Factors associated with acutely elevated serum creatinine following radical tumour nephrectomy: the Correlates of Kidney Dysfunction–Tumour Nephrectomy Database study
Introduction
Tumour nephrectomy, either radical nephrectomy (RN) or partial nephrectomy (PN), is the mainstay of treatment for intrarenal neoplasms, the most common of which being renal cell carcinoma (RCC). Surgery can result in excellent oncological outcomes, especially for localised masses, however postoperative kidney dysfunction and chronic kidney disease (CKD) are recognised as potentially important complications (1). Studies comparing RN and PN have concluded that the latter has favourable renal functional outcomes and, accordingly, PN is considered standard of care when technically feasible (1).
One of the major risk factors for developing a postoperative estimated glomerular filtration rate (eGFR) below 60 mL/min per 1.73 m2 is postoperative acute kidney injury (AKI) (2). AKI is characterised by rapidly reduced kidney function and subsequent accumulation of metabolic by-products. It is staged according to serum creatinine (SCr) concentration change (percentage of baseline or absolute) and urine output, based on Kidney Disease: Improving Global Outcomes (KDIGO) criteria (3). In the USA, AKI following tumour nephrectomy has been reported more frequently in recent times than it was prior to 2004, potentially due to updated and standardised classification systems (4). AKI following nephrectomy is associated with increased morbidity and mortality, lower long-term eGFR values, postoperative CKD, and prolonged hospitalisation (2,4-6). Previous studies have indicated that older age, higher body mass index (BMI), male gender, hypertension, and smaller tumour size associate with AKI (2,4,5,7). Higher preoperative eGFR in RN patients is also associated with increased risk of AKI (2), however this was not found to be the case in analyses combining patients undergoing both RN and PN (4). Surgical factors associated with postoperative AKI are RN compared to PN, inferior vena cava (IVC) clamp time >20 min, surgery duration (4,5).
The aims of the present study were to identify predictors of kidney functional impairment in the first week following RN.
Methods
Study population
Subjects were recruited from the Correlates of Kidney Dysfunction–Tumour Nephrectomy Database (CKD–TUNED) study, an ongoing single-centre prospective observational study evaluating renal functional outcomes in tumour nephrectomy patients. The study utilised a convenience sample of 227 consecutive cases managed between June 2013 and December 2016. All patients were ≥18 years old and provided written informed consent. Inclusion criteria for the present study were patients with a preoperative eGFR >15 mL/min per 1.73 m2 who were undergoing RN. Exclusion criteria for the present study were: PN (n=74), missing baseline data (n=2), perioperative mortality (n=1), stage V CKD (n=5), renal transplant recipients (n=10) or any previous renal ablative procedure (n=5). A final sample of 130 subjects was analysed. The study was conducted at the Princess Alexandra Hospital, a large tertiary referral centre in Brisbane, Australia. Approval was granted by the Metro South Human Research Ethics Committee (HREC/05/QPAH/95; HREC/16/QPAH/353).
Data collection
Histories were collated from structured patient interviews and corroborated through chart review to minimise recall bias. Hypertension and diabetes mellitus (any aetiology) were recorded if formally diagnosed. Obesity was defined as BMI ≥30 kg/m2. Smoking history was self-reported; for regression analysis this was dichotomised as never vs. ever smoked. Age and BMI were recorded at preadmission or preanaesthetic evaluation. Preoperative biochemistry and haematological data were retrieved from hospital records; values are from the most recent available instance within one preoperative month. Patients for whom data were not available within one month prior to surgery were not included in these analyses (n=2). Symptomatic presentation encompassed local symptoms, constitutional/paraneoplastic manifestations, symptomatic metastases, or other clinical findings directly attributable to the renal tumour. Kidney function was evaluated by SCr and eGFR (determined using the CKD-EPI equation) (8). The zenith SCr within the first 7 postoperative days was recorded for each patient as a surrogate measure of nadir GFR. Urine samples were collected intraoperatively, and albumin-creatinine ratio (ACR) was reported and log-transformed for regression analysis. Urine creatinine was evaluated using the Jaffe rate method and urinary albumin was assessed using a turbidimetric method; both measured using a Beckman D × C 800 general chemistry analyser (Beckman Coulter, Brea, Ca, USA). Grade, tumour (T)-stage, tumour size (largest dimension) and histological subtype were recorded from pathology reports. For analytical purposes, T-stage was dichotomised as ‘Localised’ (Benign/T1/T2) or ‘Advanced’ (T3/T4); grade was dichotomised as ‘Low Grade’ (≤2 or n/a) or ‘High Grade’ (≥3); tumour subtype was dichotomised as clear cell (cc) RCC or non-ccRCC.
