Can active surveillance really reduce the harms of overdiagnosing prostate cancer? A reflection of real life clinical practice in the PRIAS study
Introduction
Since the use of prostate specific antigen (PSA) testing, the majority of newly diagnosed prostate cancer (PCa) patients are considered to have low risk of progression (1). Although PSA screening leads to a PCa mortality reduction, overdiagnosis and overtreatment of these low-risk PCa patients are still a substantial concern (1-4). To prevent overtreatment, active surveillance (AS) is increasingly recognized as a favorable alternative to direct radical therapy for men with low-risk PCa (5-8). Considering all relevant data, the US Preventive Services Task Force (USPSTF) has recently proposed to endorse physicians to counsel men on PSA screening (9). This change in the USPSTF recommendation is heavily based on a modeling study which suggests that greater use of AS for low-risk PCa may tilt the balance of benefits and harms in PSA screening in favor of screening (10). However, this study models a conservative management program without considering the harms of repeated biopsies nor considering the harms of unnecessary treatments which occur despite an AS strategy. Moreover, patients who initially start an AS strategy may switch to active treatment based on signs of progression or other reasons. Furthermore, there is no consensus around the appropriate conduct of AS and differences may exist between strictly controlled cohorts and real life clinical practice (11). In this study, we analyze long-term follow-up data from the first 500 patients enrolled in the Prostate Cancer Research International Active Surveillance (PRIAS) study, representing real life clinical practice in both community and academic centers from around the world.
Methods
The PRIAS study is a prospective observational study, initiated in December 2006. It facilitates centers around the world in performing AS, by providing a web-based register (www.prias-project.org) with automatic evidence-based and individualized strategy recommendations (12). Data entering is done by each participating physician and monitored by the coordinating study center (Erasmus MC, Rotterdam, The Netherlands).
Study population
We selected the first 500 PRIAS patients, all enrolled before July 2008, to analyze their long-term follow-up data. Patients who already were under surveillance before official initiation of PRIAS were only included if the PRIAS follow-up protocol had been applied. These 500 patients have been part of previous reports (13-15).
PRIAS protocol
The recommended criteria for inclusion were Gleason score (GS) ≤3+3, stage ≤ cT2c, PSA ≤10 ng/mL, ≤2 cores positive for PCa, PSA-density ≤0.2 ng/mL/cm3, and fitness for curative treatment (13). The recommended follow-up strategy during the first 2 years after diagnosis was a PSA test every 3 months and a digital rectal examination (DRE) every 6 months. Thereafter, a PSA test every 6 months and a DRE once yearly was recommended. Standard repeat biopsies were scheduled 1, 4, 7, and 10 years after diagnosis and subsequently every 5 years. Yearly biopsies were only recommended if PSA-doubling time (PSA DT) was between 0 and 10 years. A bone scan was recommended if the PSA level was ≥20 ng/mL. The recommended criteria to switch to active treatment were GS >3+3 or more than two positive cores on biopsy, and stage higher than cT2. A PSA DT between 0 and 3 years was used to recommend a switch to active treatment until the end of 2014, but was dropped afterwards (13). Furthermore, the criteria for a switch to active treatment were adapted to incorporate magnetic resonance imaging (MRI) targeted biopsy findings in 2015, as described in a recent publication (13).
Statistical analyses
We performed descriptive statistics to report baseline characteristics, biopsy outcomes, reasons for discontinuation, treatments after discontinuation, outcomes on radical prostatectomy (RP), and biochemical recurrence (BCR), metastases and death rates. Biopsy outcomes were divided in two categories: reclassification based on GS ≥3+4 only and reclassification based on GS ≥3+4 or ≥2 cores positive. RP outcomes were divided in 4 categories: low-risk PCa (GS 3+3, ≤cT2), intermediate-risk PCa Grade Group 2 (GS 3+4, ≤cT2), intermediate-risk PCa Grade Group 3 (GS 4+3, ≤cT2), and high-risk or locally advanced PCa (GS ≥4+4 or ≥T3). BCR was defined as a PSA level ≥0.2 ng/mL after RP or a PSA level 2.0 ng/mL above the nadir after radiation therapy (RT).
