Do we have biomarkers to predict response to neoadjuvant and adjuvant chemotherapy and immunotherapy in bladder cancer?
Introduction
Bladder cancer (BC) is the fourth and ninth most common malignancy amongst men and women in the western world (1). Depending on invasion pattern, BC can be distinguished in non-muscle-invasive BC (NMIBC) which represents the majority of primary BC with approximately 85% and muscle invasive BC (MIBC). Radical cystectomy (RC) is the standard of care treatment for muscle-invasive BC in the absence of metastatic disease (2,3). However, cancer-specific survival after cystectomy is relatively low, ranging from 72% to 25% 5 years postoperatively (4,5). Nearly 50% of patients harboring T2b–T4a disease develop metastases within 2 years implying that micro-metastases might be present at the time of surgery (4,5).
Therefore, perioperative systemic treatment might be of use to achieve better disease control and improve survival. The chemotherapeutic agent of choice for first line therapy is cisplatin (2,3), usually embedded in a regimen combined with gemcitabine due to lower toxicity compared to a combination with methotrexate, vinblastine and doxorubicin (MVAC) (6). There is an ongoing debate on the time schedule for surgery and chemotherapy (3). Two large, randomized trials and two meta-analyses demonstrated survival benefit for neoadjuvant chemotherapy (NAC) compared with surgery alone in patients with MIBC (7-10).
Despite high-level evidence (7-10), cisplatin-based NAC met resistance in medical communities around the world (11,12), due to concerns regarding delay of surgery in NAC non-responders, the potential toxicity, and especially the inability to predict the chance of response. However, current guidelines recommend the application of chemotherapy in a neoadjuvant setting before RC (2,3) as the literature clearly supports NAC, demonstrating a 5–10% increase in 5-year cancer-specific survival in MIBC compared with surgery alone (7-10). The rationale of NAC being the achievement of a down-staging of the primary tumor in BC-patients with clinically organ-confined or locally advanced BC (cT2–T4a N0 M0) as well as early eradication of micro-metastases (7,8). Moreover, NAC might be better tolerated than adjuvant chemotherapy (AC) after RC, due to the relevant post-cystectomy morbidity which might prevent a reasonable amount of patients from receiving AC (13-15). Finally, using NAC, the effect of systemic treatment can be verified pathologically. This information has important prognostic value as the 5-year cancer-specific survival for responders to NAC (<ypT2) is 90%, in contrast to 30–40% for non-responders. Contrarily, evidence supporting AC is less robust (10).
To date, there is no reliable method for the prediction of response to chemotherapy resulting in a possible overtreatment in non-responders with unnecessary toxicity that might render patients in a deteriorated physical condition without the opportunity for additional, alternative therapy. Therefore, the identification of chemotherapy responders before initiating systemic therapy would be a very helpful clinical asset. Multiple molecular biomarkers have been studied for prediction of response to chemotherapy such as mutations in deoxyribonucleic acid (DNA) damage repair pathways, receptor tyrosine kinases, gene expression markers, regulators of apoptosis, cellular mechanisms of drug uptake and transport,
The aim of the present review is to summarize and discuss the current literature on biomarkers for the prediction of response to systemic therapy in MIBC.
Molecular subtyping
The introduction of molecular characterization of tumors in recent years using whole transcriptome gene arrays or next-generation sequencing has generated large datasets leading to a new understanding of the genomic landscape of urothelial carcinoma (UC). With reliable clinical markers missing to discriminate a response to chemotherapy, molecular characterization holds great promise to identify and select only those patients who benefit most from chemotherapy and spare predictable non-responders from unnecessary cytotoxic side effects.
Expression profiling and molecular subtyping identifies gene signatures responding to chemotherapy
In a study from 2007, Als and colleagues performed gene expression profiling using the Affymetrix platform on 30 UC specimens and validated their findings immunohistochemically in a cohort of 124 patients (16). They identified emmprin (CD147), a membrane protein and a modulator of matrix metalloproteinases and survivin, a member of the inhibitor of apoptosis (IAP) protein family, as independent prognostic factors for overall survival (OS). Intriguingly, negative expression of both markers was predictive of response to palliative cisplatin-based chemotherapy (16). In a later retrospective validation study including 250 patients receiving NAC, negative emmprin expression assessed by immunohistochemistry (IHC) resulted in a significantly higher OS than those with positive expression (71% vs. 38%, P<0.001). Emmprin negative patients had after NAC an absolute risk reduction of 25% in OS and a number needed to treat of 4. Patients with emmprin positive tumors (approx. 30% of tumors) had no survival benefit through chemotherapy and may be directed to alternative therapies in future trials (17).
Basal-like tumors may benefit most from chemotherapy
The promise to use whole transcriptome profiling by ribonucleic acid (RNA) sequencing and microarray data as biomarkers was substantiated by recent reports from several independent groups who identified intrinsic subtypes of BC (18-26).
Expression profiling datasets revealed similarities between UC of the bladder (UCB) and breast cancer, where distinct molecular subtypes (basal-like, HER2, luminal A and B) have already been established and integrated into clinical routine for treatment selection (27).
There is a general consensus that gene expression patterns in UCB can roughly discriminate between basal and luminal cancers (28), expressing markers corresponding to less differentiated basal and terminally differentiated cell phenotypes in normal urothelium, respectively (29,30).
Although published subtype-classifications in UCB differ between the different datasets, similarities exist regarding their clusters with respect to invasion, prognosis and response to therapy.
The Höglund group from Lund firstly described different molecular subtypes with respect to gene expression, mutation pattern, genomic instability and disease aggressiveness (31). The same group later published a refined taxonomy using integrated genomics consisting of 5 subtypes: genomically unstable (GU), urobasal (Uro) A and B, infiltrated, and squamous cell carcinoma like (SCCL) (18) (Figure 1).
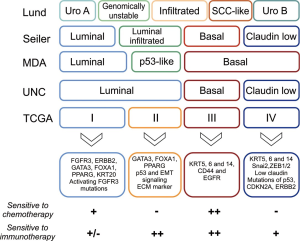
Uro A and B tumors were characterized by expression of differentiation-associated markers and showed high FGFR3 gene expression, high frequency of FGFR3 activating mutations, and high p63 levels. While Uro A tumors had a good prognosis, aggressive and more invasive Uro B tumors could be distinguished by a more basal-like phenotype and a presence of p53, PIK3CA, CDKN2A and ERBB mutations. It is assumed that Uro B tumors may represent progressed forms of the Uro A cancers (32).