Outcomes
The primary outcome of this study was zenith postoperative SCr ≥1.5-times that of baseline (normal preoperative SCr), hereafter referred to as AKI, as per KDIGO clinical practice guidelines (3).
The secondary outcome was percentage increase in SCr (%ΔSCr):
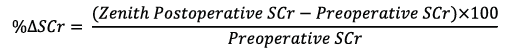
Statistical analyses
Demographic characteristics are presented overall and grouped by the primary outcome. Categorical data were reported as count (percentage). Continuous data were reported as mean ± standard deviation or median (inter-quartile range), depending on whether or not the data were normally distributed. Patients grouped by the primary outcome were compared on categorical data using Pearson’s chi-square test, and on continuous data by a Mann-Whitney U-test or Student’s t-test, depending on data distribution.
The primary outcome of AKI was evaluated by univariable and multivariable logistic regression analyses. The multivariable logistic regression model was fitted to identify independent associations with AKI following univariable analysis. Preoperative SCr was not included in regression analyses due to collinearity with eGFR. T-stage was not adjusted for due to significant association with tumour size. Pre-specified biologically/clinically relevant variables included in the model were age, gender, smoker status, preoperative eGFR, urine ACR, tumour size, surgical approach, and the presence of diabetes mellitus, hypertension, and obesity. Other variables were also included in the final model if associated with AKI with a P value <0.25 on univariable logistic regression analysis. Variables were screened for collinearity using a Draughtsman’s plot, however no additional collinearity was detected. Standardised residuals were used to identify outliers (defined as values ≥2) and the Δχ2 influence statistic was used to identify influential cases (defined as values ≥7). To assess the effect of outliers and influential cases on the final model, percentage change in adjusted odds ratio (OR) was compared between the final model and a multivariable model run without outliers and influential cases. To evaluate goodness-of-fit, a receiver operating characteristic curve was fitted to the model and the c-statistic reported.
The secondary outcome of SCr percentage increase was evaluated using linear regression. The multivariable linear regression model retained the same predictors included in the multivariable logistic regression model. Outliers were assessed as described previously. The model was run without these cases and compared by percentage change in the β coefficient. To evaluate goodness-of-fit, the coefficient of determination was reported.
All analysis was performed using Stata 14 (StataCorp, College Station, TX, USA) and α was set at 0.05.
Results
Patient characteristics
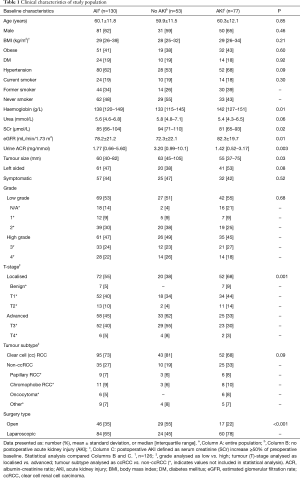
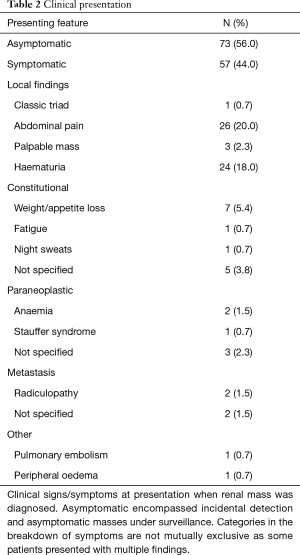
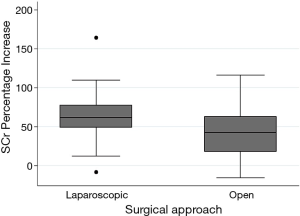