Results
The first 500 patients included in PRIAS were followed prospectively by 30 centers across 8 countries (Table S2) until November 20, 2017. At diagnosis, the median age was 65.9 years, the median PSA was 5.3 ng/mL, and most patients had one positive biopsy core (69%) with GS 3+3 (100%) and a clinical stage T1c (80%) (Table 1). Fifteen patients (3%) did not comply to the recommended inclusion criteria and either had a PSA-density just above 0.2 ng/mL/mL, a PSA just above 10 ng/mL or 3 cores positive for GS 3+3.
Full table
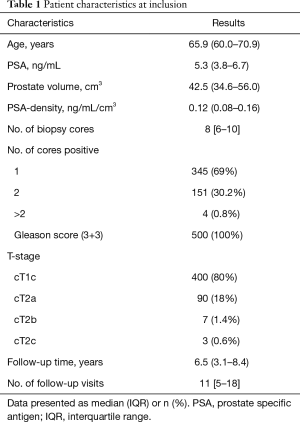
Full table
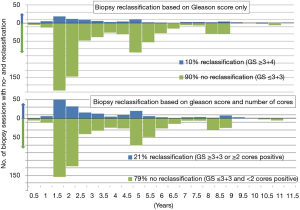
During follow-up, a total of 838 biopsies sessions were performed in 427 patients, of which 48% underwent 2 or more surveillance biopsies (range 1–5). Of the remaining 73 patients who did not have a surveillance biopsy, 50 patients discontinued AS based on protocol advice (rising PSA, n=28), anxiety (n=15) or unknown reason (n=7); and 23 patients were lost to follow-up after a median of 3.4 years. Based on the criteria GS ≥3+4 or >2 cores positive, 79% of the 838 biopsies did not lead to reclassification; based on the criterion GS ≥3+4, 90% of the biopsies would not have led to reclassification (Figure 1).
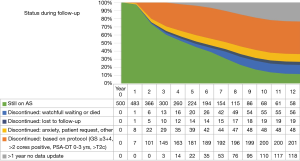
The median follow-up time, the time between first and last visit, was 6.5 years. After 5 and 10 years of follow-up, respectively, 224 (45%) and 68 (13.6%) patients were still on AS; 181 (36%) and 200 (40%) patients discontinued AS based on protocol advice; 39 (8%) and 48 (9.6%) patients discontinued due to anxiety, on own request or other reasons; 14 (3%) and 19 (3.8%) patients discontinued and were lost to follow-up; 20 (4%) and 55 (11%) patients were switched to watchful waiting (WW) or died; and 22 (4%) and 110 (22%) patients had no recent (>1 year) data-update (Figure 2).
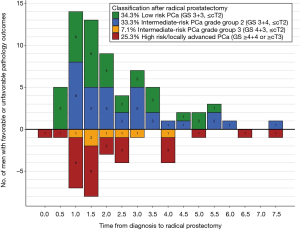
Among the 500 patients, 325 (65%) discontinued AS after a median of 2.3 years. Subsequently, 112 patients underwent RP, 126 underwent radiotherapy, 57 switched to WW or died, and 30 had another or unknown treatment. RP results of 99 patients were available for analysis. Of the 99 patients, 34% of patients had low-risk PCa (GS 3+3, ≤ cT2) and 33% of patients had intermediate-risk PCa Grade Group 2 (GS 3+4, ≤ cT2), 7% of patients had intermediate-risk PCa Grade Group 3 (GS 4+3, ≤ cT2), and 25% of patients had high-risk or locally advanced PCa (GS ≥4+4 or ≥ T3). Of the patients in the latter two groups, 62.5% had their active treatment within the first 2 years (Figure 3).
Of the total 500 patients, 16 (3.2%) patients had BCR after a median of 2.8 years after RT (n=4) and RP (n=12). Four (0.8%) patients developed metastasis after a median of 1.6 years after discontinuing AS (2 patients had switched to RP, 1 to RT, and 1 to WW). Of the 30 (6%) patients who died, after a median of 4.8 years after diagnosis, 1 patient died due to PCa.
