Online tools for patient counseling in bladder and kidney cancer—ready for prime time?
Introduction
Estimating prognosis is a key element throughout the cancer continuum and as such the foundation of treatment decisions that physicians and their patients have to face. In addition to the challenge of gauging prognosis, advances in the medical field have led to multiple treatment options, among which patients and their families have to choose—adding to the complexity of information to process and thereby creating a potential barrier for non-medically trained subjects. Formerly, the doctor-patient relationship placed physicians in a paternal role whereas patients assumed a rather passive, dependent role when facing clinical decisions (1). However, lack of patient involvement in treatment decision is a major risk factor for regret of treatment choice (2) and throughout the last four decades the idea of patient-centered care or patient-centeredness has emerged—a concept which takes into consideration personal preferences, needs, and values, and actively engages patients in clinical decision-making (3). A vast body of evidence supports this approach and has demonstrated that patient-centered care improves disease-related outcomes and quality of life (3). Central to the concept of patient-centeredness is the idea of shared-decision making, which in turn necessitates conveying a great volume of information to the patient (4). Providers of cancer care can rely on a number of counseling tools to provide such information. These include estimates and recommendations based on their own clinical experience and intuition, scientific publications (i.e., data from clinical trials), cancer registry data [i.e., Surveillance Epidemiology and End Results (SEER)], look-up tables, and prediction models such as nomograms. The latter hold distinct advantages: They perform with greater accuracy than physicians´ estimations or stage based predictions. Moreover, can they integrate multiple disease and patient characteristics and thereby provide patient-tailored estimates of a given outcome (5).
With the availability of the Internet to an ever-increasing patient population, online access to counseling tools, such as prediction models and registry data is gaining in importance. In fact, it has been estimated, that 4.5% of all web-based search queries are conducted for health-related issues (6) and that 62–80% of cancer patients are interested obtaining web-based information (7). While online prognostic tools are readily available in breast and colon cancer (8,9) their availability to patients with bladder and kidney cancer has not been evaluated to date. The aim of this semi-systematic review, therefore, was to evaluate the availability of online prognostic tools intended for patient use in bladder and kidney cancer, as well as to describe their content and format.
Methods
First, a nonsystematic literature search was conducted using the MEDLINE/PubMed database to identify original articles, review articles and editorials. Searches were limited to the English language, and used the keywords urothelial carcinoma; transitional cell carcinoma; muscle-invasive, non-muscle invasive bladder cancer; kidney cancer; renal cell carcinoma in combination with prognostic factor; predictive tool; nomogram; risk stratification; survival; recurrence. All abstracts were reviewed and the corresponding full-length articles for those that were most relevant to each subsection were analyzed. Articles written before 1995 were excluded from analysis. Secondly, we performed an Internet search, using the search engine Google and employed similar terms as in our literature search. For each search, the first five pages of results for relevant tools were reviewed. Predictive tools identified in this search approach were only selected for further review if they were accessible in an online format.
Results
Bladder cancer
With an estimated 79,030 new diagnoses and 16,870 deaths in 2017, bladder cancer is the 5th most common cancer in the U.S. alone and conveys the highest mortality among urological malignancies (10). Non-muscle invasive (NIMBC) and muscle-invasive (MIBC) disease vary in prognosis and generally are amenable to several treatment regimens. Contrary, in the metastatic setting of bladder cancer, therapeutic options are limited at poor prognosis with little change seen over the last decades (11).
Cancer registry data
The SEER program, sponsored by the National Cancer institute, consists of multiple statewide tumor registries that cover cancer incidence and survival of approximately 28% of the US population (12). Since its inception in 1973, these population-based data are made available to the public on an annual base and in the current web-based format offer a wide array of information to patients (Table 1). These include information about the incidence, as well as the relative overall and stage-specific (defined as in situ, localized, regional, distant) survival, stratified by gender, age, and race (available as “statistical summaries”) (35). In addition to these cancer statistics, the website offers several interactive tools, such as "know your chances”, that offer survival estimations in the context of competing risks, and under stratification of age, gender and race, albeit without stage-specific stratification (35).