Baseline characteristics are outlined in Table 1 and mode of presentation is shown in Table 2. Following nephrectomy, the mean SCr percentage increase in the first week was 55%±29%. Of 130 patients, 77 (59%) experienced AKI with a SCr increase ≥1.5-times the preoperative value, and 6 (5%) experienced AKI with a SCr increase ≥2-times the preoperative value. Compared with patients who did not develop AKI in the first postoperative week, those who developed AKI were more likely to have lower baseline SCr and higher baseline eGFR values, higher baseline haemoglobin values, lower urine ACR, localised tumours, and undergone laparoscopic nephrectomy. There was a weak inverse association between preoperative eGFR and tumour size [β: ‒0.10; 95% confidence interval (95% CI): ‒0.204; 0.002], which did not reach statistical significance (P=0.06) and had a small coefficient of determination (R2=0.03). There was no significant difference in tumour size between ccRCC and non-ccRCC groups (P=0.8). ACR was significantly higher with advanced tumours [3.1 (1.1–8.3) mg/mmol], compared to localised tumours [1.2 (0.5–2.9) mg/mmol; P<0.001], and in patients with a preoperative eGFR <60 mL/min per 1.73 m2 [5.1 (1.63–8.92) mg/mmol], compared to those with a preoperative eGFR ≥60 mL/min per 1.73 m2 [1.5 (0.5–3.8) mg/mmol; P=0.005]. In patients with tumours ≥40 mm, a laparoscopic approach, compared to open, associated with a higher percentage increase SCr when grouped by tumours 40–70 mm (P=0.01) and >70 mm (P=0.02) (Table 3). The relative risks of developing AKI after laparoscopic surgery (compared to open) were 1.16 and 1.94 in patients with T1/2 and T3/4 tumours, respectively.
Full table
Full table

Full table
Predictors of AKI
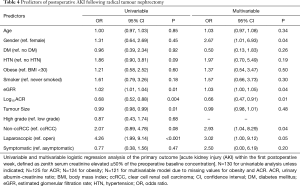
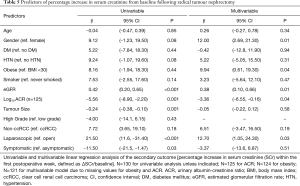
The results of the univariable and multivariable logistic regression analyses are presented in Table 4. The independent predictors of AKI post-nephrectomy were male gender (reference female; OR: 2.67; 95% CI: 1.01, 6.93), urine ACR (OR: 0.66; 95% CI: 0.47, 0.91), preoperative eGFR (OR: 1.03; 95% CI: 1.00, 1.05), laparoscopic nephrectomy (reference open nephrectomy; OR: 3.02; 95% CI: 1.00, 9.12), and non-ccRCC (reference ccRCC; OR: 2.93, 95% CI: 1.04, 8.29). The c-statistic for this model was 0.8.
Full table
Predictors of SCr Percentage Increase
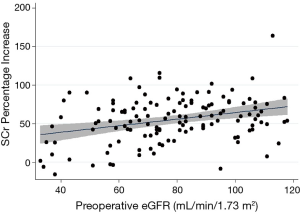
The results of the univariable and multivariable linear regression analyses are presented in Table 5. The independent predictors of a higher SCr percentage increase were male gender (reference female; β: 12.0; 95% CI: 2.69, 21.3), urine ACR (β: ‒3.36; 95% CI: ‒6.55, ‒0.16), preoperative eGFR (β: 0.38; 95% CI: 0.10, 0.66), laparoscopic nephrectomy (reference open nephrectomy; β: 12.7; 95% CI: 1.05, 24.3) and obesity (reference not obese; β: 9.94, 95% CI: 0.61, 19.3). For the multivariable model, R2=0.34. When outliers were removed from the multivariable model, the effect size of obesity reduced significantly (β: 4.66; 95% CI: ‒3.65, 12.9). The relationship between preoperative eGFR and postoperative SCr percentage increase is presented in Figure 1 and a box plot of SCr percentage increase values compared by surgical approach is presented in Figure 2.