GU and SCCL tumors were characterised by abnormal expression patterns of differentiation-associated biomarkers. GU tumors showed an absence of activating FGFR3 mutations and the presence of p53 and ERCC2 mutations, RB1 deletions and amplification of PPARG, GATA3 and ERBB2. SCCL tumors showed high expression of EGFR, basal-type cytokeratins (i.e., KRT5/6) and squamous differentiation-associated cytokeratin 14. The infiltrated subtype showed markers of immune and stromal cell type infiltration (18,32).
Other groups performed independent studies of molecular profiling in UCB cohorts. From the MD Anderson Cancer Center (MDA) group, Choi and colleagues generated two whole transcriptome datasets inpatient cohorts comprising 73 and 57 muscle-invasive UC, respectively. They discriminated basal-like and luminal-like gene signatures resembling those from previously characterized breast cancer cohorts and therefore named them basal and luminal. A third subtype showed expression of stromal biomarkers and an active p53 gene expression signature and was termed “p53-like” (21). This subtype had similarities to the infiltrated subtype from the Lund classification with expression of stromal markers, infiltration of fibroblasts and expression of extracellular matrix (ECM) proteins. Basal-like tumors, with characteristics of previously described SCCL subtype (18), had the poorest prognosis in this classification. Interestingly, none of the seven p53-like tumors in the discovery cohort treated with cisplatin-based NAC responded to treatment. This was further explored in other validation cohorts and confirmed that most p53-like tumors were chemotherapy-resistant. In contrast, about half of the basal tumors were pathologically downstaged by NAC. Notably, many of the tumors initially classified as basal or luminal showed a shift in gene expression towards a p53-like phenotype after NAC (21). This could reflect an acquired mechanism of chemoresistance or a selection of small chemoresistant subclones of KRT5/14 positive cells in the bulk tumor (33,34).
A later study, applying the same molecular subtyping strategy to a patient cohort from a phase 2 study receiving MVAC NAC, also suggested that basal -like tumors benefit most from cisplatin-based chemotherapy (26).
Damrauer et al. from the University of North Carolina (UNC) applied consensus gene clustering on a meta-dataset of 262 high-grade tumors and a new dataset from 49 tumors to group tumors into basal and luminal subtypes. Basal tumors were associated with poorest outcome. A subset of basal tumors expressed markers of epithelial mesenchymal transition (EMT) and low claudin. This subgroup was therefore termed “claudin-low” (22).
The Cancer Genome Atlas (TCGA) group published in 2014 the first 131 tumors analyzed by whole genome, RNA/microRNA (miRNA) sequencing and reverse phase protein array (RPPA) (20). They defined four distinct expression clusters (I–IV). Cluster I showed papillary morphology and enrichment of FGFR3 mutations. Clusters I and II expressed urothelial differentiation-associated markers, i.e., FOXA1, UPK3A, ERBB2 and GATA3. Both clusters together correspond to the MDA/UNC luminal subtype (21,22). Cluster III featured squamous-basal characteristics expressing high levels of KRT5, KRT14, KRT6A and EGFR, corresponding to SCCL and basal subtypes as described above (18,21) and had the worst prognosis. Cluster IV was also basal, and showed similarities to the infiltrated claudin-low subtype described by the UNC group (22).
In a recent study, Seiler et al. (24) assembled several retrospective cohorts from above described groups to perform whole transcriptome profiling on 343 TURB specimens before NAC. The group established, harmonized and validated a single-sample genomic subtyping classifier (GSC) to assign muscle invasive UC to one of four consensus subtypes based on biological characteristics and impact on prognosis: luminal and basal-type tumors were subclassified into tumors with and without EMT and immune infiltration: luminal and luminal infiltrated, basal and claudin-low. The authors analyzed the subtype-specific survival rates and compared them to a cross-cohort comparison with published datasets of patients undergoing cystectomy without NAC. The authors showed that NAC downstaged and improved OS mainly in patients with basal tumors, but not in tumors of the other three subtypes. Although luminal tumors had a favorable survival outcome and claudin-low tumors were associated with poor survival, NAC had no relevant impact on the outcome of these subtypes.
While gene expression profiling is technically well-established on formalin-fixed and paraffin-embedded tissue with similar quality compared to fresh frozen tissue or qPCR (35), RNA sequencing or gene arrays are costly and can only be performed in centers with specific technical facilities.
The use of specific antibodies to define molecular subtypes would facilitate the implementation of a molecular classification in the routine histopathologic workup.
Dadhania and colleagues investigated the MDA cohort samples and performed a tissue microarray to identify protein markers to discriminate UC subtypes (36). They found that a 2-marker set using GATA3 and KRT5/6 was able to discriminate between basal and luminal tumors with over 90% accuracy.
The rapid progress of genomic characterization of UC in recent years has resulted in a variety of subtypes taxonomies. Although a consensus meeting made a first step towards a standardized taxonomy (28), further harmonization will be required in the future for a better understanding and clinical applicability. Nevertheless, consensus exists for defining basal-squamous-like tumors expressing KRT5/6 and KRT14 (28). Patients with such tumor characteristics may in future be advised to receive cisplatin-based NAC while other patients may require novel, genomic-driven target therapies.
Mutations in DNA damage repair pathways confer platinum sensitivity
Cisplatin damages cellular DNA through formation of platinum-DNA crosslinks. DNA repair, such as nucleotide excision repair (NER), homologous recombination (HR) or mismatch-repair (MMR) mechanism can correct such chemotherapy-induced defects. Platinum-based chemotherapy therefore can exploit DNA repair deficiency of tumors harboring defects in DNA damage response (DDR) genes (37).
DDR-associated genes may therefore present promising biomarkers to predict response to platinum-based chemotherapy.
Bellmunt and colleagues were one of the first to report in 2007 an association between activity of DNA repair and clinical response to cisplatin chemotherapy in patients with advanced BC. The authors measured mRNA levels of several DDR genes and found that median survival was significantly longer in patients with tumors that had low expression levels of the NER gene ERCC1 (38).
Van Allen et al. performed whole exome sequencing in a cohort of 50 MIBC patients (39). They found that inactivating mutations of ERCC2, a NER effector gene, was exclusively found and enriched in responders [9 of 25 responders (36%),] while none of the non-responders (0 of 25) defined by pT0/pTis status at cystectomy carried an ERCC2 missense mutation. The authors also showed that loss of function of ERCC2 resulted in increased cisplatin sensitivity in vitro.
