eHealth and mHealth in prostate cancer detection and active surveillance
eHealth and mobile health (mHealth)
Information and communication technologies (ICT) offer patients and healthcare providers new ways to improve wellness, practice prevention and reduce suffering from diseases. eHealth is defined by the World Health Organization as “the use of ICT for health” (1). ICT represents a new opportunity to enhance care, which is also true for the field of Urology. The term eHealth was first used in 1999 and has become a neologism, i.e., an umbrella term that includes many items ranging from the infrastructure to access the images from a computer tomography scan via the picture archiving and communication system (PACS), to the implementation of telemedicine, and even the use of augmented reality or machine learning algorithms (2).
In 2012 the European Commission published an eHealth Action Plan 2012–2020 as a roadmap to empower patients and healthcare workers, to link up devices and technologies, and to invest in research towards the personalized medicine of the future (3). The European Commission feels eHealth holds great potential as “it can benefit citizens, patients, health and care professionals, as well as health organizations and public authorities”. When eHealth is applied effectively, it is thought to deliver more personalized ‘citizen-centric’ healthcare, i.e., healthcare that is more targeted, effective and efficient and helps reduce errors, as well as the length of hospitalization. It furthermore facilitates socio-economic inclusion and equality, quality of life and patient empowerment through greater transparency, access to services and information, and the use of social media for health (3).
mHealth is a subset of eHealth which can be characterized as “mobile wireless technologies for public health” (1). Because of its ease of use and broad acceptance, mHealth is considered a valuable tool in the implementation of patient-centered care (patient-reported preferences, experiences and outcomes), which has become a goal of contemporary healthcare systems and international standards (4). There is evidence of successful implementations of mHealth in different contexts, ranging from mobile phone-based clinical guidance for rural health providers in India, to apps that help pregnant women with gestational diabetes in Oxford (5,6). Moreover, its demographic reach transcends generations with various successful examples, including the promotion of physical activity and its acceptance by both young and older adults (7,8).
One of the most popular aspects of mHealth are smartphone applications (“apps”). Currently, there are almost 300,000 mHealth apps available in the Apple App Store and Google Play Store (9). These two virtual stores cover more than 90% of the smartphone ecosystem (9). mHealth interventions can furthermore be implemented using basic phones (e.g., sending health advice via SMS), tablets (e.g., replacing bedside paper-based medical charts) and wearables (e.g., fitness monitoring with an Apple Watch). The total mHealth market revenue alone is expected to reach US $26 billion at the end of 2017 (9).
Advantages and concerns related to the use of eHealth and mHealth
eHealth and mHealth can be useful for treating patients, but also for conducting research, educating professionals, monitoring public health, and tracking chronic diseases. They are thought to be cost-effective alternatives to more traditional face-to-face ways of providing medical care and therefore hold a great potential in the ever growing world of healthcare expenditure. mHealth has the ability to provide access to healthcare as well as timely sharing of data. Real-time monitoring devices can gather live data from sensors and send inputs into a mobile medical app on a smartphone, a server or network to support clinical decision making. It does so regardless of geographical barriers, environmental circumstances and traditional infrastructures; currently there are places where people are more likely to have access to a mobile phone than to clean water or electricity (10). However, to avoid harm, it is critical that, among other concerns, scientific accuracy, patient privacy and user safety of mHealth applications are assured.
Literature has shown a lack of involvement of healthcare professionals in app development in all medical specialties, including Urology. This is concerning as it has also been proven that their participation and contribution in the elaboration of apps increases content accuracy, app downloads and buy-in (11-13). Because most mHealth apps are not considered medical devices by their developers, they bypass strict regulation such as the European Union MEDDEV 2.1/6 (July 2016) “Guidelines on the qualification and classification of stand alone software used in healthcare within the regulatory framework of medical devices”, which states: “it is necessary to clarify that software in its own right, when specifically intended by the manufacturer to be used for one or more of the medical purposes set out in the definition of a medical device, is a medical device”. Few mHealth apps have been scientifically reviewed and/or approved by the European Medicines Agency or the USA Food and Drug Administration (14). This can have serious clinical consequences. As an example, in Dermatology, where smartphones are commonly used as clinical diagnostic tools—and therefore would be a medical device according to MEDDEV for which certification is necessary—an app that claimed to quantify skin cancer risk mislabeled 80% of textbook melanomas (15,16).
