Being on active surveillance: the patient perspective
Introduction
Common wisdom dictates that the curative treatment of localised prostate cancer (PCa) is possible by a radical surgical removal of the prostate or a complete eradication of the tumor by radiotherapy. Excellent improvements in both treatments have been achieved. In surgery by the development of robot-assisted surgery and in radiation by a number of technical improvements.
Still management of expectation is accepted in the increasing treatment options of watchful waiting (WW) and active surveillance (AS).
WW is related to the slow growth of PCa and the co-morbidity of old age. The combination of both factors allows WW up to less than 10 years of life expectancy. Depending on the optimism of patient and doctor alike it would be reasonable from the age of 75 to stop looking for a diagnosis of PCa. Please note that symptoms are always treated and WW in reality delays palliative treatment.
There is now consensus that AS is indicated in patients with the lowest risk of cancer progression: >10 years survival, prostate specific antigen (PSA) <10 mg/mL, Gleason score <7 or better ISUP grade 1 (1,2) and cT1 T2, < two positive biopsies, ≤50% cancer per biopsy.
AS definitely removes the burden of side-effects of invasive primary treatments and maintains health related quality of life (HRQoL). However, uncertainty remains on reclassification and the anxiety of living with untreated cancer (3).
We aim for evidence-based logic to increase the certainty of the AS decision by using informative, aids support in choice and shared decision techniques to decide on the primary treatment of PSA-detected PCa. The final decision is always made together with the tumor board [Multidisciplinary Oncology Commission (MOC)] and attended with a trusted companion of the patient. The emphasis is on a correct diagnosis and procedure with ample time between consultations filled with health literacy and communication with the house doctor and the prostate tumor unit representative. One reason to move carefully is the understanding of the patient on the different options and patient-related outcomes (PROMS). The second reason is to absorb possible delays in the work-up as a magnetic resonance imaging (MRI) request or a surgery date. Third one builds up the confidence by putting the celebration of a wedding anniversary over the planning of an MRI in the prediagnostic phase.
Last, we build up extra evidence by referring to the literature especially screening (4,5) and health results in oncology over the last two decades.
Welch made it clear that screening always leads to overdiagnosis and subsequent overtreatment. Especially the overdetection in the European Randomised Study of Screening for Prostate Cancer (ERSPC) is impressive by showing that over half of the cancers detected will not lead to symptomatic diseases in the lifetime of the patient.
The reverse proof is provided by a number of historical autopsy studies of the prostate on patients that died of other causes and observational studies on the mortality of PCa in the previous century.
Natural (untreated) history of PCa
The clinical studies on PCa really started in the 20th century. Before 1900 PCa was a rare disease due to the fact that most of these cancers are detected after 50 years of age while life expectancy hovered around 46 years in these days.
This situation changed rapidly with increasing life expectancy and a number of pathologists reported on the remarkable percentages of PCa found in autopsies on patients who never had prostate problems.
In summary the incidence of small, latent cancer started early in the fifties of their patients’ life expectancy increasing till the last decades of life. No treatment was advocated in patients after the detection of T1a (A1 US classification) post mortem by Gaynor [1938] (6), Nesbit [1951] (7) and Franks [1954] (8).
These latent (discovered mostly in TUR-resected tissue as incidental) were recovered as T1a and T1b in the 1987 issue of “the UICC TNM classification of malignant tumors”. The distinction to grade and local extent (>5% of tissue) to designate T1b was published by Jewett in 1975 (9). This latter form confirming some volume was treated.
These concepts opened the door to delayed treatment. An analysis on the cause of death in 360 A2, B and C1 clinical stages of PCa were followed till death. Where most studies reported a low incidence of death (4% to 7%) in patients initially diagnosed with localised disease, Lerner et al. reported that PCa was a major cause of mortality in these patients. They concluded that aggressive curative therapy regardless of treatment modality should be considered for localised cancer in men with a life expectancy of 10 and more years (10).
At the end of the century there was consensus in the USA that the clinical course of T1a PCa is variable and that patients with low volume prostates (30 g) have an excellent prognosis and can be managed conservatively (11).
Confirmation of these subtle differences were previously reported by Adolfsson focusing on the relative death ranges after radical prostatectomy and deferred therapy (12).