Full table
Discussion
The present study demonstrated that male gender, lower levels of albuminuria, higher preoperative eGFR and laparoscopic nephrectomy were independently associated with a higher likelihood of reduced kidney function following RN, whether analysed as occurrence of AKI or as percentage increase in SCr. Non-ccRCC was also associated with a higher risk of AKI, whilst obesity was associated with SCr percentage increase.
The present study found that higher preoperative eGFR and lower preoperative albuminuria were associated with a higher risk of AKI and postoperative SCr rise. Whilst these observations seem somewhat counterintuitive, given that higher eGFR and lower albuminuria predict a lower long-term risk of CKD (9,10), there is support in the literature for their validity. In a single-centre retrospective cohort study of 519 patients undergoing RN in Korea, Cho et al. identified that higher baseline eGFR independently associated with AKI within seven postoperative days (OR: 1.49, 95% CI: 1.27, 1.73) (2). This counterintuitive association between pre- and postoperative kidney function has also been reported at 12 postoperative months. In a Korean single-centre retrospective cohort study of 543 RN patients grouped by preoperative CKD stage, patients with stage I, II and III CKD, respectively, experienced 31%, 27% and 13% reductions in eGFR, which was partially attributed to greater functional hyperfiltration in the stage III group (11).
The finding that albuminuria was negatively associated with AKI and SCr rise has not been reported previously and is contrary to findings from other populations (12). A possible explanation is that patients without AKI may have also had a greater degree of glomerular hyperfiltration as a compensatory response to greater functional parenchymal loss in patients with higher T-stage, resulting in albuminuria. In the present study, advanced tumours were associated with a higher ACR compared to localised tumours (P<0.001), which mirrors results from a study of 51 RCC patients (managed with immunotherapy) which identified a positive trend between albuminuria and T-stage (13). Although there was minimal association between ACR and eGFR, patients with low preoperative eGFR were more likely to have a higher ACR than those with a normal eGFR (P=0.005), which may have also contributed to this association, where patients with preoperative kidney functional impairment also had albuminuria. Conclusions cannot be drawn based on these associations alone and further investigation into this phenomenon is warranted.
The association between male gender and postoperative SCr elevation is supported by previous studies evaluating RN patients. In a large retrospective study of 253,046 RN and PN patients managed in the USA, female gender was independently associated with lower odds of postoperative AKI relative to male gender (OR: 0.64, 95% CI: 0.59, 0.69). Similarly, Cho et al. demonstrated male gender was independently associated with increased risk of AKI relative to female gender (OR: 3.13; 95% CI: 1.91, 5.12). Indeed, male gender is associated with postoperative AKI regardless of surgical site or indication (14). For example, a retrospective analysis of 57,080 patients in an American multicentre surgical registry identified that male gender was an independent predictor of AKI compared to female gender (hazard ratio: 1.4; 95% CI: 1.2, 1.7) (14). Males may be at increased risk of SCr rise relative to females due to greater skeletal muscle mass on average leading to more creatinine being generated (15).
The observation that laparoscopic nephrectomy was associated with increased risk of AKI and the percentage increase in SCr following RN was unexpected. Patients were subgrouped by tumour size and T-stage to evaluate model validity, however, the differences between patients managed with laparoscopic and open surgery did not dissipate (Table 3). A possible physiological explanation for this relationship may be the increased intra-abdominal pressure associated with establishing a pneumoperitoneum. During laparoscopy, healthy normotensive, normovolaemic patients can experience oliguria intraoperatively as a manifestation of reduced kidney function (16). Modest increases in intra-abdominal pressure can lead to venous congestion, causing interstitial oedema, increased intracapsular pressure, and a decreased renal blood flow gradient, which may facilitate acute reductions in kidney function and explain our findings (17,18). In patients undergoing RN and IVC thrombectomy, IVC clamp time >20 min is associated with increased risk of postoperative AKI (5), which would contribute similarly to venous congestion, depending on the degree of established collateral drainage. Reduced renal perfusion following insufflation may also be a contributing factor. For example, in a porcine CO2 pneumoperitoneum model, reduced renal perfusion, creatinine clearance and urine production were reported as consequences of prolonged pneumoperitoneum (19).