Discussion
AS is increasingly being used and is considered a solution to the widely recognized problem of overtreatment of screening detected low-risk PCa (8,9,16). The mortality outcomes of AS patients seem comparable to patients who choose direct radical treatment, but possibly with a higher risk of metastasis (17-19). Although evidence is not yet conclusive, these findings have led to several guidelines endorsing PSA screening and statements that overdiagnosis does not necessarily lead to overtreatment when AS is used (9,20).
In this study, we show that AS indeed seems a safe alternative to direct radical therapy, with only 3.2% of patients having BCR after a switch to radical treatment, 0.8% of patients developing metastasis after discontinuing AS, and only one PCa death. However, there are some substantial drawbacks with AS which limit its capability to mitigate all harms associated with overdiagnosing low-risk PCa. In this current analysis, we found that at least 79% of surveillance biopsy sessions performed during follow-up can retrospectively be considered redundant, as they did not show reclassification. During this time period, the criteria for reclassification on biopsy were GS ≥3+4 or ≥2 cores positive. However, in 2016 the PRIAS study omitted the ≥2 positive cores criteria for reclassification because it did not significantly predict unfavorable outcomes on RP (13). Therefore, retrospectively, the 90% of biopsy sessions without GS ≥3+4 can be considered redundant. Other AS cohorts show similar high rates of biopsy sessions which did not lead to reclassification based on the criteria of GS ≥3+4 (21,22). Following a prostate biopsy, 0.5% to 6.9% of patients require hospital admission due to severe urinary tract infection or sepsis, and up to 25% experience other complaints (23), demonstrating that prostate biopsies should be avoided as much as possible.
Furthermore, of the 99 patients who underwent RP, we found that at least one third of patients had favorable pathology (GS3+3, ≤ pT2) which even if left untreated, would not progress (24). Another third of patients appeared to have intermediate risk PCa Grade Group 2 (GS 3+4, ≤ pT2), for at least some of whom it remains unclear whether they will have benefit from their switch to RP (25). The remaining one third of patients had unfavorable pathology (GS ≥4+3 or ≥ pT3), for whom a switch to active treatment was appropriate. However, as more than half of these patients had their RP within 2 years after diagnosis, we can assume that these patients were misclassified at diagnosis. As shown in previous analyses of PRIAS patients and other AS cohorts, risk stratification during AS lacks specificity to detect progression or misclassification within the window of curability, with under- and overtreatment as a result (13,26).
Finally, after 5 and 10 years of follow-up, respectively, 51% and 64% of the 500 patients had discontinued AS. Ten years after diagnosis, only 14% of the patients who originally started AS were confirmed to be still on AS. Of the remaining 22% of patients, no recent update (>1 year) was available, and these patients should be considered lost to follow-up with the possibility of actually still being on AS or in the meantime having discontinued AS. In other AS cohorts, higher rates of patients still being on AS are reported, with 50% to 63.5% after 10 years (17,18,27), possibly explained by differences in inclusion and follow-up criteria, and the less strictly controlled PRIAS protocol. Moreover, not all patients who switch to active treatment do so because of protocol based signs of progression. In this study, 48 (18%) of patients switched to active treatment because of anxiety or other reasons, comparable to the 23% of patients in the Johns Hopkins cohort (18).
This study has some limitations inherent to the initial facilitating setup of this observational study. Some men, for example, were lost to follow-up or did not have a recent data-update. As this is not a strictly controlled cohort, events might have occurred out of our scope. Furthermore, during the follow-up period of these patients, MRI was largely unavailable. Although MRI is more frequently used in contemporary AS strategies, including PRIAS, more data and longer follow-up are needed to evaluate the additional value of MRI in AS to detect misclassification and especially to detect progression (28-30). At least for now, MRI is not accurate enough to replace systematic re-biopsies (31). Future ways to improve the conduct of AS most likely involve incorporation of more sophisticated individualized risk stratification methods, based on MRI and other biomarkers (32,33).