Therefore, deferred therapy may be an alternative to active therapy in patients with localised disease and with a life expectancy of less than 10 years. A word of caution is needed. The term latent is not clearly defined in terms of cancer volume and we prefer to define low grade, low volume (as postulated by Mc Neal JE and by Monique Roobol) and organ confined as a possible clinically innocent cancer ready for AS (13).
A complete overview of the results of conservative management of localised PCa has been summarized in a meta-analysis on the national history of these cancers (14).
The clinical application of the serum PSA test brought a revolution into the diagnosis of PCa by introducing the painless transrectal biopsies to detect PCa in prostatic tissue based on raised serum levels of PSA. Even confused as resulting of benign prostatic hyperplasia (BPH) or PCa a great surge in PCa incidence was the clinical result. Worse a great proportion of these cancers was not visible by then standard radiological/ultrasonic techniques nor palpable. This resulted in the introduction of a new category of T1 category PCa in the 1992 TNM classification (15).
The box of Pandora was opened and we deal clinically with organ confined cancer, graded if enough material is available and measured if it can be made visible by the new MRI techniques. Back to square 1 where the new ISUP grading is easier than the Gleason grading and where constant improvement is searched in the visualisation of these small lesions.
Patients face real time uncertainty in prognosis
Until now the results of AS in different studies are very encouraging but for the patients it still feels like crossing the Rubico. The dice are thrown (Alea iacta est), they fulfil the criteria to enter the treatment to protect their chance for curative treatment. Prospective trials are ongoing (cfr. PRIAS) to evaluate the diagnostic criteria as prognostic factors balancing between optimal or permanent delay of invasive treatment or missing the window of opportunity for cure although we only expect final failure in 10 to 15 years.
One randomized study (ProtecT) has been published with a follow-up of 10 years (16) simulating AS.
In this study the primary outcome of cancer death, eight occurred in the active monitoring group, five in the radical surgical group and four in the radiotherapy group as cancer specific deaths that was low irrespective of treatment. There was more disease progression (3×) in the active monitoring group. However, the active monitoring protocol differs from contemporary AS practice (lax monitoring protocol, inclusion of patients with intermediate and high risk PCa) so that the monitoring results cannot be compared with contemporary results of AS.
The reality remains that the patients with low risk PCa aiming to avoid or delay the usual primary treatments face tests on a regular schedule and may cross over in their choice based on changes in the diagnostic, prognostic factors towards further objective cancer growth or dedifferentiation or personal anxiety.
The entrance AS criteria serve prognostic factors in PCa (17) (Table 1).

Full table
Based on our actual prognostic factors it is still difficult to predict clinical outcomes. It remains a limited but real risk of evaluating possible long-term survival vs. QoL expressed in impotence, incontinence and bowel problems. A change of the biopsies towards higher grade is a major cause for intervention while negative biopsy controls are obviously a relief (real or not).
The tumor volume is obviously a recognized prognostic parameter and it would be useful to recognize a preclinical assessment of the total amount of tumor.
Recent ultrasound and MRI techniques promise another straightforward solution for the hesitating patient. The simple deduction that more tumor present leads to more differentiation and metastatic disease.
It is obvious that relative or absolute rises in PSA values, including density and doubling time, are the most frequent real or pretending excuse to leave the deferred treatment time for surgery or radiation.
Application of the prognostic factors help to predict the outcome of the individual patient, help to understand the course of the disease (metastatic or local extension), aims to select the treatment modality (brachytherapy) and does explain variations in treatment outcome.
We are experiencing more evidence-based decisions in AS but are still of target. Precision medicine counting on optimal, evidence-based, medical treatment is prevailing but personalised, holistic care based on psycho-social and emotional motivations plays still a major role. The importance given to the new research treatment of focal treatment leaves the AS patient with the impression that the urological sciences are impressed but not totally convinced of the AS value of treating low grade, low volume cancer: do nothing.
Objective evaluation of tumor progression can be stimulated by accepting the validated ISUP 2014 pathological classification to replace the Gleason system. It comes back to the WHO simple grading system of five grades, offers more accurate stratification and a lowest grade of one as apposed to six is attractive to overcome overtreatment (18).