In PN patients, Marszalek and colleagues demonstrated that acutely elevated SCr was associated with laparoscopic PN compared to an open approach in a retrospective matched-pairs analysis of patients from two separate centres in Austria (n=100 undergoing either laparoscopic or open PN in each group) (20). Patients in this study were matched on age, sex and tumour size, and all tumours were incidentally detected pT1 lesions. Kidney function was evaluated by eGFR (calculated using the Mayo Clinic Quadratic Equation) 24-hours postoperatively and on follow-up. Mean eGFR decline within 24 postoperative hours in the laparoscopic and open groups was 8.8% and 0.8%, respectively, P<0.001. However, longitudinal evaluation of these patients indicated no difference between open and laparoscopic approach; mean eGFR decline from preoperative values was 10.9% and 10.6% in the laparoscopic and open groups, respectively, with a mean follow-up of 3.6 years. This indicates that both groups had similar long-term outcomes, but instead of resolution of kidney function in the laparoscopic group, kidney function in the open group had a progressive negative trend which matched the laparoscopic group’s follow-up kidney function (20).
Similarly, in a retrospective observational study of 116 RN patients managed at a single centre in the USA, there were no differences in SCr comparing patients managed with an open vs. laparoscopic approach at a median follow-up of 4.3 years (21). This discordance with the results of the present study is likely due to the fact that any early changes in kidney function attributable to laparoscopy are likely transient and relatively insignificant, in comparison to the much more pronounced effect that cortical parenchymal loss following renal ablation contributes to decline in kidney function.
An assumption of this analysis is that unmeasured confounders were not present. This limits the models used and further investigation into whether laparoscopy is causal in reducing postoperative kidney function reduction is necessary. Further conclusions may be able to be drawn from extended follow-up of this cohort; however, at face-value it is unlikely that benefits of forgoing laparoscopy outweigh any risks associated with subsequent short-term impairment of kidney function.
The finding that obesity associated with SCr percentage increase is supported by studies previously discussed. Cho et al. found that BMI was positively associated with postoperative AKI risk in RN patients (OR: 1.16; 95% CI: 1.01, 1.34) (2), and Schmid et al. found obesity was associated with increased risk of postoperative AKI in an analysis combining RN and PN patients (OR: 1.51; 95% CI: 1.33, 1.71) (4), which supports the validity of the positive result. However, obesity was not associated with AKI in the present study (Table 4), and effect size was reduced substantially when outliers were removed from the linear model, therefore this finding may represent a type 1 statistical error. Notwithstanding, reasons for obesity associating with SCr percentage increase may be related to structural and functional changes within the kidney as a consequence of obesity, often manifesting as altered renal haemodynamics which effectively reduce renal functional reserve, limiting the kidney’s ability to compensate for increased functional demand (22).
The finding that non-ccRCC was an independent predictor of AKI is more difficult to explain. Given that non-ccRCC was not significantly associated with percentage increase in SCr (P=0.20), it is possible that the association with AKI also represented a type 1 statistical error. Tumour size was unlikely to account for the findings as there was no difference in tumour size when comparing ccRCC with non-ccRCC (P=0.8), and no independent statistically significant association observed between tumour size and either SCr percentage increase or AKI (Tables 4,5). It is noteworthy that all patients who had benign lesions (oncocytoma n=6 and cystic nephroma n=1, constituting 20% of the non-ccRCC group) experienced postoperative AKI. There are no previously reported associations between benign renal tumours and worse postoperative kidney function relative to malignant lesions, however, given the very small subgroup size, generalised inferences cannot be made from these results.