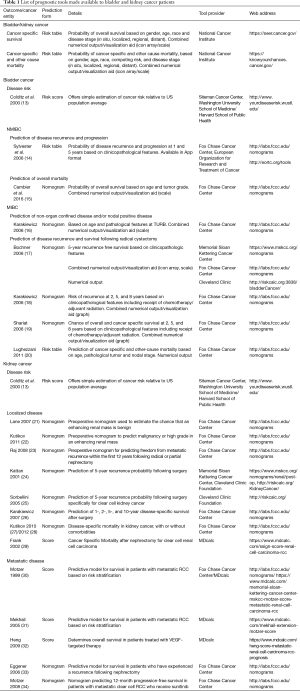
Full table
Overall disease risk
Our search identified one predictive model for the overall risk of BC that has been made available in a patient-friendly, web-based format. The Washington University School of Medicine/Harvard Cancer Risk Index (13) is based on a simple scoring system in dichotomous format (Table 1). Based on individual risk factors, such as age, family and smoking history, and environmental exposure (including exposure to aromatic amines and water chlorination) a score is built. The score is then compared to the population average to provide the user with a relative risk estimate (“low”, “average”, “high”). This comparison is based on Surveillance Epidemiology and End Results (SEER) published data. A limitation includes the lack of providing absolute risk estimates. Further, this model was developed and validated in a US population and may therefore not be applicable in a European cohort.
Non-muscle invasive bladder cancer (NMIBC)
The European Organization for Research and Treatment of Cancer (EORTC) Genito-Urinary Cancers Groups has developed a scoring system and risk tables to predict the 1- and 5-year probabilities of disease recurrence and progression in patients diagnosed with pathological Ta/T1 NMIBC (Table 1). The model was developed using previous trial data of 2,596 patients and incorporates six clinico-pathological features: Tumor stage and grade, number of tumors, tumor size, concomitant Carcinoma in situ, and history of prior disease recurrence (14). To date, the model has been externally validated in a number of cohorts and has been incorporated into international guidelines (36,37). Further, the risk-tables are available as application software (App) for handheld devices. Notably, the model has some distinct limitations, which mainly pertain to the accrual period of patients (1979 to 1989), which is not reflective of current standards of treatment. For example, a second look transurethral resection of the bladder was not performed, and fewer than 10% of the patients received immediate intravesical instillation therapy. Consequently, the model tends to overestimate the risk of disease recurrence and progression (38).
In an effort to address these shortcomings, a recent report has developed a nomogram and a risk grouping system for NMIBC in patients that were treated with 1–3 years of intravesical Bacillus Calmette-Guerin (Table 1). The model was developed in two contemporary EORTC trial cohorts [1992–2013] to predict disease recurrence, progression, disease-specific and overall survival and subsequently (15). The according nomogram consists of the two factors age and grade and predicts 1- and 5-year survival probability (15). Limitations include the lack of patients with Carcinoma in Situ as well as pending external validation.
Muscle-invasive bladder cancer (MIBC)
Prediction of non-organ confined disease
Karakiewicz et al. developed a preoperative nomogram to predict advanced pathological disease stage and presence of lymph node metastasis at the time of radical cystectomy (16). The model was developed in a cohort of 731 patients and incorporates age, stage, grade, and presence of carcinoma in situ at transurethral resection of the bladder, and further has recently been externally validated (39) (Table 1).
Prediction of disease recurrence and survival following radical cystectomy
Our web search identified four nomograms that predict survival in patients undergoing radical cystectomy and which have been made available in a patient-friendly web format (Table 1) (17-20). The International Bladder Cancer Nomogram Consortium (IBCNC), developed a postoperative nomogram predicting the 5-year risk of disease recurrence following radical cystectomy and pelvic lymph node dissection (17). The nomogram was developed in a multicenter cohort of more than 9,000 patients and has subsequently been externally validated in a large European cohort (40). Variables included are gender, age, pathological tumor stage and grade, histologic type (transitional cell and squamous cell carcinoma, adenocarcinoma), pathological nodal stage, and time from diagnosis of disease to radical cystectomy. Similarly, Karakiewicz et al. (18) in a multicenter cohort of 728 patients developed a nomogram to predict disease recurrence after radical cystectomy with bilateral lymphadenectomy. The model differs from the IBCNC in that it requires the presence of lymphovascular invasion or carcinoma in situ, as well as adjuvant chemo- or radiotherapy in addition to a pathological tumor and nodal stage. Shariat et al. (19) relying on the same cohort, developed a nomogram to predict all-cause and cancer-specific survival at 2, 5, and 8 years after radical cystectomy. External validation of both models is pending to date.