Notably, ERCC2 mutations are found more frequently in UC 13% than in any other solid cancer (all less than 4%) (39).
The results were validated in a small separate cohort (40). Again, ERCC2 mutations were found in a significant higher proportion in cisplatin responders (40%, 8 of 20) but only in 2 of 28 non-responders (7%). In addition, in both the discovery and validation cohorts, patients with ERCC2-mutated tumors receiving cisplatin-based NAC had a significantly longer OS than patients with wild-type ERCC2 tumors (40).
In the same cohort, Plimack and colleagues tested a 3-DDR gene signature (ATM, RB1, FANCC). In a discovery cohort of 34 patients, mutations in at least one of the tested genes predicted pathologic response in 13/15 (87%) patients after NAC, whereas none (0%) of the non-responders had an alteration in any of these genes. In a small validation cohort the results could be confirmed, although less strikingly, with 7/11 (64%) of responders but only 2/13 (15%) of the non-responders with residual disease carried DDR gene mutations (41).
Another cohort of 43 patients who received AC was investigated by whole-exome sequencing. Twenty-five of 43 tumors carried mutations in at least one DDR gene such as ATM, ERCC2, BRCA1, and BRCA2. The presence of somatic DDR gene mutations was associated with significantly enhanced recurrence-free survival (RFS) after AC (median 32.4 vs. 14.8 months; hazard ratio of 0.46) and was an independent predictor of RFS. The impact on OS, however, was not reported (42).
Groenendijk et al. investigated predictive mutations for NAC response using NGS and selected complete pathologic responders (ypT0N0) and non-responders (higher than ypT2) from a cohort of MIBC. Nine of 38 (24%) of the complete responders (ypT0N0) to cisplatin-based NAC had ERBB2 missense mutations, whereas none of 33 non-responders (higher than ypT2) had ERBB2 mutations. ERBB2 is a member of the epidermal growth factor receptor family and is over-expressed in a subpopulation of UCs correlating with prognosis (43). Interestingly, ERBB2 amplifications (rather than loss of function mutations) were identified in both responders and non-responders. The authors hypothesized that cisplatin-based therapy is appropriate in patients carrying the ERBB2 mutations, whereas anti-HER2 therapies may be effective in those with ERBB2 amplification, although this remains controversial (44,45).
The study also revealed discrepancies between the previous above-mentioned reports as mutations in DDR genes, namely ATM, RB1 and ERCC2 were not significantly predictive of pathologic response and were found both in responders and non-responders (46). These inconsistencies might be explained by small sample cohorts and use of different chemotherapy regimens. Furthermore, pathologic response may not be the optimal criterion to assess efficacy of NAC. Seiler et al. showed in a larger series of patients that the effect of NAC on OS was independent of pathologic response (24).
Teo et al. investigated predictive mutations in patients with advanced or metastatic urothelial cancer receiving palliative chemotherapy (47). They performed NGS analyzing 341 genes including 34 DDR-associated genes in a cohort of 100 patients. A total of 47 of the 100 patients included in this study harboured at least one DDR mutation. Patients with DDR gene alterations receiving platinum-based chemotherapy had a significantly longer PFS (9.3 versus 6.0 months) and OS (23.7 versus 13.0 months) compared with patients with wild-type DDR genes. In contrast to the studies reported by Van Allen (39) and Plimack (41), mutations in ERCC2, ATM, and FANCC were only detected in 5%, 9%, and 1% of patients, respectively, and were individually not significantly associated with clinical outcomes.
Nevertheless, these data collectively suggest better outcome of platinum-based chemotherapy in tumors with deleterious DNA repair mechanisms and render mutations in DDR-associated genes (and potentially ERBB2) attractive biomarkers for clinical decision-making. Further validation with larger sample sizes, homogeneous chemotherapy regimens and longer follow up are required to establish molecular evaluation in clinical practice.
Clinical trials are underway, i.e., the COXEN trial (NCT02177695) using a co-expression extrapolation analysis to assess the use of biomarkers for treatment personalization.
Biomarkers to predict response to programmed death 1/programmed death-ligand 1 (PD-1/PD-L1) checkpoint-inhibitors
Immune-checkpoint inhibitors targeting the PD-1/PD-L1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) pathways have shown significant effectiveness and tolerable safety profiles in the treatment of advanced or metastatic UC (48-51). Atezolizumab, pembrolizumab, nivolumab, avelumab and durvalumab are approved by the FDA as second-line treatments after chemotherapy or as first-line therapy in cisplatin ineligible patients. However, overall response rates (ORRs) usually do not exceed 30% and the phenomenon of hyperprogression of tumors under treatment PD-1/PD-L1 inhibition with worsened prognosis has been reported (52). Biomarkers for prediction of therapy response would significantly improve clinical management.
PD-L1 expression
Expression of PD-1 or PD-L1 by tumor cells or T cells has been under investigation as a biomarker to predict response to therapy.
In the IMvigor trial using Atezolizumab for treatment of UC, high PD-L1 expression was predictive of a significantly higher response rate in the phase-1 study (53). In the phase-2 validation cohort (second-line treatment after chemotherapy or frontline in cisplatin-ineligible patients), high PD-L1 expression levels (IC2/3) on immune cells were associated with higher response rates to Atezolizumab and longer OS in patients pre-treated with chemotherapy. By contrast, PD-L1 expression on tumor cells did not correlate with outcome (49). In the cisplatin ineligible patient cohort receiving first line atezolizumab, there was no significant association between PD-L1 expression levels and clinical response (50). These differences between the patient cohorts may be coincidental or caused by shifting molecular gene expression patterns through chemotherapy (21).
The Checkmate 275 phase 2 study used the same IHC assay, but instead used a cutoff point of 5% to define PD-L1 expression subgroups. Similarly, there was no association between PD-L1 expression and response to nivolumab (48).
In the Keynote-045 phase 3 study evaluating pembrolizumab as second line treatment, response rates, disease progression or survival were independent of PD-L1 expression on tumor and immune cells (54).
Due to the use of different methods and cut points between the studies, the role of PD-L1 expression on immune or tumor cells as a biomarker to predict response in UC remains unclear. Low/missing PD-L1 expression, however, does not preclude durable response to checkpoint inhibition.
