Active surveillance review: contemporary selection criteria, follow-up, compliance and outcomes
Active surveillance (AS) is of growing interest as an alternative to definitive therapy as more and more men are being diagnosed with low-risk, Gleason 6 or Grade Group 1 prostate cancer (PCa). Current insights into the limited metastatic potential of histologically low-grade tumors (1,2) have enhanced the appeal of deferred treatment, especially in light of demonstrable detriment to health-related quality of life secondary to definitive therapy.
The introduction of prostate-specific antigen (PSA) level as a screening test for PCa for the past ≥20 years has increased the incidence of the disease but reduced PCa related mortality (3). The increased incidence has been secondary to more low-risk diagnoses leading to overtreatment and diminished quality of life (4). Since AS is a compelling antidote to the overtreatment phenomena, there has been a particularly strong push supporting more conservative treatment approaches for men with low-risk PCa following since the 2011 United States Preventive Services Task Force (USPSTF) recommendation. And while the rationale for AS in patients with low-risk PCa is well established (5-8), there is significant variation in existing protocols and guidelines across different institutions. In total 16 international guidelines advocate the use of AS as an initial option for localized PCa management, and each is slightly different.
The primary goal of AS is to prevent overtreatment by selecting patients with low-risk PCa and closely monitoring them such that definitive treatment can be offered when needed with a curative intent (9). The difficulty lies in identifying patients who are truly at low risk of PCa related death. The various eligibility protocols that identify patients for whom AS would be an appropriate treatment differ in the strictness of their inclusion criteria, variables that are considered and monitoring procedures. For example, maximum PSA values for inclusion might range from ≤10 ng/mL (8,10,11) to ≤15 ng/mL (12). Similarly, the number of allowed positive biopsy cores ranges from 1 to 2, >2 or a proportion of total cores, depending on the AS entry definition. In consequence, very wide differences in the proportions of patients that are deemed AS-eligible might ensue and might translate into a wide range of pathological characteristics and/or might yield a wide spectrum of biochemical recurrence (BCR) survival-free rates.
The trade-off between more liberal and strict criteria is misattribution of grade versus overtreatment. Approximately 25–30% of men diagnosed with low-risk disease will be reclassified on repeat biopsy (13,14) as they in fact harbor high-grade PCa. These cases have been described as the “wolf in sheep’s clothing”. In these cases, men who would likely have benefited from upfront definitive treatment would be followed on AS initially but later undergo treatment, potentially missing the opportunity for cure. Co-existent higher-grade cancer is common; however, spontaneous grade progression from Gleason 3 to 4 or 5 is uncommon. In most cases grade progression occurs in high volume Gleason 6 cancers (15,16).
The dilemma of AS is in including too many patients to AS versus excluding men from AS who truly have indolent disease and could be spared unnecessary treatment. Treating patients who are at increased risk of death from other causes or for whom PCa would never cause any symptoms ultimately diminishes their quality of life. The stricter the criteria, the less likely unrecognized high-grade disease becomes, but more men are precluded from AS who may have otherwise never suffered any symptoms or consequences of their PCa.
Nevertheless, AS for carefully selected men with low-risk, localized disease translates into benefits that include, but are not limited to, avoidance of treatment-induced side effects such as erectile dysfunction, urinary incontinence, bowel dysfunction and diminished quality of life. These benefits must be weighed against the risk of cancer progression. The intention of all AS eligibility criteria is to differentiate between men with truly low-risk PCa and those at greater risk of a PCa-related death. Understanding all the contemporary protocols for AS, and their differences, may help in choosing the appropriate treatment approach for men with PCa.
This review will examine contemporary guidelines and institutional protocols for AS.
Guidelines
Of all published guidelines for AS of men with PCa identified here, half were developed in Europe [eight; EAU (17,18), NICE (19-21), GSU (22), DUA (23), KCE (24), FCCG (25), SCAN (26), I+CS (27)], three in Canada [CCO (28), AHS (29), CCNS (30)], two in the US [AUA (31), NCCN (32-35)], one in Asia [NCCS (36)), Australia (PCFA (37)], and New Zealand [PCT (38)]. All were published between 2006 and 2015, and most of them have undergone subsequent updates. A detailed description of the guidelines is summarized by Bruinsma et al. (39). All guidelines were assessed according to The Appraisal of Guidelines for Research and Evaluation (AGREE II) Instrument (40), and according to this assessment twelve of sixteen guidelines were of “good” quality and four (DUA, AHS, SCAN, NCCS) were deemed of “moderate” quality (39). The assessment considered the following factors: scope and purpose; stakeholder involvement; rigor of development; clarity and presentation; applicability; and editorial independence (41). Alas, inadequate reporting or lack of available data may account for lower quality scores when using AGREE II tool.
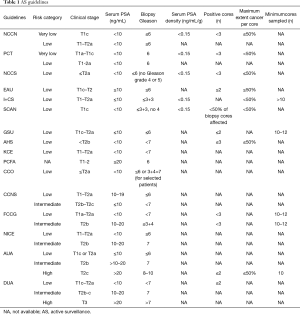
All guidelines include several key variables in their eligibility criteria: clinical stage, PSA parameters and biopsy Gleason grade (see Table 1).
Full table
The DUA and AUA guidelines for AS eligibility are most inclusive as both recommend AS (not watchful waiting) for selected “high” risk patients. The strictest guidelines are the NCCN and PCT. However, some guidelines are stricter in one or two parameters and more inclusive in others and some guidelines include parameters that may not be mentioned in others (i.e., positive cores, maximum core involvement, etc.).