Based on our findings here, however, AS seems not to solve the problem of overtreatment sufficiently. Therefore, improvement of the diagnostic pathway is an absolute must. With PSA-based screening, benefits (mortality reduction) and harms (unnecessary testing and overdiagnosis) go hand in hand (34). These harms could, however, be reduced by smarter screening using risk prediction tools to aid in the decision to have a PSA-test or to undergo a prostate biopsy (35). In addition, other biomarkers and MRI could be incorporated into the diagnostic pathway to secure the detection of potentially lethal PCa (36,37).
Conclusions
Although AS seems a favorable alternative to direct radical therapy for men with low-risk PCa, the ability of AS to prevent the harms associated with overdiagnosis has not yet been clearly defined in practice. During AS, despite its aim, many in retrospect-unnecessary biopsies are performed and risk stratification methods lack the specificity to prevent all of the overtreatment. Therefore, it remains important to avoid overdiagnosing PCa as much as possible.
Acknowledgements
The work was funded by Prostate Cancer Research Foundation (SWOP), Rotterdam, the Netherlands.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The medical ethical committee of the Erasmus University Medical Centre and, dependent of local regulations, local committees, approved the PRIAS study (MEC number 2004-339). All participants provided written informed consent.
Full table
References
- Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol 2014;65:1046-55. [Crossref] [PubMed]
- de Koning HJ, Gulati R, Moss SM, et al. The efficacy of prostate-specific antigen screening: Impact of key components in the ERSPC and PLCO trials. Cancer 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-8. [Crossref] [PubMed]
- Andriole GL, Crawford ED, Grubb RL 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009;360:1310-9. [Crossref] [PubMed]
- Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990-2013. JAMA 2015;314:80-2. [Crossref] [PubMed]
- Loeb S, Folkvaljon Y, Curnyn C, et al. Uptake of Active Surveillance for Very-Low-Risk Prostate Cancer in Sweden. JAMA Oncol 2017;3:1393-8. [Crossref] [PubMed]
- Womble PR, Montie JE, Ye Z, et al. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol 2015;67:44-50. [Crossref] [PubMed]
- Tosoian JJ, Carter HB, Lepor A, et al. Active surveillance for prostate cancer: current evidence and contemporary state of practice. Nature reviews Urology 2016;13:205-15. [Crossref] [PubMed]
- Bibbins-Domingo K, Grossman DC, Curry SJ. The US Preventive Services Task Force 2017 Draft Recommendation Statement on Screening for Prostate Cancer: An Invitation to Review and Comment. JAMA 2017;317:1949-50. [Crossref] [PubMed]
- Roth JA, Gulati R, Gore JL, et al. Economic Analysis of Prostate-Specific Antigen Screening and Selective Treatment Strategies. JAMA Oncol 2016;2:890-8. [Crossref] [PubMed]
- Bruinsma SM, Bangma CH, Carroll PR, et al. Active surveillance for prostate cancer: a narrative review of clinical guidelines. Nature reviews Urology 2016;13:151-67. [Crossref] [PubMed]
- van den Bergh RC, Roemeling S, Roobol MJ, et al. Prospective validation of active surveillance in prostate cancer: the PRIAS study. Eur Urol 2007;52:1560-3. [Crossref] [PubMed]
- Bokhorst LP, Valdagni R, Rannikko A, et al. A Decade of Active Surveillance in the PRIAS Study: An Update and Evaluation of the Criteria Used to Recommend a Switch to Active Treatment. Eur Urol 2016;70:954-60. [Crossref] [PubMed]
- Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol 2013;63:597-603. [Crossref] [PubMed]
- van den Bergh RC, Vasarainen H, van der Poel HG, et al. Short-term outcomes of the prospective multicentre 'Prostate Cancer Research International: Active Surveillance' study. BJU Int 2010;105:956-62. [Crossref] [PubMed]