Lastly, Lughezzani et al., (20) using the SEER registry, developed competing risk tables to provide cancer-specific and overall mortality, based on stratification by pathological tumor and nodal stage, as well as age at surgery.
Kidney cancer
Kidney cancer is the 10th most common cancer overall and affects nearly 63,330 patients in the US alone per year (10). The differentiation between localized and metastatic disease is crucial for making treatment decisions. Especially for metastatic disease stages, treatment options have expanded tremendously over the past decade. Within this environment, urologists have to increasingly rely on tools to counsel their patients.
Localized kidney cancer
These days, localized kidney cancer is almost exclusively diagnosed at an asymptomatic stage through the increased use of radiographic imaging. These incidental lesions pose a challenge for radiologists and urologists alike: First, imaging is not perfectly reliable while providers and patients need an accurate estimation of malignant potential. Second, patients are inherently eager to know about potential risks of local or systemic recurrence before and after surgery.
Preoperatively, to estimate the chance of a radiologic lesion to be malignant, Lane et al. proposed a model based on age, radiographic tumor size, smoking history, symptoms at presentation and gender. Although parameters from 862 patients were considered, the nomogram had a relatively low concordance index of 0.64 (21). Kutikov and colleagues evaluated whether radiographic features of renal masses could predict tumor pathology. In a comprehensive institutional cohort of 525 patients, they found that the RENAL nephrometry score (41) could quantitate the preoperative likelihood of malignant and high-grade pathology (22). Based on readily available preoperative parameters (gender, mode of presentation, radiographic lymphadenopathy, radiographic evidence of necrosis and tumor size), Raj used a multi-institutional cohort of >2,000 patients with either radical or partial nephrectomy to help counsel patients regarding their 12-year metastasis-free survival (23).
Similarly, there exist several prognostic calculators after surgery for kidney cancer. The Karakiewicz nomogram helps estimate cancer-specific survival 1-, 2-, 5-, and 10-year after local surgery by utilizing pathological (T stage, nodal status, presence of metastases, tumor size, tumor grade) and clinical (symptoms at presentation) (26). Kattan et al. (24) proposed a nomogram based on histological (chromophobe, conventional, papillary, none), clinical symptoms (none, incidental, local, systemic), tumor stage (AJCC Version 5) to calculate the 5-year recurrence free survival after surgery. The same group proposed a nomogram exclusively for clear cell renal cell carcinoma, the most common subtype, in 2005 (25). Parameters include tumor size in centimeter, the pathological stage according to the 2002 TNM classification, pathological grading, necrosis, vascular invasion and stage at presentation (incidental, local, metastatic). The latter nomogram was updated in 2016 and validated in a larger cohort (42). In elderly patients, death from disease is more unlikely than death from other causes. A simple nomogram consisting of information about race, gender, age and tumor size is available to calculate the competing risk unadjusted (27) or adjusted for relevant comorbidities (28). In clear cell kidney cancer, the stage, size, grade, and necrosis (SSIGN) score (29) is used to estimate cancer-specific survival after radical nephrectomy. Its reliance on the overcome 2002 TNM classification hampers its current clinical applicability.
