crossref] [PubMed]
- Meng MV, Greene KL, Turek PJ. Surgery or assisted reproduction? A decision analysis of treatment costs in male infertility. J Urol 2005;174:1926-31; discussion 1931.
- Hsieh MH, Meng MV, Turek PJ. Markov modeling of vasectomy reversal and ART for infertility: how do obstructive interval and female partner age influence cost effectiveness? Fertil Steril 2007;88:840-6. [Crossref] [PubMed]
- Lee R, Li PS, Goldstein M, et al. A decision analysis of treatments for obstructive azoospermia. Hum Reprod 2008;23:2043-9. [Crossref] [PubMed]
- Kavoussi PK, Bird ET. Validation of a vasoepididymostomy predictor model: is vasoepididymostomy truly predictable preoperatively? Fertil Steril 2009;92:180-1. [Crossref] [PubMed]