Mutational load
Cancers with higher rates of somatic mutations were shown to respond better to immunotherapy (55-57). TCGA ranks UC as the third highest mutated cancer after melanoma and lung carcinoma (20). This translates into higher neoantigen burden playing a role in tumor cell recognition of CD8+-infiltrating lymphocytes and better response to immunotherapy (58). Checkpoint inhibitors may therefore have a substantial impact in the treatment of UC.
The correlation of mutational load with survival and response to atezolizumab in patients with UC was examined in the IMvigor 210 study. The authors could indeed show that higher mutational load was associated with significantly better response rates (cohort 1&2) and longer OS (cohort 1) (50).
Studies investigating biomarkers for response to chemotherapy in UC reported that tumors harboring DDR gene mutations had a higher mutational burden and copy number alterations, making these tumors vulnerable to both chemo- and immunotherapy (42,47), although this has yet to be confirmed.
Molecular characterization
Gene expression markers investigated in the above mentioned IMvigor study revealed that the immune-related and interferon-γ-induced chemokines CXCL9 and CXCL10 were positive predictors of response to atezolizumab (49). The same factors were recently confirmed as predictive markers for OR in a phase 2 trial Checkmate 275 (48). This is in context with experimental studies showing that defects in interferon-γ signalling caused resistance to immunotherapy and that PD-L1/PD-L2 expression is regulated through the interferon-Jak/Stat-IRF axis (59,60). Manguso and colleagues recently reported their results of an in vivo-screen using CRISPR-Cas9 genome editing in mice to knockout genes expressed in melanoma. They could show that deletion of the protein tyrosine phosphatase PTPN2 in tumor cells increased the efficacy of immunotherapy by enhancing interferon-γ-mediated effects on antigen presentation (59). Such targets, however, require validation in UCB and in prospective clinical studies.
The tumor samples from the IMvigor 210 trial were clustered by gene expression into the four TCGA subtypes. Although responses were observed across all expression subtypes, the ORR was highest at 34% in the cluster II subtype, compared to cluster I (10%), cluster III (16%) and cluster IV (20%) subtypes. Cluster II presents a gene signature of activated effector T cells and an infiltrated phenotype. However, the equally “infiltrated” cluster IV tumors did not respond equally well (49).
In contrast, in the nivolumab CheckMate 275 trial, patients with basal 1-type tumors (represents TCGA cluster III) showed the highest response rate of 30% (7 from 23). Furthermore, gene expression analysis in this trial showed that tumors expressing a high interferon-γ signature and CD8 had a higher ORR (48).
However, the gene expression data of those two trials have not been made publicly available and the methodology for assigning TCGA clusters was not revealed. Final conclusions on the impact of subtyping on response to checkpoint blockade can therefore not be drawn.
The results of larger phase-3 trials are awaited to better identify predictors of clinical response.
Neoadjuvant immunotherapy is not yet established in the treatment of UC. A potentially strong rationale for neoadjuvant immunotherapy has been provided by an experimental study in metastatic breast cancer mouse models (61). The authors demonstrated a significantly greater therapeutic efficacy of neoadjuvant compared to adjuvant immunotherapy and showed that distant metastatic lesions could be eradicated following primary tumor resection after neoadjuvant immunotherapy.
Clinical trials are currently underway using checkpoint inhibitors in a neoadjuvant (i.e., NCT02451423, NCT02736266, NCT0236576, NCT02812420, NCT02989584, NCT 02690558, NCT02365766) and adjuvant (NCT02450331, NCT02632409) setting before or after cystectomy in patients with urothelial cancer.
Of note, some patients show accelerated tumor growth rate under PD1/PDL1-inhibition, so called hyperprogression. Kato and colleagues found a time to treatment failure <2 months in 49 of 155 patients with various cancers (31.6%). Hyperprogressors harbored MDM2/4 or EGFR alterations. All 6 patients, including 1 case of UCB, with MDM2/4 mutations showed hyperprogression. Further studies are required to define risk factors for hyperprogression in UCB and to spare patients from immunotherapies.
Apoptosis
Apoptosis is necessary for physiologic homeostasis and can be initiated via two pathways: the extrinsic pathway, mediated by death receptors on the surface of cells and the intrinsic pathway, mediated by mitochondria. Both pathways lead to activation of effector caspases, which induce cell death (62). Abnormal regulation of apoptotic pathways has been linked to carcinogenesis as well as to resistance to chemotherapy (63,64). Unfortunately, there is only few clinical data about in vivo effects targeting apoptosis in BC so far but multiple preclinical therapeutic biomarkers have been identified.
Different studies showed promising results by targeting IAP with second mitochondria-derived activator of caspases (SMAC) mimetics, which bind to IAPs and induce apoptosis (64-67) and several SMAC mimetics are under preclinical and clinical development as anti-cancer drugs (64). Wang et al. showed that targeting IAPs with a synthetic SMAC peptide (SmacN7) in T24 BC cells induced a down-regulation of the X-linked IAP protein (XIAP) expression with an up-regulation of caspase-3 and it could sensitize T24 BC cells to mitomycin C chemotherapy. It was concluded that SmacN7 could act as a cell-permeable IAP inhibitor, which induces apoptosis and enhances chemo-sensitivity of BC cells (68).
Metwalli and coworkers could demonstrate in a panel of seven different BC cell lines that combination therapy of the SMAC mimetic compound-A with TNF-related apoptosis-inducing ligand (TRAIL) or with standard chemotherapy (gemcitabine/cisplatin) enhances the antitumoral effects. Immunoblotting showed that combination treatment with compound-A and TRAIL or chemotherapy resulted in enhanced apoptosis. Immunoprecipitation of XIAP showed displacement of active caspase-3 fragments from XIAP, supporting the proposed mechanism of action (69). Mizutani et al. showed synergistics effects of administration of TRAIL with cisplatin and adriamycin in vitro (70,71). Bilim et al. demonstrated that XIAP can be downregulated by antisense oligodeoxyribonucleotides (AS-ODN) followed by an enhanced sensitivity of BC cell lines to adriamycin (72).
Overexpression of pro-survival Bcl-2 family member proteins has been associated with poor chemotherapeutic response in BC (73). Mani et al. investigated the response of BC cell lines to treatment with the BH3 mimetic gossypol. The cell lines were either chemo-sensitive or showed a specific acquired resistance against gemcitabine- or cisplatin. They used the small-molecule pan-Bcl-2 inhibitor gossypol (AT-101) that is known to induce apoptosis and that is also able to induce autophagy through release of the pro-autophagic BH3 only proteins. Gossypol concomitantly triggered apoptosis combined with a cytoprotective type of autophagy. Therefore, autophagy may at least partly be a reason for the acquired resistance against gemcitabine or cisplatin. Simultaneous targeting of Bcl-2 proteins and autophagy might be therefore a reasonable therapeutic option to circumvent chemoresistance in an NAC or AC setting (74).