In contrast to the present findings, previous reports of short- and long-term follow-up of patients undergoing RN identified that smaller tumours were associated with increased risk of poorer postoperative kidney function in patients managed with RN. Cho et al. found tumour size to be negatively associated with AKI within seven postoperative days on multivariable analysis (OR: 0.86; 95% CI: 0.81, 0.93) (2). Zabor et al. identified that tumour size was positively associated with eGFR recovery 24-months postoperatively (hazard ratio: 1.07; 95% CI: 1.04, 1.10) (23). On subgroup analysis, this effect was only statistically significant for patients with a preoperative eGFR ≥60 mL/min per 1.73 m2. In a Korean single-centre retrospective study of 1,371 RN patients, tumour sizes <4 and 4–7 cm were independently associated with the development of CKD at three postoperative years (reference >7 cm; OR: 2.37; 95% CI: 1.56, 3.60; and OR: 2.24; 95% CI: 1.49, 3.38, respectively) (24). It is plausible that tumour size does not greatly impact post-nephrectomy kidney function because larger tumours reduce the effective functional mass of the ipsilateral kidney through parenchymal destruction more so than smaller tumours would. Therefore removal of a kidney with a larger tumour and less functional parenchyma contributes less to the reduction in kidney function than removal of a kidney containing a small tumour with more functional nephrons. A second possible explanation is that, due to the gradual loss of functional parenchyma in the ipsilateral kidney with a large and growing tumour, compensatory function in the contralateral kidney progressively increases, and precedes surgical resection—thereby blunting the impact of subsequent parenchymal loss. Given the fact that eGFR was minimally correlated with tumour size in this cohort, it is proposed that the latter effect is greater than the former, and any predictive value of tumour size was insignificant relative to the compensatory change in eGFR in the present study.
The definition of AKI in this study is based on the lowest stage of AKI in KDIGO clinical practice guidelines, based only on the criteria of relative increase in SCr (3). As these patients are concurrently undergoing functional nephron removal, it is difficult to distinguish between patients for whom this definition encompasses true injury, and those in which it simply indicates a reduced capacity to compensate for functional nephron loss. Either way, evaluating this outcome is justified based on previous literature. To the authors’ best knowledge, only five other studies capturing RN patients have evaluated AKI as a primary outcome (2,4-7). Cho et al. retrospectively evaluated 519 RN patients with a preoperative eGFR ≥60 mL/min per 1.73 m2, and defined AKI as zenith SCr elevated ≥1.5-times the preoperative value within seven postoperative days. They found that CKD (defined as eGFR <60 mL/min per 1.73 m2) at three years postoperatively was more prevalent in patients with postoperative AKI (50%) compared to those without (32%), and that AKI was an independent predictor of CKD (OR: 4.24; 95% CI: 2.28, 7.89) (2). Considering this, the primary outcome of the present study is likely to have clinical relevance.
The strengths of the present study include its prospective design (which resulted in minimal missing baseline data and no missing postoperative data), its use of standardized renal function outcomes, and its generally robust findings across different statistical methodologies. The study was limited by its modest sample size (n=130) and number of AKI events (n=77), which necessitated the use of parsimonious multivariable statistical models. Controlling for outliers showed minimal influence on effect sizes in multivariable models. The observational nature of this study also prevented causal inferences being drawn about the relationships between clinical factors and post-nephrectomy kidney function. Finally, the study did not capture information regarding intraoperative factors (e.g., hydration status, blood pressure and surgery duration), patient lifestyle (e.g., exercise and diet), medications, or contralateral renal functional volume, which may have significantly impacted on postoperative kidney function.
Conclusions
The present study identified that male gender, lower levels of albuminuria, higher preoperative eGFR and laparoscopic nephrectomy were independently associated with a higher likelihood of poorer postoperative kidney function. Further studies are warranted to determine whether optimisation of surgical approach can play an important role in mitigating postoperative AKI and any attendant risks of morbidity and mortality.
Acknowledgements
RJ. Ellis was supported by an Australian Government Research Training Scholarship. DW. Johnson is a current recipient of a National Health and Medical Research Council Practitioner Fellowship.
Footnote
Conflicts of Interest: DW. Johnson reports grants and personal fees from Baxter Healthcare and Fresenius Medical Care, travel sponsorship from Amgen, and personal fees from Astra Zeneca during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: Approval was granted by the Metro South Human Research Ethics Committee (HREC/05/QPAH/95; HREC/16/QPAH/353). All patients were ≥18 years old and provided written informed consent.
References
- Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67:913-24. [Crossref] [PubMed]
- Cho A, Lee JE, Kwon GY, et al. Post-operative acute kidney injury in patients with renal cell carcinoma is a potent risk factor for new-onset chronic kidney disease after radical nephrectomy. Nephrol Dial Transplant 2011;26:3496-501. [Crossref] [PubMed]
- Kidney Disease; Improving Global Outcomes AKI Workgroup. KDIGO clinical practice guidelines for acute kidney injury. Kidney Int Suppl 2012;2:1-138.