Because of the intrinsic nature of eHealth and, in particular mHealth, sensitive health data can be exchanged via wireless networks, which raises new safety concerns. Cyber security attacks are a contemporary concern: in a recent European Union Agency for Network and Information Security (ENISA) study, two thirds of the European Member States considered healthcare a critical sector (17). Therefore, measures should be taken to protect the data integrity, assure data protection and guarantee patient confidentiality. This can be assured in various ways, depending on the specific scenario, but may include cryptography (i.e., saving the information in a coded form), role-based access control (i.e., each user can only read and/or edit certain data, according to his/her professional role) or watermarking (i.e., embedding hidden medical data in medical images). In the European Union the Data Protection Regulation Act (EU2016/679) reforms the data protection rules on processing personal data of natural persons and on the free movement of such data. Together with the MEDDEV 2.1/6 (July 2016) guideline, they form some sort of a European Union legal framework, providing some legal clarity on the status of health and wellbeing mobile applications. Because of the legal aspects related to patient security and data privacy governments are, based on the European Union legal framework, expected to introduce clear cyber security guidelines for the protection of eHealth infrastructures and services. Therefore, eHealth providers should assure that they respect these guidelines. Finally, the importance of cyber security training and specific recommendations should also be promoted among healthcare organizations and users.
Implementation of eHealth and mHealth—clinicians and patients perspective
The integration of eHealth and mHealth into clinical practice has to be tailored to a specific goal and try to meet the patients’ and the healthcare professionals’ expectations. Research in the Netherlands has shown that the uptake of eHealth and mHealth applications is only to increase when applications are built with a vision and fulfil a certain necessity (18). From the patients’ perspective, in the specific case of prostate cancer, depending on his level of comfort with technology and willingness for eHealth/mHealth interactions, this can range from a simple appointment reminder sent via SMS to virtual evaluation as an alternative to in-person interaction. For the healthcare professional, eHealth and mHealth may be another opportunity to provide care, as a complement to the standard clinical appointment or perhaps even as a replacement to some outpatient visits.
As with social media, caution is needed in the interaction between the clinician and patient through eHealth or mHealth. To assure a high level of professionalism and setting boundaries, scientific organizations are issuing guidelines and publishing recommendations on how to communicate with patients through eHealth and mHealth (19). For example, it is recommended that all direct patient-professional contact should take place during regular working hours (19).
eHealth and mHealth in prostate cancer and active surveillance
Prostate cancer has the second highest incidence among men worldwide and is a concern in many healthcare systems. Several studies have been designed to improve the current care paradigm, and the European Randomized study of Screening for Prostate Cancer (ERSPC) showed that mortality could be lowered via screening. After 13 years of follow-up, the results confirm a reduction of 21% in prostate cancer mortality attributable to screening with prostate specific antigen (PSA). The absolute risk reduction of death from prostate cancer at 13 years was 0.11 per 1,000 person-years or 1.28 per 1,000 men randomized, which is equivalent to 1 prostate cancer death averted per 781 screened men and 1 per 27 diagnosed men. Moreover, there was a substantial increased absolute effect compared with findings after 9- and 11-year of follow-up (20).
The ERSPC study has also shown that population-based screening would lead to overdiagnosis (i.e., detecting cancers that would not cause symptoms or death), and consequently overtreatment (i.e., overdiagnosed cancers that are treated and their possible side-effects, namely incontinence and erectile dysfunction) (21). While research is focusing on how to improve the screening algorithm and reduce the rate of overdiagnosis, active surveillance was developed as an alternative to immediate radical treatment. Active surveillance aims to delay or completely avoid unnecessary treatment of potentially indolent tumors (e.g., Gleason 3+3) and avoid treatment related side-effects, and consequently preserve the patients’ quality of life.