- Klotz L. Active surveillance for low-risk prostate cancer. Curr Opin Urol 2017;27:225-30. [Crossref] [PubMed]
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015;33:272-7. [Crossref] [PubMed]
- Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol 2015;33:3379-85. [Crossref] [PubMed]
- Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 2016;375:1415-24. [Crossref] [PubMed]
- Murphy DG, Ahlering T, Catalona WJ, et al. The Melbourne Consensus Statement on the early detection of prostate cancer. BJU Int 2014;113:186-8. [Crossref] [PubMed]
- Jain S, Loblaw A, Vesprini D, et al. Gleason Upgrading with Time in a Large Prostate Cancer Active Surveillance Cohort. J Urol 2015;194:79-84. [Crossref] [PubMed]
- Porten SP, Whitson JM, Cowan JE, et al. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J Clin Oncol 2011;29:2795-800. [Crossref] [PubMed]
- Loeb S, Vellekoop A, Ahmed HU, et al. Systematic review of complications of prostate biopsy. Eur Urol 2013;64:876-92. [Crossref] [PubMed]
- Ross HM, Kryvenko ON, Cowan JE, et al. Do adenocarcinomas of the prostate with Gleason score (GS)</=6 have the potential to metastasize to lymph nodes? Am J Surg Pathol 2012;36:1346-52. [Crossref] [PubMed]
- Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol 2016;40:244-52. [PubMed]
- Loeb S, Bruinsma SM, Nicholson J, et al. Active surveillance for prostate cancer: a systematic review of clinicopathologic variables and biomarkers for risk stratification. Eur Urol 2015;67:619-26. [Crossref] [PubMed]
- Welty CJ, Cowan JE, Nguyen H, et al. Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol 2015;193:807-11. [Crossref] [PubMed]
- Schoots IG, Petrides N, Giganti F, et al. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol 2015;67:627-36. [Crossref] [PubMed]
- Meng X, Rosenkrantz AB, Taneja SS. Role of prostate magnetic resonance imaging in active surveillance. Transl Androl Urol 2017;6:444-52. [Crossref] [PubMed]
- Barrett T, Haider MA. The Emerging Role of MRI in Prostate Cancer Active Surveillance and Ongoing Challenges. AJR Am J Roentgenol 2017;208:131-9. [Crossref] [PubMed]
- Alam R, Carter HB, Epstein JI, Tosoian JJ. Active surveillance of prostate cancer: Current state of practice and utility of multiparametric magnetic resonance imaging. Rev Urol 2017;19:77-88. [PubMed]
- Nieboer D, Steyerberg E, Bruinsma S, et al. 796 - Risk-based selection for active surveillance: Results of the Movember Foundation’s Global Action Plan prostate cancer active surveillance (GAP3) initiative. European Urology Supplements 2017;16:e1383-e1385. [Crossref]
- Venderbos LD, Roobol MJ, Bangma CH, et al. Rule-based versus probabilistic selection for active surveillance using three definitions of insignificant prostate cancer. World J Urol 2016;34:253-60. [Crossref] [PubMed]
- Auvinen A, Moss SM, Tammela TL, et al. Absolute Effect of Prostate Cancer Screening: Balance of Benefits and Harms by Center within the European Randomized Study of Prostate Cancer Screening. Clin Cancer Res 2016;22:243-9. [Crossref] [PubMed]
- Roobol MJ, Verbeek JFM, van der Kwast T, et al. Improving the Rotterdam European Randomized Study of Screening for Prostate Cancer Risk Calculator for Initial Prostate Biopsy by Incorporating the 2014 International Society of Urological Pathology Gleason Grading and Cribriform growth. Eur Urol 2017;72:45-51. [Crossref] [PubMed]
- Grönberg H, Adolfsson J, Aly M, et al. Prostate cancer screening in men aged 50-69 years (STHLM3): a prospective population-based diagnostic study. Lancet Oncol 2015;16:1667-76. [Crossref] [PubMed]
- Auvinen A, Rannikko A, Taari K, et al. A randomized trial of early detection of clinically significant prostate cancer (ProScreen): study design and rationale. Eur J Epidemiol 2017;32:521-7. [Crossref] [PubMed]