In addition, further anti-apoptotic molecules like Bcl-xL, survivin and XIAP were found to be frequently overexpressed in different cancer entities including BC. Overexpression of these molecules seems to be associated with a poor outcome (66,75-78). Kunze et al. showed that siRNA mediated suppression of these anti-apoptotic proteins in BC cells mediated significant reductions in cell viability and cell counts as well as an increased induction of apoptosis (79). The same study group was able to sensitize BC cells to conventional chemotherapeutics with Bcl-xL-targeting AS-ODNs. In different cell lines, combined treatment with AS-ODNs and cisplatin caused an additional inhibition of cell viability and an increased activity of apoptosis (80).
Several biomarkers with an impact on apoptotic pathways have been identified. However, few of these markers were proven in clinical trials and none has been adopted into routine practice.
Drug transporter
Multidrug resistance (MDR) to chemotherapeutic drugs is one of the main causes of chemotherapy failure in cancer treatment. It frequently results from expression of ATP-dependent efflux pumps such as the drug transporter family ATP-binding cassette transporter (ABC) with its subfamily B, member 1 (ABCB1) (81). MDR reversal agents typically act by inhibiting the drug efflux activity of drug transporters such as ABCB1, and thereby increasing intracellular drug levels (81). Resistance to chemotherapy is frequently caused by overexpression of ABCB1 in tumor cells, which develops mostly as a specific response to ABCB1 substrates (e.g., vinca alkaloids, taxanes, or anthracyclines) (74,82-85). Cisplatin, as it is frequently used as a chemotherapeutic agent in BC, is not an ABCB1 substrate (86,87).
However, a study recently showed that BC cells with a specific acquired resistance to gemcitabine after long-term treatment can also display ABCB1 upregulation (82). That is why, ABCB1 expression may affect the efficacy of candidate drugs for NAC, AC or second-line therapies of BC after failure of first line therapy with gemcitabine and cisplatin. In addition, overexpression of drug pumps may also modulate further malignant properties of cancer cells (e.g., cell survival, cell proliferation, cell invasion) independently of the transporter-mediated drug efflux (88,89). Therefore, it is not surprising that ABCB1 expression correlates with an advanced tumor grade or with an increasing risk of recurrence in UCB patients (90).
Besides ABC family drug transporter members, other transporters are also involved in chemoresistance. MicroRNA-218 (miR-218) is downregulated in many malignancies. The glucose transporter 1 (GLUT-1) is a target of miR-218. In BC cells T24 and EJ, it was shown that over-expression of miR-218 significantly reduced the rate of glucose uptake and total level of glutathione and enhanced the chemo-sensitivity of BC cells to cisplatin (66). In addition, overexpression of GLUT-1 was shown to be a predictor of poor survival in BC (91).
Another example is the copper transporter receptor 1 (CTR1) that plays an important role in cisplatin uptake. The level of CTR1 expression may influence cisplatin sensitivity. Kilari et al. demonstrated that tumor CTR1 expression correlated with pathological outcome (92).
Concerning gemcitabine, as another frequently used agent for NAC or AC, North and coworkers evaluate the relevance of the deoxycytidine kinase and the human equilibrative nucleoside transporter 1. Both molecules have been validated as predictive markers for a benefit of a gemcitabine therapy in pancreatic cancer. Unfortunately, the predictive value could not be shown for BC patients (93).
Inhibition of drug-transporters by low molecular weight compounds has been extensively investigated in clinical trials in different cancer entities but the results have been disappointing and none of the biomarkers has been introduced into daily practice (94).
Discussion
RC combined with platinum-based NAC is the standard of care treatment for clinically localized MIBC (2,3). To date, there is no reliable method for the prediction of response to chemotherapy resulting in a possible overtreatment in non-responders. Therefore, the identification of chemotherapy responders before the start of systemic therapy would be a very helpful clinical asset. Multiple molecular biomarkers have been studied for prediction of response to chemotherapy such as mutations in DNA damage repair pathways, receptor tyrosine kinases, gene expression markers, regulators of apoptosis, cellular mechanisms of drug uptake and transport.
From a molecular point of view, UCB is a widely heterogenic tumor entity and next generation therapies will be guided by genetic testing. So far only a few prediction tools based on readily available clinical or pathological parameters have been developed to identify patients who might benefit from chemotherapy in a neoadjuvant setting relying on high risk features such as cT3b-T4adisease, hydroureteronephrosis, lymphovascular invasion, neuroendocrine or micropapillary subtypes (95) or relying on a high proliferation rate in the initial chemotherapy-naive BC (96). In the future, multivariable prediction models incorporating biomarkers might be helpful to identify patients that could possibly benefit from chemotherapy in a clinical setting with an improved predictive accuracy compared to existing models (97).
NAC before radical surgery represent an ideal setting to study resistance mechanisms as well as to identify biomarkers for the prediction of response to chemotherapy. Especially the comparison of pretreatment tumor tissue (TUR-BT) compared to residual tumor tissue after NAC in RC specimen may provide a valuable resource to analyze histological as well as molecular features of resistant and responsive cellular clones.
The rapid progress of genomic characterization of UCB in recent years has resulted in a first standardized taxonomy which requires further harmonization to allow clinical applicability (28). In times to come, analyses of histopathological and molecular features of each subtype might provide insight on mechanisms underlying treatment response or resistance. Nevertheless, consensus exists for defining basal-squamous-like tumors expressing KRT5/6 and KRT14 (28). Patients with such tumor characteristics may in future be advised to receive cisplatin-based NAC while other patients may require novel, genomic-driven target therapies. However, molecular driven clinical decision making requires validation in prospective trials. Consequently, mutation driven individualized medicine approaches have already resulted in the initiation of clinical trials with the aim to improve patient care and efficacy of individual therapy by molecular patient selection (i.e., NCI-MATCH Trial, NCT02465060).
Despite reports that demonstrated mutations in DDR genes (ATM, RB1 and ERCC2) in responders as well as non-responders and that did not show an association with pathologic response (46), the literature suggests better outcome of platinum-based chemotherapy in BC with DDR mutations. Clinical trials such as the COXEN trial (NCT02177695) are underway to assess the use of these potential biomarkers for clinical decision-making.