With active surveillance, patients with apparent low-risk tumors enter a strict follow-up schedule consisting of clinical visits, PSA, multi-parametric magnetic resonance imaging (mpMRI) and re-biopsy, to ensure that if there is disease progression (i.e., clinically significant prostate cancer Gleason ≥3+4) the patient can switch to active treatment while the disease is still in a “curable” stage (i.e., before the cancer has grown or spread beyond control).
Rotterdam prostate cancer risk calculator (RPCRC)
One way of reducing overdiagnosis is to apply risk stratification in the prostate cancer diagnostic phase. Based on data from 3,624 previously unscreened men and 2,896 men with a previous negative prostate biopsy in ERSPC Rotterdam, the RPCRC nomogram was developed. The RPCRC predicts the risk of a biopsy-detectable prostate cancer and also of potentially high-risk prostate cancer (Gleason score ≥7 and clinical stage >T2b). The different RPCRC algorithms, combining information on PSA level, previous negative prostate biopsy, digital rectal examination (DRE), prostate volume measurement, and transrectal ultrasonography (TRUS), provide an increasingly accurate risk estimation [area under the curve (AUC)]. In previously unscreened men, the AUCs ranged from 0.69 to 0.79 for any prostate cancer, and from 0.74 to 0.86 for serious prostate cancer (22). In the previously screened group (men with at least one previous negative prostate biopsy), applying the same models, AUCs ranged from 0.62 to 0.69 for predicting prostate cancer and from 0.72 to 0.81 for predicting serious prostate cancer (22). By applying the RPCRC, 30–35% of prostate biopsies are averted, while missing only a small percentage of cancers and none of the high risk prostate cancers (22). The RPCRC risk predictions aid in decreasing the rate of overdiagnosis and overtreatment, and has been externally validated multiple times, confirming its good predictive capability (23).
The nomogram was designed into a graphical device and published online (www.prostatecancer-riskcalculator.com) in 2007 (24). After its publication, an implementation study in five Dutch hospitals was initiated to assess the value of the RPCRC in daily clinical practice and whether the RPCRC recommendations were followed by urologists and patients. In 83% of cases, both urologists and patients complied with the RPCRC recommendation (25). If a man is diagnosed with prostate cancer, risk calculator five calculates the chance of having an indolent prostate cancer. An indolent tumor is a cancer that may not require immediate treatment. Such a man can start and continue active surveillance as long as no upgrading is seen. When the probability of having indolent disease was >70%, active surveillance was recommended, and active treatment otherwise. 82% of patients with an active surveillance recommendation were compliant with that recommendation while 29% of patients with an active treatment recommendation chose active surveillance instead (26).
Both studies indicate that the RPCRC is a valuable eHealth tool which can inform decision-making and decrease the rate of overdiagnosis and potential subsequent overtreatment. Furthermore, the RPCRC has been externally validated to assess its capabilities in other patient cohorts and healthcare systems. Results confirm the good discriminative ability of the risk calculator and show how such an eHealth tool positively influences cross border healthcare, which is one of the pillars of the European Unions’ eHealth Action Plan 2012–2020.
Validation studies confirm that the use of the RPCRC should be favored in the decision of whether or not to perform prostate biopsies over the conventional diagnostic pathway. This advice has been incorporated into the European Association of Urology prostate cancer guideline, as well as the Dutch General Practitioners guideline. It confirms that the RPCRC is an example of an eHealth tool with a vision, a true added value in daily clinical practice that positively influences cross border healthcare.
To increase the usability and accessibility of the web-based RPCRC, it has been redesigned into an app, using the same algorithms as for the available web-based risk calculators (Figure 1). While the web-based RPCRC was working with a graphical display, the app uses a decision tree structure. The amount of information available induces which algorithm is used. Ninety-two participants, including urologists, medical students, and general practitioners, evaluated the usability of the app through the Post-Study System Usability Questionnaire (PSSUQ, developed by IBM). Scores on system usefulness ranged from 88–98%, information quality from 78–92%, and interface quality from 80–95% (27). These results show that overall the participants were satisfied with the usability of the app. In 2015, the RPCRC app won the British Journal of Urology International award for Best Urology App, which was presented at the American Urological Association Annual Meeting. In clinical practice, numerous urologists worldwide use the RPCRC-app on a daily basis.