- Schmid M, Krishna N, Ravi P, et al. Trends of acute kidney injury after radical or partial nephrectomy for renal cell carcinoma. Urol Oncol 2016;34:293.e1-e10. [Crossref] [PubMed]
- Shin S, Han Y, Park H, et al. Risk factors for acute kidney injury after radical nephrectomy and inferior vena cava thrombectomy for renal cell carcinoma. J Vasc Surg 2013;58:1021-7. [Crossref] [PubMed]
- Schmid M, Abd-El-Barr AE, Gandaglia G, et al. Predictors of 30-day acute kidney injury following radical and partial nephrectomy for renal cell carcinoma. Urol Oncol 2014;32:1259-66. [Crossref] [PubMed]
- Fantin JP, de Carvalho Neiva R, Gatti M, et al. Risk factors for acute renal failure in nephrectomized patients treated in a university hospital. Transl Androl Urol 2017;6:277-81. [Crossref] [PubMed]
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12. [Crossref] [PubMed]
- Kidney Disease; Improving Global Outcomes CKD Workgroup. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1-150.
- O'Donnell K, Tourojman M, Tobert CM, et al. Proteinuria is a predictor of renal functional decline in patients with kidney cancer. J Urol 2016;196:658-63. [Crossref] [PubMed]
- Choi DK, Jung SB, Park BH, et al. Compensatory structural and functional adaptation after radical nephrectomy for renal cell carcinoma according to preoperative stage of chronic kidney disease. J Urol 2015;194:910-5. [Crossref] [PubMed]
- Grams ME, Astor BC, Bash LD, et al. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol 2010;21:1757-64. [Crossref] [PubMed]
- Vaglio A, Buzio L, Cravedi P, et al. Prognostic significance of albuminuria in patients with renal cell cancer. J Urol 2003;170:1135-7. [Crossref] [PubMed]
- Kheterpal S, Tremper KK, Heung M, et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology 2009;110:505-15. [Crossref] [PubMed]
- Thomas ME, Blaine C, Dawnay A, et al. The definition of acute kidney injury and its use in practice. Kidney Int 2015;87:62-73. [Crossref] [PubMed]
- Goren O, Matot I. Perioperative acute kidney injury. Br J Anaesth 2015;115 Suppl 2:ii3-14. [Crossref] [PubMed]
- Patel DM, Connor MJ Jr. Intra-abdominal hypertension and abdominal compartment syndrome: an underappreciated cause of acute kidney injury. Adv Chronic Kidney Dis 2016;23:160-6. [Crossref] [PubMed]
- Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol 2014;10:37-47. [Crossref] [PubMed]
- London ET, Ho HS, Neuhaus AM, et al. Effect of intravascular volume expansion on renal function during prolonged CO2 pneumoperitoneum. Ann Surg 2000;231:195-201. [Crossref] [PubMed]
- Marszalek M, Meixl H, Polajnar M, et al. Laparoscopic and open partial nephrectomy: a matched-pair comparison of 200 patients. Eur Urol 2009;55:1171-8. [Crossref] [PubMed]
- Colombo JR Jr, Haber GP, Jelovsek JE, et al. Seven years after laparoscopic radical nephrectomy: oncologic and renal functional outcomes. Urology 2008;71:1149-54. [Crossref] [PubMed]
- Eknoyan G. Obesity and chronic kidney disease. Nefrologia 2011;31:397-403. [PubMed]
- Zabor EC, Furberg H, Mashni J, et al. Factors associated with recovery of renal function following radical nephrectomy for kidney neoplasms. Clin J Am Soc Nephrol 2016;11:101-7. [Crossref] [PubMed]
- Jeon HG, Choo SH, Sung HH, et al. Small tumour size is associated with new-onset chronic kidney disease after radical nephrectomy in patients with renal cell carcinoma. Eur J Cancer 2014;50:64-9. [Crossref] [PubMed]