Metastatic disease
Patients with metastatic kidney cancer have an array of treatment options currently available. However, until the middle of the last decade, traditional immunotherapy was the treatment of choice. The Motzer score was developed to model survival in metastatic kidney cancer in these patients and is traditionally based on the levels of LDH, Hb, corrected serum calcium, Karnofsky performance status and a disease-free interval of <1 year (30). In the Mekhail extension, performance status is dropped with the addition of prior radiation treatment and the number of metastasis (≥2) (31). With similar parameters, Eggener et al. have developed a nomogram to estimate survival in patients with a recurrence following nephrectomy (33). Probably the most useful score in the treatment of metastatic renal cell carcinoma in the current treatment environment is the Heng score. Developed in a large, multi-institutional cohort, it uses the time from diagnosis to the initiation of systemic therapy, the performance index, Hb, Calcium, Neutrophil count and platelet count to estimate overall survival. Most importantly, it was developed in a cohort of patients treated with VEGF-therapy, specifically (32). Another useful application was described by Motzer et al. in 375 patients on Sunitinib therapy for metastatic kidney cancer. 12-month progression-free survival is calculated using the backbone of the traditional Motzer score (Hb, time to treatment, LDH and corrected calcium) with the addition of a number of metastatic sites, the presence of lung or liver metastases, ECOG PS, thrombocytosis, Alkaline Phosphatase and prior nephrectomy (34).
Uncharted territory of online tools for patient counseling with kidney cancer
At the dawn of personalized medicine, tremendous opportunities and challenges lie ahead. An interesting online tool without current clinical evidence might be the Genetic Data Commons Data Portal of the National Institute of Health. In this robust data-driven online repository, cancer researchers and bioinformaticians can browse through over 1,600 kidney cancer cases with various information on genes, alterations and gene mutations (https://portal.gdc.cancer.gov/). Another source, which by now is mainly research-driven, is the clear cell renal cell carcinoma metabolomics data explorer as provided by the MSKCC (http://sanderlab.org/kidneyMetabProject/). Metabolograms are a visual tool for exploring metabolic pathways using both gene expression and metabolite abundance data. In an app, users can review metabolite data between tumor and normal samples, or see how the metabolic data line up against the gene expression data obtained from the Cancer Genome Atlas (https://cancergenome.nih.gov/). Ultimately, these tools can help identify new treatment targets.
Discussion
Prognostic tools pose a valuable option to facilitate shared decision-making throughout the cancer continuum, as they incorporate individual patient, disease, and possibly treatment characteristics to provide tailored estimates of prognosis. While such tools are readily available in breast and colon cancer treatment, their availability for bladder and kidney cancer has not been evaluated. Therefore, this semi-systematic review aimed to identify and describe predictive tools for patients undergoing treatment for the latter two malignancies, available in a web-based format.
Our search identified a total of twenty-three tools, which assessed a total of six (bladder cancer) and five (kidney cancer) different outcomes. These tools were developed in a number of populations, ranging from single-/multi-institutional to large population-based datasets such as SEER. Interestingly, despite the relative variability in the number of tools, the variability in terms of providers was limited to eight. While we find this indicative of a relative lack of availability of online tools, we also observed that among these providers, “user-friendliness” differed, which could particularly affect patients with limited health literacy/numeracy. For example, while all websites provided legal disclaimers for the intended use, only three providers offered an introductory/explanatory page to provide a description of the tools intent, data elements used, and outcomes provided. In this regard, only one of the tools was made available in an app format that would support its use on a handheld device, despite evidence of a growing desire for such applications among patients and providers (43).
Aside from a lack of user-availability and -friendliness we observed that none of the tools incorporated genomic or molecular markers, which we feel will be a critical future step as personalized medicine is evolving. Further, only one of the tools incorporated modifiable risk factors, such as smoking behavior or weight loss into prognosis (13). However, given the “teachable moment” associated with cancer diagnosis, incorporation of modifiable risk factors could function as a motivator of behavioral change (8). Lastly, none of the tools identified in this review used quality of life or adverse treatment effects as a predicted outcome. Yet, the prevalence of cancer survivors has been rising throughout recent decades and as such, treatment associated quality of life is emerging as valuable measurement of treatment success (44). For example, studies among patients receiving treatment for head and neck cancer show that almost a quarter of patients rank cure as secondary to functional outcome and health-related quality of life (45).
The current review has to be considered within its limitations, such as its non-systematic approach. In addition, we restricted our search to tools available in web-based format. Taken together, patients and care providers in bladder and kidney cancer care can rely on over 20 different online tools to provide estimates of prognosis and to aid clinical decision making. However, limited variability in providers and user-friendliness, lack of app-based formats, and incorporation of outcomes other than survival demonstrate that online tools for patient counseling in bladder and kidney cancer care are only beginning to align with a growing need in clinical reality. Further and future avenues include incorporation of health-related quality of life as well as genomic and biomarkers into prediction tools.