Europa Uomo is involved, with a great number of patient advocacy groups and scientific centres, in a project on multidimensional stratification of PCa patients eligible for AS. The objective is to improve patient stratification, better exploitation of parameters, the prospective impact of recently developed genomic classifier, a quality of life evaluation and a freely available internet platform to facilitate decision making.
Progether is the PCa patient platform monitoring the clinical course of all AS. Our official policy favours candidate AS members to join prospective international [PRIAS (19)] or national (Royal Marsden, UK) studies with a strong organisational back-up.
Still in many local and regional hospitals patients join a version of AS. Our educational and training programs ensure that AS receives the same interest as surgery/radiotherapy. Obviously, the results of ongoing AS studies are published in our journals and newsletters (20). Citing a John’s Hopkins study on AS with 0.5% mortality over 15 years.
Multidisciplinary treatment favours AS in general. However, most of these patients are around seventy and the time has come to recognize that AS has favourable outcomes in younger men and should not be denied on the basis of young age (21).
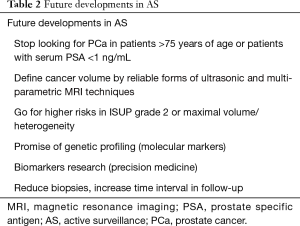
This open policy on AS and the interaction with participating patient offers a rather objective response towards a needed decision to quit the program based on objective facts which is especially useful in the PSA variations in the follow-up of an individual patient. Despite overall success of AS (see previous chapters) it is still difficult to predict any tumor progression and to assess life expectancy in individual patients. Future developments are listed in Table 2.
Full table
Psychological associations in AS
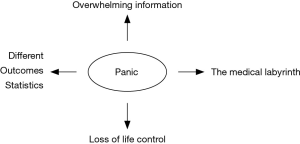
Next to survival the great problem remains quality of life the better in survivorship (remaining life) where the patient aims to regain his previous life, life style, function in civil society. This sounds like mission impossible if the simple diagnosis of the C word reduces most patients to panic and total loss of life control. A simplistic version of this transition is presented in Figure 1.
The challenge to look at PCa as a biological phenomenon that should be treated according to its progressive or indolent clinical course was triggered by the impact of the absolute figures. An indolent course for low grade, low volume PCa was already recognized around 1930 but representing almost half of all screened diagnosis based on PSA in the ERSPC study could not be overlooked in terms of overdetection and resulting overdiagnosis.
PCa is not the only cancer with indolent disease. In 2006 BIG (22) labelled 10% to 20% of breast cancer as indolent and its biggest problem in controlling breast cancer. This challenge simply disappeared by the introduction of a simple tumorectomy and if needed breast reconstruction. Nowadays there is only a message of overtreatment with chemotherapy.
Applying this principle to PCa, focal disease destruction is still research treatment and the AS and WW policy seems to determine future treatments of PCa.
The best quote on the subject is provided by WF Whitmore Jr in 1985: “Appropriate treatment implies that therapy be applied neither to those patients for whom it is unnecessary not to those for whom it will prove ineffective. Furthermore, the therapy should be that which will most assuredly permit the individual a qualitatively and quantitatively normal life. It need not necessarily involve an effort at cancer cure!”
The persisting uncertainty (diminished by clinical observation and discipline) still leaves a chance for anxiety and depression in about 10% of the selected AS patients. Age, marital status and education were found to be predictors of HRQoL in men on AS (23). Basically, living with an untreated cancer is a problem but also individual (personality, mental health, ability to cope with cancer) and clinical (PSA, co-morbidities) were reported in some studies (24). About 13% to 20% of patients presented low scores in the physical health domain. Older patients were more likely to report poor HRQoL. Only a specific subpopulation of patients on AS reported poor QoL. Ad hoc psycho-educational interventions should be designed to meet the unique.
These subtle challenges of uncertainty and anxiety are the two most common psychological challenges in men on AS. Sometimes facing a doctor, or relatives, in favour of action feel a reduction in QoL if connected with constant fear of the disease. The AS decision is loaded with stress, and support groups to relax may be a major gain in the overall treatment.
A last point is that AS patients go for alternative treatment in about 25% of cases. It should be discussed with their doctor and family. As long as no harm is perceived it only means some extra expenses.