The Prostate cancer Research International Active Surveillance (PRIAS) study
Men diagnosed with low-risk prostate cancer (PSA ≤10, Gleason 3+3, T1c-T2a) can choose between active treatment and active surveillance. In 2006 the PRIAS study was initiated to validate the management of prostate cancer with active surveillance. More information on the PRIAS-protocol can be found on www.prias-project.org.
The PRIAS study is an entirely web-based study. Physicians can log in the website to enter patient inclusion and follow-up data. Urological clinical practice can benefit from the use of this tool; the follow-up data entered by the physician generates a graphical display of the PSA measurements and PSA-doubling time. Furthermore, a recommendation on whether the patient should continue on active surveillance or switch to curative treatment is automatically presented. Such information facilitates direct evidence-based decision making for the patient and the physician when considering active surveillance (28).
As said, active surveillance is a monitoring strategy and consists of a range of clinical visits including PSA measurement, mpMRI, and re-biopsy. The low-risk nature of the tumor combined with the long follow-up trajectory makes it more of a chronic condition. The diagnosis of disease and the medical mills they end up in can cause patients to feel they have lost control over the situation and their bodies. This may cause restlessness for patients and their spouses/partners/families and a feeling of uncertainty. Within the PRIAS study the ‘Follow MyPSA’-app is being developed to guide patients. Such an eHealth application provides patients with the opportunity to monitor their disease, plan and manage appointments and questions for their urologists. It is hypothesized that the use of such a tool will encourage active participation and can have a positive effect on the quality of life of the patient. Furthermore, it can improve the quality of care as it can focus on patients’ needs more specifically (29).
Urology apps
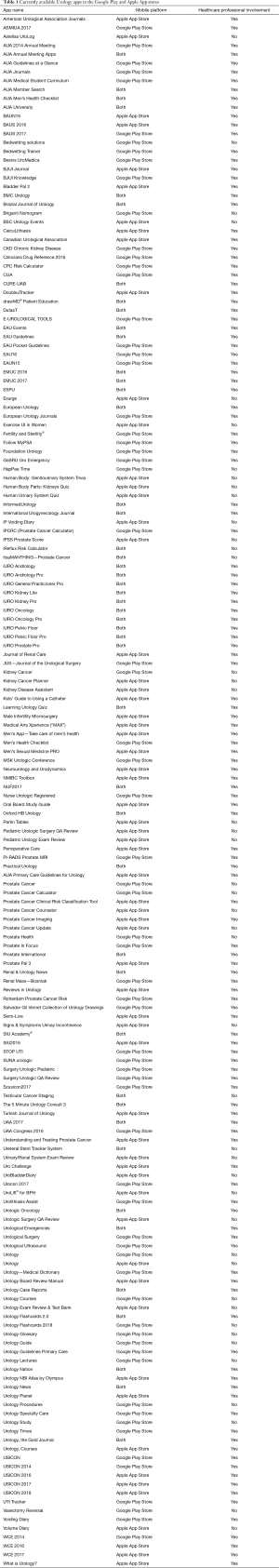
The RPCRC (website and app), the PRIAS website and the ‘Follow MyPSA’-app are three examples of eHealth applications in the field of Urology. In 2015, Pereira-Azevedo et al. reviewed the number of Urology apps available in the Apple App Store and Google Play Store. They identified 150 unique Urology apps, of which 34 were urological cancer apps. It should be noted that these 150 Urology apps represented less than 1% of the total number of smartphone medical apps available. At the time, there seemed to be an untapped potential for Urology apps, especially taking into account that there were more breast cancer apps (n=178; 118 for Android, 59 for iOS) than all available Urology apps in total (30).