The role of PD-1 and PD-L1 expression on immune or tumor cells as a biomarker to predict response to immunotherapy in UC remains unclear which is partially caused by the use of different methods and cut points between studies (48-51). Low or missing PD1 and PD-L1 expression patterns do not preclude durable response to checkpoint inhibition. Ongoing clinical trials using checkpoint inhibitors in a neoadjuvant or adjuvant setting in UCB patients undergoing RC might deliver additional evidence on the interpretation of PD-1 and PD-L1 expression patterns.
Moreover, abnormal regulation of apoptotic pathways has been linked to carcinogenesis as well as to resistance to chemotherapy (63,64) Unfortunately, there has been only few clinical data on targeting apoptosis in UCB so far even though multiple preclinical therapeutic biomarkers have been identified. However, few of these markers were proven in clinical trials and none has been adopted into routine practice.
Furthermore, MDR to chemotherapeutic drugs is one of the main causes of chemotherapy failure in cancer treatment, and it frequently results from expression of ATP-dependent efflux pumps (ABC, ABCB1) (81). Inhibition of drug-transporters by low molecular weight compounds has been extensively investigated in clinical trials in different cancer entities but the results have been disappointing and none of these biomarkers has been introduced into daily practice (94).
All published studies on biomarkers for prediction of response to chemotherapy have important limitations such as the small number of patients and the heterogeneity of chemotherapy regimens. Further validation of these markers is required to establish molecular evaluation in clinical practice by using homogeneous chemotherapy regimens in large prospective studies with sufficient follow up. Ideally, these studies should compare NAC treated patients with patients receiving RC only to distinguish whether their role is predictive or rather prognostic.
In summary, to date, cisplatin-based NAC before RC is the standard of care for MIBC. Despite tremendous efforts to identify predictive genetic and molecular characteristics of response to chemotherapy in UCB, these potential biomarkers have not yet translated into clinically useful tools. However, ongoing clinical trials examining the benefit of individual therapies in UCB by molecular patient selection hold promise to shed light on this question.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Naishadham D, Jemal D. Cancer statistics 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Chang SS, Boorjian SA, Chou R, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol 2016;196:1021-9. [Crossref] [PubMed]
- Alfred Witjes J, Lebret T, Comparat EM, et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol 2017;71:462-75. [Crossref] [PubMed]
- Ploussard G, Shariat SF, Dragomir A, et al. Conditional survival after radical cystectomy for bladder cancer: Evidence for a patient changing risk profile over time. Eur Urol 2014;66:361-70. [Crossref] [PubMed]
- Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001;19:666-75. [Crossref] [PubMed]
- von der Maase H, Sengelov L, Roberts JT, et al. Long-Term Survival Results of a Randomized Trial Comparing Gemcitabine Plus Cisplatin, With Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin in Patients With Bladder Cancer. J Clin Oncol 2005;23:4602-8. [Crossref] [PubMed]
- Grossman HB, Tangen CM, Speights VO, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859-66. [Crossref] [PubMed]
- International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 trial. J Clin Oncol 2011;29:2171-7. [Crossref] [PubMed]
- Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 2003;361:1927-34. [Crossref] [PubMed]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 2005;48:202-5. [Crossref] [PubMed]
- Schiffmann J, Sun M, Gandaglia G, et al. Suboptimal use of neoadjuvant chemotherapy in radical cystectomy patients: A population-based study. Can Urol Assoc J 2016;10:E82-6. [Crossref] [PubMed]
- Burger M, Mulders P, Witjes W. Use of neoadjuvant chemotherapy for muscle-invasive bladder cancer is low among major European centres: results of a feasibility questionnaire. Eur Urol 2012;61:1070-1. [Crossref] [PubMed]
- Roghmann F, Trinh QD, Braun K, et al. Standardized assessment of complications in a contemporary series of European patients undergoing radical cystectomy. Int J Urol 2014;21:143-9. [Crossref] [PubMed]
- Shabsigh A, Korets R, Vora KC, et al. Defining Early Morbidity of Radical Cystectomy for Patients with Bladder Cancer Using a Standardized Reporting Methodology. Eur Urol 2009;55:164-74. [Crossref] [PubMed]
- Johar RS, Hayn MH, Stegemann AP, et al. Complications after robot-assisted radical cystectomy: Results from the international robotic cystectomy consortium. Eur Urol 2013;64:52-7. [Crossref] [PubMed]
- Als AB, Dyrskjot L, von der Maase H, et al. Emmprin and Survivin Predict Response and Survival following Cisplatin-Containing Chemotherapy in Patients with Advanced Bladder Cancer. Clin Cancer Res 2007;13:4407-14. [Crossref] [PubMed]
- Hemdan T, Malmström PU, Jahnson S, et al. Emmprin expression predicts response and survival following cisplatin containing chemotherapy for bladder cancer: A validation study. J Urol 2015;194:1575-81. [Crossref] [PubMed]
- Sjödahl G, Lauss M, Lövgren K, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res 2012;18:3377-86. [Crossref] [PubMed]
- Sjödahl G, Lövgren K, Lauss M, et al. Toward a molecular pathologic classification of urothelial carcinoma. Am J Pathol 2013;183:681-91. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507:315-22. [Crossref] [PubMed]
- Choi W, Porten S, Kim S, et al. Identification of Distinct Basal and Luminal Subtypes of Muscle-Invasive Bladder Cancer with Different Sensitivities to Frontline Chemotherapy. Cancer Cell 2014;25:152-65. [Crossref] [PubMed]
- Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A 2014;111:3110-5. [Crossref] [PubMed]