Last but not least we advice all patients with PCa, especially those on AS, to adapt to the recommended lifestyle. Regular exercise is most important and our physical exercise unit known as Feel+ became a concrete part of patient support (25). A balanced diet is recommended (26) with a definitive stop to tobacco and alcohol products. However, one myth remains standing that two glasses of red wine (remember resveratrol) are good for your male health and prostate.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71:618-29. [Crossref] [PubMed]
- Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol 2016;40:244-52. [PubMed]
- Denis LJ, Roobol M, Dourcy-Belle-Rose B. Prostate cancer from the horizon of the patient. Acta Oncol 2011;50 Suppl 1:148-54. [Crossref] [PubMed]
- Welch HG. Should I be tested for Cancer? Los Angeles: University of California Press, 2004:224.
- Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst 2009;101:374-83. [Crossref] [PubMed]
- Gaynor EP. Zur Frage des Prostatakrebs. Virchow’s Arch Path Anat 1938;301:602-52. [Crossref]
- Nesbit RM, Baum WC. Management of occult prostatic carcinoma. J Urol 1951;65:890-4. [Crossref] [PubMed]
- Franks LM. Latent carcinoma of the prostate. J Pathol Bacteriol 1954;68:603-16. [Crossref] [PubMed]
- Jewett HJ. The present status of radical prostatectomy for stages A and B prostatic cancer. Urol Clin North Am 1975;2:105-24. [PubMed]
- Lerner SP, Seale-Hawkins C, Carlton CE Jr, et al. The risk of dying of prostate cancer in patients with clinically localized disease. J Urol 1991;146:1040-5. [Crossref] [PubMed]
- Cheng L, Bergstralh EJ, Scherer BG, et al. Predictors of cancer progression in T1a prostate adenocarcinoma. Cancer 1999;85:1300-4. [Crossref] [PubMed]
- Adolfsson J, Carstensen J. Natural course of clinically localized prostate adenocarcinoma in men less than 70 years old. J Urol 1991;146:96-8. [Crossref] [PubMed]
- Denis L, Murphy GP. Prostate Cancer 2000, ESO Monograph. Berlin: Springer Verlag, 2000:94.
- Chodak GW, Thisted RA, Gerber GS, et al. Results of conservative management of clinically localized prostate cancer. N Engl J Med 1994;330:242-8. [Crossref] [PubMed]
- Schröder FH, Hermanek P, Denis L, et al. The TNM classification of prostate cancer. Prostate Suppl 1992;4:129-38. [Crossref] [PubMed]
- Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 2016;375:1415-24. [Crossref] [PubMed]
- Denis LJ, Warde PR. Prognostic Factors in Cancer. Prostate Cancer. 3rd ed. London: Wiley-Liss, 2006:247-51.
- Epstein JI, Zelefsky MJ, Sjoberg DD, et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur Urol 2016;69:428-35. [Crossref] [PubMed]
- Prostate Cancer Research International Active Surveillance (PRIAS). Available online: www.prias-project.org
- Hoekx L. Low risk PCa. PROSTAATinfo 2017;16:5.
- Leapman MS, Cowan JE, Nguyen HG, et al. Active Surveillance in Younger Men With Prostate Cancer. J Clin Oncol 2017;35:1898-904. [Crossref] [PubMed]
- Straetele C, Decker N, Loi S, et al. Tailoring Treatment for Breast Cancer. Dublin: Transbig poster, 2006.
- Bellardita L, Villa S, Valdagni R. Living with untreated prostate cancer: predictors of quality of life. Curr Opin Urol 2014;24:311-7. [Crossref] [PubMed]
- Bellardita L, Rancati T, Alvisi MF, et al. Predictors of health-related quality of life and adjustment to prostate cancer during active surveillance. Eur Urol 2013;64:30-6. [Crossref] [PubMed]
- Feel+ program. Antwerp, Belgium: Oncologic Centre Antwerp, 2017. Available online: http://www.wijook.be
- UCSF Helen Diller Family Comprehensive Cancer Center. Health and Wellness: Living with Prostate Cancer. PART 2: Diet recommendations. Available online: https://www.pcf.org/wp-content/uploads/2017/12/UCSF-PCF_Diet_GuideIIweb.pdf