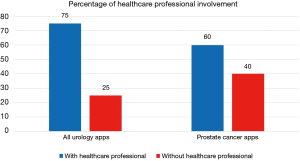
For the current article, an updated review of urology apps available in the Apple App Store and Google Play Store was performed, using the methods previously described (30). One hundred and seventy-six unique urology apps were found (+17%); 67 (38%) for Android, 62 (35%) for iOS, and 47 (27%) available on both stores (Table 1). Only 20 (11%) apps were related to prostate cancer, and the majority (60%) were developed with a healthcare professional (Figure 2): Briganti Nomogram, CPC Risk Calculator, Follow MyPSA, IPCRC (Prostate Cancer Calculator), itsaMANTHING—Prostate Cancer, iURO Prostate Pro, Partin Tables, PI-RADS Prostate MRI, Prostate Cancer, Prostate Cancer Calculator, Prostate Cancer Clinical Risk Classification Tool, Prostate Cancer Counselor, Prostate Cancer Imaging, Prostate Cancer Update, Prostate Health, Prostate In Focus, Prostate International, Prostate Pal 3, RPCRC, and Understanding and Treating Prostate Cancer. Even though there was in increase in the number of apps, there still seems to persist an untapped potential for the participation of the urological community in app development, as 1 in 4 apps were developed without a healthcare professional, which is slightly worse than in 2015 (30).
Full table
Conclusions
eHealth and mHealth are becoming ubiquitous in our day-to-day life. Possible use in Urology ranges from educational, clinical or surgical purposes, and may include such diverse tools as health promoting apps, electronic diaries that aid in treatment monitoring or augmented reality apps. The future will include the use of innovative and ground-breaking ICT solutions and the challenge will be to define a clear vision for them. To increase the uptake of eHealth applications, it is important that healthcare professionals are involved in their design, assuring usability, and also their development, promoting evidence-based views. To reach their full potential healthcare apps must integrate seamlessly into urological practice, while fulfilling the clinical needs of professionals and patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- World Health Organization. eHealth at WHO. Available online: http://www.who.int/ehealth/about/en/
- Oh H, Rizo C, Enkin M, et al. What is eHealth (3): a systematic review of published definitions. J Med Internet Res 2005;7:e1. [Crossref] [PubMed]
- European Commission. eHealth Action Plan 2012-2020: Innovative healthcare for the 21st century. Available online: https://ec.europa.eu/digital-single-market/en/news/ehealth-action-plan-2012-2020-innovative-healthcare-21st-century
- Witteman HO, Dansokho SC, Colquhoun H, et al. User-centered design and the development of patient decision aids: protocol for a systematic review. Syst Rev 2015;4:11. [Crossref] [PubMed]
- Gautham M, Iyengar MS, Johnson CW. Mobile phone-based clinical guidance for rural health providers in India. Health Informatics J 2015;21:253-66. [Crossref] [PubMed]
- Hirst JE, Mackillop L, Loerup L, et al. Acceptability and user satisfaction of a smartphone-based, interactive blood glucose management system in women with gestational diabetes mellitus. J Diabetes Sci Technol 2015;9:111-5. [Crossref] [PubMed]
- Hong Y, Goldberg D, Dahlke DV, et al. Testing Usability and Acceptability of a Web Application to Promote Physical Activity (iCanFit) Among Older Adults. JMIR Hum Factors 2014;1:e2. [Crossref] [PubMed]
- Al Ayubi SU, Parmanto B, Branch R, et al. A Persuasive and Social mHealth Application for Physical Activity: A Usability and Feasibility Study. JMIR Mhealth Uhealth 2014;2:e25. [Crossref] [PubMed]