Acknowledgements
P Gild is supported by the University Medical Center Hamburg-Eppendorf (UKE) (Hamburg, Germany).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Brody DS. The patient's role in clinical decision-making. Ann Intern Med 1980;93:718-22. [Crossref] [PubMed]
- Clark JA, Wray NP, Ashton CM. Living with treatment decisions: regrets and quality of life among men treated for metastatic prostate cancer. J Clin Oncol 2001;19:72-80. [Crossref] [PubMed]
- Epstein RM, Fiscella K, Lesser CS, et al. Why the nation needs a policy push on patient-centered health care. Health Aff (Millwood) 2010;29:1489-95. [Crossref] [PubMed]
- Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med 2012;366:780-1. [Crossref] [PubMed]
- Shariat SF, Karakiewicz PI, Godoy G, et al. Use of nomograms for predictions of outcome in patients with advanced bladder cancer. Ther Adv Urol 2009;1:13-26. [Crossref] [PubMed]
- Kirkovits T, Schinkoethe T, Drewes C, et al. eHealth in Modern Patient-Caregiver Communication: High Rate of Acceptance Among Physicians for Additional Support of Breast Cancer Patients During Long-Term Therapy. JMIR Cancer 2016;2:e14. [Crossref] [PubMed]
- Smits R, Bryant J, Sanson-Fisher R, et al. Tailored and Integrated Web-Based Tools for Improving Psychosocial Outcomes of Cancer Patients: The DoTTI Development Framework. J Med Internet Res 2014;16:e76. [Crossref] [PubMed]
- Rabin BA, Gaglio B, Sanders T, et al. Predicting cancer prognosis using interactive online tools: a systematic review and implications for cancer care providers. Cancer Epidemiol Biomarkers Prev 2013;22:1645-56. [Crossref] [PubMed]
- Shachar SS, Muss HB. Internet tools to enhance breast cancer care. NPJ Breast Cancer 2016;2:16011. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Abdollah F, Gandaglia G, Thuret R, et al. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol 2013;37:219-25. [Crossref] [PubMed]
- Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40:IV-3-18. [Crossref] [PubMed]
- Colditz GA, Atwood KA, Emmons K, et al. Harvard report on cancer prevention volume 4: Harvard Cancer Risk Index. Risk Index Working Group, Harvard Center for Cancer Prevention. Cancer Causes Control 2000;11:477-88. [Crossref] [PubMed]
- Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:466-5; discussion 475-7.
- Cambier S, Sylvester RJ, Collette L, et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance Bacillus Calmette-Guerin. Eur Urol 2016;69:60-9. [Crossref] [PubMed]
- Karakiewicz PI, Shariat SF, Palapattu GS, et al. Precystectomy Nomogram for Prediction of Advanced Bladder Cancer Stage. Eur Urol 2006;50:1254-60; discussion 1261-2. [Crossref] [PubMed]
- Bochner BH, Kattan MW, Vora KC. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol 2006;24:3967-72. [Crossref] [PubMed]
- Karakiewicz PI, Shariat SF, Palapattu GS, et al. Nomogram for predicting disease recurrence after radical cystectomy for transitional cell carcinoma of the bladder. J Urol 2006;176:1354-61; discussion 1361-2. [Crossref] [PubMed]
- Shariat SF, Karakiewicz PI, Palapattu GS, et al. Nomograms provide improved accuracy for predicting survival after radical cystectomy. Clin Cancer Res 2006;12:6663-76. [Crossref] [PubMed]
- Lughezzani G, Sun M, Shariat SF, et al. A population-based competing-risks analysis of the survival of patients treated with radical cystectomy for bladder cancer. Cancer 2011;117:103-9. [Crossref] [PubMed]
- Lane BR, Babineau D, Kattan MW, et al. A preoperative prognostic nomogram for solid enhancing renal tumors 7 cm or less amenable to partial nephrectomy. J Urol 2007;178:429-34. [Crossref] [PubMed]
- Kutikov A, Smaldone MC, Egleston BL, et al. Anatomic features of enhancing renal masses predict malignant and high-grade pathology: a preoperative nomogram using the RENAL Nephrometry score. Eur Urol 2011;60:241-8. [Crossref] [PubMed]