- Choi W, Czerniak B, Ochoa A, et al. Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer. Nat Rev Urol 2014;11:400-10. [Crossref] [PubMed]
- Seiler R, Ashab HAD, Erho N, et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur Urol 2017;72:544-54. [Crossref] [PubMed]
- Rebouissou S, Bernard-Pierrot I, de Reyniès A, et al. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci Transl Med 2014;6:244ra91. [Crossref] [PubMed]
- McConkey DJ, Choi W, Shen Y, et al. A Prognostic Gene Expression Signature in the Molecular Classification of Chemotherapy-naïve Urothelial Cancer is Predictive of Clinical Outcomes from Neoadjuvant Chemotherapy: A Phase 2 Trial of Dose-dense Methotrexate, Vinblastine, Doxorubicin, and Cisp. Eur Urol 2016;69:855-62. [Crossref] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61-70. [Crossref] [PubMed]
- Lerner SP, McConkey DJ, Hoadley KA, et al. Bladder Cancer Molecular Taxonomy: Summary from a Consensus Meeting. Bladder Cancer 2016;2:37-47. [Crossref] [PubMed]
- Southgate J, Harnden P, Trejdosiewicz LK. Cytokeratin expression patterns in normal and malignant urothelium: a review of the biological and diagnostic implications. Histol Histopathol 1999;14:657-64. [PubMed]
- Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 2009;297:F1477-501. [Crossref] [PubMed]
- Lindgren D, Frigyesi A, Gudjonsson S, et al. Combined gene expression and genomic profiling define two intrinsic molecular subtypes of urothelial carcinoma and gene signatures for molecular grading and outcome. Cancer Res 2010;70:3463-72. [Crossref] [PubMed]
- Choi W, Ochoa A, Mcconkey DJ, et al. Urothelial Cancer Genetic Alterations in the Molecular Subtypes of Bladder Cancer: Illustration in the Cancer Genome Atlas Dataset. Eur Urol 2017;72:354-65. [Crossref] [PubMed]
- Kurtova AV, Xiao J, Mo Q, et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 2015;517:209-13. [Crossref] [PubMed]
- McConkey DJ, Choi W, Dinney CP. Genetic subtypes of invasive bladder cancer. Curr Opin Urol 2015;25:449-58. [Crossref] [PubMed]
- Mitra AP, Lam LL, Ghadessi M, et al. Discovery and validation of novel expression signature for postcystectomy recurrence in high-risk bladder cancer. J Natl Cancer Inst 2014.106. [PubMed]
- Dadhania V, Zhang M, Zhang L, et al. Meta-Analysis of the Luminal and Basal Subtypes of Bladder Cancer and the Identification of Signature Immunohistochemical Markers for Clinical Use. EBioMedicine 2016;12:105-17. [Crossref] [PubMed]
- Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer 2012;12:801-17. [Crossref] [PubMed]
- Bellmunt J, Paz-Ares L, Cuello M, et al. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Ann Oncol 2007;18:522-8. [Crossref] [PubMed]
- Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4:1140-53. [Crossref] [PubMed]
- Liu D, Plimack ER, Hoffman-Censits J, et al. Clinical Validation of Chemotherapy Response Biomarker ERCC2 in Muscle-Invasive Urothelial Bladder Carcinoma. JAMA Oncol 2016;2:1094. [Crossref] [PubMed]
- Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer. Eur Urol 2015;68:959-67. [Crossref] [PubMed]
- Yap KL, Kiyotani K, Tamura K, et al. Whole-exome sequencing of muscle-invasive bladder cancer identifies recurrent mutations of UNC5C and prognostic importance of DNA repair gene mutations on survival. Clin Cancer Res 2014;20:6605-17. [Crossref] [PubMed]
- Bolenz C, Shariat SF, Karakiewicz PI, et al. Human epidermal growth factor receptor 2 expression status provides independent prognostic information in patients with urothelial carcinoma of the urinary bladder. BJU Int 2010;106:1216-22. [Crossref] [PubMed]
- Powles T, Huddart RA, Elliott T, et al. Phase III, Double-Blind, Randomized Trial That Compared Maintenance Lapatinib Versus Placebo After First-Line Chemotherapy in Patients With Human Epidermal Growth Factor Receptor 1/2-Positive Metastatic Bladder Cancer. J Clin Oncol 2017;35:48-55. [Crossref] [PubMed]
- Oudard S, Culine S, Vano Y, et al. Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur J Cancer 2015;51:45-54. [Crossref] [PubMed]
- Groenendijk FH, de Jong J, Fransen van de Putte EE, et al. ERBB2 Mutations Characterize a Subgroup of Muscle-invasive Bladder Cancers with Excellent Response to Neoadjuvant Chemotherapy. Eur Urol 2016;69:384-8. [Crossref] [PubMed]
- Teo MY, Bambury RM, Zabor EC, et al. DNA Damage Response and Repair Gene Alterations Are Associated with Improved Survival in Patients with Platinum-Treated Advanced Urothelial Carcinoma. Clin Cancer Res 2017;23:3610-8. [Crossref] [PubMed]
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312-22. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67-76. [Crossref] [PubMed]
- Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol 2017;18:212-20. [Crossref] [PubMed]
- Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin Cancer Res 2017;23:4242-50. [Crossref] [PubMed]
- Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558-62. [Crossref] [PubMed]
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376:1015-26. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Colli LM, Machiela MJ, Myers TA, et al. Burden of nonsynonymous mutations among TCGA cancers and candidate immune checkpoint inhibitor responses. Cancer Res 2016;76:3767-72. [Crossref] [PubMed]
- McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016;351:1463-9. [Crossref] [PubMed]
- Manguso RT, Pope HW, Zimmer MD, et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature 2017;547:413-8. [Crossref] [PubMed]
- Garcia-Diaz A, Shin DS, Moreno BH, et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep 2017;19:1189-201. [Crossref] [PubMed]
- Liu J, Blake SJ, Yong MC, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 2016;6:1382-99. [Crossref] [PubMed]
- Kerr JF. Shrinkage necrosis: a distinct mode of cellular death. J Pathol 1971;105:13-20. [Crossref] [PubMed]
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature 2001;411:342-8. [Crossref] [PubMed]
- Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov 2012;11:109-24. [Crossref] [PubMed]
- Chai J, Du C, Wu JW, et al. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 2000;406:855-62. [Crossref] [PubMed]