- Research 2 Guidance. mHealth App Developer Economics 2016: The Current Status and Trends of the mHealth App Market. Available online: https://research2guidance.com/r2g/r2g-mHealth-App-Developer-Economics-2016.pdf
- The World Bank. ‘Maximizing Mobile’ Report Highlights Development Potential of Mobile Communications. Available online: http://web.worldbank.org/WBSITE/EXTERNAL/TOPICS/EXTINFORMATIONANDCOMMUNICATIONANDTECHNOLOGIES/0,,contentMDK:23242711~pagePK:210058~piPK:210062~theSitePK:282823,00.html
- Pereira-Azevedo N, Osório L, Cavadas V, et al. Expert Involvement Predicts mHealth App Downloads: Multivariate Regression Analysis of Urology Apps. JMIR Mhealth Uhealth 2016;4:e86. [Crossref] [PubMed]
- Subhi Y, Todsen T, Ringsted C, et al. Designing web-apps for smartphones can be easy as making slideshow presentations. BMC Res Notes 2014;7:94. [Crossref] [PubMed]
- Barton AJ. The regulation of mobile health applications. BMC Med 2012;10:46. [Crossref] [PubMed]
- Pelletier SG. Explosive Growth in Health Care Apps Raises Oversight Questions. Available online: https://www.aamc.org/newsroom/reporter/october2012/308516/health-care-apps.html
- Brewer AC, Endly DC, Henley J, et al. Mobile applications in dermatology. JAMA Dermatol 2013;149:1300-4. [Crossref] [PubMed]
- Wolf JA, Moreau JF, Akilov O, et al. Diagnostic inaccuracy of smartphone applications for melanoma detection. JAMA Dermatol 2013;149:422-6. [Crossref] [PubMed]
- Mattioli R, Levy-Bencheton C. Methodologies for the identification of Critical Information Infrastructure assets and services. 2014. ENISA.
- Nictiz. EHEALTH-MONITOR 2017. Available online: https://www.nictiz.nl/ehealth/ehealth-monitor/ehealth-monitor-2017
- Hilty DM, Chan S, Torous J, et al. New frontiers in healthcare and technology: Internet- and web-based mental options emerge to complement in-person and telepsychiatric care options. J Health Med Informatics 2015;6:1-14.
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014;384:2027-35. [Crossref] [PubMed]
- Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst 2003;95:868-78. [Crossref] [PubMed]
- Roobol MJ, van Vugt HA, Loeb S, et al. Prediction of prostate cancer risk: the role of prostate volume and digital rectal examination in the ERSPC risk calculators. Eur Urol 2012;61:577-83. [Crossref] [PubMed]
- Louie KS, Seigneurin A, Cathcart P, et al. Do prostate cancer risk models improve the predictive accuracy of PSA screening? A meta-analysis. Ann Oncol 2015;26:848-64. [Crossref] [PubMed]
- Kranse R, Roobol M, Schröder FH. A graphical device to represent the outcomes of a logistic regression analysis. Prostate 2008;68:1674-80. [Crossref] [PubMed]
- van Vugt HA, Roobol MJ, Busstra M, et al. Compliance with biopsy recommendations of a prostate cancer risk calculator. BJU Int 2012;109:1480-8. [Crossref] [PubMed]
- van Vugt HA, Roobol MJ, van der Poel HG, et al. Selecting men diagnosed with prostate cancer for active surveillance using a risk calculator: a prospective impact study. BJU Int 2012;110:180-7. [Crossref] [PubMed]
- Pereira-Azevedo N, Osório L, Fraga A, et al. Rotterdam Prostate Cancer Risk Calculator: Development and Usability Testing of the Mobile Phone App. JMIR Cancer 2017;3:e1. [Crossref] [PubMed]
- van den Bergh RC, Roemeling S, Roobol MJ, et al. Prospective validation of active surveillance in prostate cancer: the PRIAS study. Eur Urol 2007;52:1560-3. [Crossref] [PubMed]
- Venderbos L, Roobol M. m-PRIAS: an e-health technology for men on active surveillance for prostate cancer. Qual Life Res 2017;26:1.
- Pereira-Azevedo N, Carrasquinho E, Cardoso de Oliveira E, et al. mHealth in Urology: A Review of Experts’ Involvement in App Development. PLoS One 2015;10:e0125547. [Crossref] [PubMed]