- Raj GV, Thompson RH, Leibovich BC, et al. Preoperative nomogram predicting 12-year probability of metastatic renal cancer. J Urol 2008;179:2146-51; discussion 2151. [Crossref] [PubMed]
- Kattan MW, Reuter V, Motzer RJ, et al. A postoperative prognostic nomogram for renal cell carcinoma. J Urol 2001;166:63-7. [Crossref] [PubMed]
- Sorbellini M, Kattan MW, Snyder ME, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol 2005;173:48-51. [Crossref] [PubMed]
- Karakiewicz PI, Briganti A, Chun FK, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol 2007;25:1316-22. [Crossref] [PubMed]
- Kutikov A, Egleston BL, Wong YN, et al. Evaluating overall survival and competing risks of death in patients with localized renal cell carcinoma using a comprehensive nomogram. J Clin Oncol 2010;28:311-7. [Crossref] [PubMed]
- Kutikov A, Egleston BL, Canter D, et al. Competing risks of death in patients with localized renal cell carcinoma: a comorbidity based model. J Urol 2012;188:2077-83. [Crossref] [PubMed]
- Frank I, Blute ML, Cheville JC, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol 2002;168:2395-400. [Crossref] [PubMed]
- Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530-40. [Crossref] [PubMed]
- Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 2005;23:832-41. [Crossref] [PubMed]
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794-9. [Crossref] [PubMed]
- Eggener SE, Yossepowitch O, Pettus JA, et al. Renal cell carcinoma recurrence after nephrectomy for localized disease: predicting survival from time of recurrence. J Clin Oncol 2006;24:3101-6. [Crossref] [PubMed]
- Motzer RJ, Bukowski RM, Figlin RA, et al. Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer 2008;113:1552-8. [Crossref] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program, Research Data (1973-2014), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission. Available online: http://www.seer.cancer.gov
- Kluth LA, Black PC, Bochner BH, et al. Prognostic and Prediction Tools in Bladder Cancer: A Comprehensive Review of the Literature. Eur Urol 2015;68:238-53. [Crossref] [PubMed]
- Babjuk M, Bohle A, Burger M, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017;71:447-61. [Crossref] [PubMed]
- Xylinas E, Kent M, Kluth L, et al. Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non-muscle-invasive urothelial carcinoma of the bladder. Br J Cancer 2013;109:1460-6. [Crossref] [PubMed]
- Ku JH, Kim M, Byun S-S, et al. External Validation of Models for Prediction of Lymph Node Metastasis in Urothelial Carcinoma of the Bladder. PLoS One 2015;10:e0120552. [Crossref] [PubMed]
- Nuhn P, May M, Sun M, et al. External validation of postoperative nomograms for prediction of all-cause mortality, cancer-specific mortality, and recurrence in patients with urothelial carcinoma of the bladder. Eur Urol 2012;61:58-64. [Crossref] [PubMed]
- Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009;182:844-53. [Crossref] [PubMed]
- Lee BH, Feifer A, Feuerstein MA, et al. Validation of a Postoperative Nomogram Predicting Recurrence in Patients with Conventional Clear Cell Renal Cell Carcinoma. Eur Urol Focus 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Pandey A, Hasan S, Dubey D, et al. Smartphone apps as a source of cancer information: changing trends in health information-seeking behavior. J Cancer Educ 2013;28:138-42. [Crossref] [PubMed]
- Rowland JH, Kent EE, Forsythe LP, et al. Cancer Survivorship Research in Europe and the United States: Where have we been, where are we going, and what can we learn from each other? Cancer 2013;119:2094-108. [Crossref] [PubMed]
- Browman GP, Berrang T, Smith S. Prognostic tools for cancer survival: a secondary role for quality-of-life measurement. J Clin Oncol 2009;27:2902-4. [Crossref] [PubMed]