- Li L. A small molecule Smac mimic potentiates TRAIL- and TNFa -mediated cell death. Science 2004;305:1471-4. [Crossref] [PubMed]
- Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene 2002;21:2283-94. [Crossref] [PubMed]
- Wang J, Zeng F, Wang L, et al. Synthetic Smac peptide enhances chemo-sensitivity of bladder cancer cells. J Huazhong Univ Sci Technol Med Sci 2008;28:304-7. [Crossref] [PubMed]
- Metwalli AR, Khanbolooki S, Jinesh G, et al. Smac mimetic reverses resistance to TRAIL and chemotherapy in human urothelial cancer cells. Cancer Biol Ther 2010;10:885-92. [Crossref] [PubMed]
- Mizutani Y, Yoshida O, Miki T, et al. Synergistic cytotoxicity and apoptosis by Apo-2 ligand and adriamycin against bladder cancer cells. Clin Cancer Res 1999;5:2605-12. [PubMed]
- Mizutani Y, Nakao M, Ogawa O, et al. Enhanced sensitivity of bladder cancer cells to tumor necrosis factor related apoptosis inducing ligand mediated apoptosis by cisplatin and carboplatin. J Urol 2001;165:263-70. [Crossref] [PubMed]
- Bilim V, Kasahara T, Hara N, et al. Role of XIAP in the malignant phenotype of transitional cell cancer (TCC) and therapeutic activity of XIAP antisense oligonucleotides against multidrug-resistant TCC in vitro. Int J Cancer 2003;103:29-37. [Crossref] [PubMed]
- Karam JA, Lotan Y, Karakiewicz PI, et al. Use of combined apoptosis biomarkers for prediction of bladder cancer recurrence and mortality after radical cystectomy. Lancet Oncol 2007;8:128-36. [Crossref] [PubMed]
- Mani J, Vallo S, Rakel S, et al. Chemoresistance is associated with increased cytoprotective autophagy and diminished apoptosis in bladder cancer cells treated with the BH3 mimetic (-)-Gossypol (AT-101). BMC Cancer 2015;15:224. [Crossref] [PubMed]
- Korkolopoulou P, Lazaris AC, Konstantinidou AE, et al. Differential expression of bcl-2 family proteins in bladder carcinomas relationship with apoptotic rate and survival. Eur Urol 2002;41:274-83. [Crossref] [PubMed]
- Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep 2006;7:988-94. [Crossref] [PubMed]
- Shariat SF, Casella R, Khoddami SM, et al. Urine Detection of Survivin is a Sensitive Marker for the Noninvasive Diagnosis of Bladder Cancer. J Urol 2004;171:626-30. [Crossref] [PubMed]
- Margulis V, Lotan Y, Shariat SF. Survivin: A promising biomarker for detection and prognosis of bladder cancer. World J Urol 2008;26:59-65. [Crossref] [PubMed]
- Kunze D, Kraemer K, Erdmann K, et al. Simultaneous siRNA-mediated knockdown of antiapoptotic BCL2, Bcl-xL, XIAP and survivin in bladder cancer cells. Int J Oncol 2012;41:1271-7. [PubMed]
- Rieger C, Huebner D, Temme A, et al. Antisense- and siRNA-mediated inhibition of the anti-apoptotic gene Bcl-xL for chemosensitization of bladder cancer cells. Int J Oncol 2015;47:1121-30. [Crossref] [PubMed]
- Chen Z, Shi T, Zhang L, et al. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett 2016;370:153-64. [Crossref] [PubMed]
- Vallo S, Michaelis M, Rothweiler F, et al. Drug-resistant urothelial canc cell lines display diverse sensitivity profiles to potential second-line therapeutics. Transl Oncol 2015;8:210-6. [Crossref] [PubMed]
- Yang LY, Trujillo JM, Siciliano MJ, et al. Distinct P-glycoprotein expression in two subclones simultaneously selected from a human colon carcinoma cell line by cis-diamminedichloroplatinum (II). Int J Cancer 1993;53:478-85. [Crossref] [PubMed]
- Yang X, Page M. P-glycoprotein expression in ovarian cancer cell line following treatment with cisplatin. Oncol Res 1995;7:619-24. [PubMed]
- Xu H, Choi SM, An CS, et al. Concentration-dependent collateral sensitivity of cisplatin-resistant gastric cancer cell sublines. Biochem Biophys Res Commun 2005;328:618-22. [Crossref] [PubMed]
- Hamaguchi K, Godwin AK, Yakushiji M, et al. Cross-resistance to diverse drugs is associated with primary cisplatin resistance in ovarian cancer cell lines. Cancer Res 1993;53:5225-32. [PubMed]
- Stordal B, Hamon M, McEneaney V, et al. Resistance to paclitaxel in a cisplatin-resistant ovarian cancer cell line is mediated by P-glycoprotein. PLoS One 2012;7:e40717. [Crossref] [PubMed]
- Fletcher JI, Haber M, Henderson MJ, et al. ABC transporters in cancer: more than just efflux pumps. Nat Rev Cancer 2010;10:147-56. [Crossref] [PubMed]
- Breier A, Gibalova L, Seres M, et al. New Insight into P-Glycoprotein as a Drug Target. Anticancer Agents Med Chem 2013;13:159-70. [PubMed]
- Serretta V, Pavone C, Allegro R, et al. Correlation between GP-170 expression, prognosis, and chemoresistance of superficial bladder carcinoma. J Cancer Res Clin Oncol 2003;129:472-6. [Crossref] [PubMed]
- Bostrom PJ, Thoms J, Van Rhijn BW, et al. Hypoxia is independently associated with poor outcome in urothelial bladder cancer patients treated with radical cystectomy. Eur Urol Suppl 2012;11:e905. [Crossref]
- Kilari D, Iczkowski KA, Pandya C, et al. Copper Transporter-CTR1 Expression and Pathological Outcomes in Platinum-treated Muscle-invasive Bladder Cancer Patients. Anticancer Res 2016;36:495-501. [PubMed]
- North S, El-Gehani F, Santos C, et al. Expression of nucleoside transporters and deoxycytidine kinase proteins in muscle invasive urothelial carcinoma of the bladder: Correlation with pathological response to neoadjuvant platinum/gemcitabine combination chemotherapy. J Urol 2014;191:35-9. [Crossref] [PubMed]
- Lage H. Gene Therapeutic Approaches to Overcome ABCB1-Mediated Drug Resistance. Recent Results Cancer Res 2016;209:87-94. [Crossref] [PubMed]
- Culp SH, Dickstein RJ, Grossman HB, et al. Refining patient selection for neoadjuvant chemotherapy before radical cystectomy. J Urol 2014;191:40-7. [Crossref] [PubMed]
- Fleischmann A, Thalmann GN, Perren A, et al. Tumor regression grade of urothelial bladder cancer after neoadjuvant chemotherapy: a novel and successful strategy to predict survival. Am J Surg Pathol 2014;38:325-32. [Crossref] [PubMed]
- Vickers AJ, Cronin AM, Kattan MW, et al. Clinical benefits of a multivariate prediction model for bladder cancer: A decision analytic approach. Cancer 2009;115:5460-9. [Crossref] [PubMed]