The Gleason score of a patient’s biopsy sample(s) is one of the key inclusion criterion in all published AS guidelines. Most guidelines allow patients to have a Gleason score of ≤6 (the EAU, NCCN, GSU, I+CS, NCCS, PCT, SCAN, PCFA, KCE and AHS). Only four guidelines (provided by NICE, CCNS, CCO, and the FCCG) consider patients with a Gleason score of 7 (mainly 3+4) as eligible for AS and two guidelines support selection of patients with a Gleason score of ≥7 (those provided by the AUA and DUA).
Overall the guidelines can be ordered as follows from most restrictive to the most liberal: NCCN, PCT, NCCS, EAU, I+CS, SCAN, GSU, AHS, KCE, PCFA, CCO, CCNS, FCCG, NICE, AUA, DUA. All published guidelines on use of AS are based on or support one of the eight protocols described below.
Description of institutional protocols and their associated AS cohorts
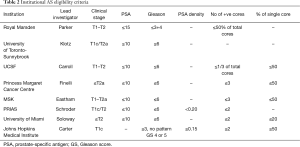
While there are numerous AS selection criteria and protocols, their effectiveness has not been compared in randomized controlled trials. The eight institutional protocols that are reviewed below are: University of California, San Francisco (UCSF), University of Toronto (Sunnybrook cohort) (UoTSB), Toronto (Princess Margaret cohort) (UoTPM), Memorial Sloan Kettering Cancer Centre (MSKCC), Prostate Cancer Research International Active Surveillance (PRIAS), University of Miami (UoM), John Hopkins (JH), and Royal Marsden (RM) (Table 2). These eight institutional cohorts of AS include a total of approximately 10,000 men. The limitation of most of these patient cohorts is the length of follow-up.
Full table
At University of California, San Francisco (UCSF) the study of AS for PCa began in 1990 (10). In 2015, the centre reported that 1,075 patients with at least 6 months of follow-up were included in the cohort of whom 810 consented for research (42). The UCSF inclusion criteria is diagnostic PSA 10 ng/mL or less, clinical stage T1/2, biopsy Gleason grade 3+3 or less, 33% or less positive cores and 50% or less tumor in any single core. A total of 556 men met the strict AS criteria and those who did not meet the criteria but chose AS were analyzed separately.
At a median follow-up of 60 months [interquartile range (IQR), 36–91 months; maximum 19 years] there were no deaths reported due to PCa. Metastatic disease developed in 1 patient (0.12%). Five-year overall survival was 98%, treatment-free survival was 60% and BCR-free survival (BFS) was 40%. Median time to treatment was 25 months (IQR, 15–45 months) and median time to reclassification was 17 months (IQR, 10–33 months). Of the 348 treated men, 240 (69%) underwent radical prostatectomy (RP), 98 (28%) received some form of radiotherapy and 10 (3%) received androgen deprivation therapy (ADT). PSA recurrence-free survival was 97% 1 year after RP.
The institutional guideline recommends monitoring by quarterly PSA testing, semiannual transrectal ultrasound and annual biopsy. The first surveillance (i.e., confirmatory) biopsy is recommended within 12 months of diagnostic biopsy. Subsequent surveillance biopsies are recommended every 12 to 24 months based on clinical risk. Surveillance biopsy sessions involve at least 12 cores with sampling from each sextant (medial and lateral) and the anterior gland. The primary trigger for treatment has been biopsy reclassification. Additional indications for consideration of treatment were patient anxiety, CAPRA risk reclassification and change in clinical stage.
A key difference between the UCSF cohort and others is that men were enrolled before repeat (confirmatory) biopsy at UCSF.
The Canadian experience with AS was first defined by Klotz et al. (43,44) as the University of Toronto (Sunnybrook) cohort (UoTSB). Beginning in 1995, Klotz and colleagues began enrolling patients in a prospective clinical trial of AS to evaluate its effectiveness and safety. The decision to intervene with definitive treatment was determined by PSA kinetics and/or histologic progression. Between 1995 and 1999, the study was offered to all low-risk patients (Gleason score <6 and PSA<10 ng/mL) and to patients older than age 70 years with PSA <15 ng/mL or Gleason score <3+4 [7]. Since January 2000, the study was restricted to low-risk patients (Gleason score <6 and PSA<10 ng/mL) or patients with favorable intermediate-risk disease (PSA 10 to 20 ng/mL and/or Gleason score 3+4) with significant comorbidities and a life expectancy of less than 10 years. The initial cohort was reported in 2002 (43) and a follow-up was reported in 2010 of 450 patients with 8-year follow-up (45). In a more recent update (45,46), 993 patients were included with maximum 16 years of follow-up. The median follow-up time from the first biopsy was reported to be 6.4 years. One hundred forty-nine (15.0%) of 993 patients died, and 844 patients are alive (censored rate, 85.0%). A total of 15 deaths (1.5%) were from PCa. The 10- and 15-year actuarial cause-specific survival rates were 98.1% and 94.3%, respectively. An additional 13 patients (1.3%) developed metastatic disease and are alive with confirmed metastases (n=9) or have died of other causes (n=4). At 5, 10, and 15 years, 75.7%, 63.5%, and 55.0% of patients remained untreated and on surveillance. Patients were 9.2 times more likely to die from non-prostate causes than from PCa.
The follow-up protocol includes PSA every 3 months for 2 years and then every 6 months in stable patients. A confirmatory biopsy is recommended to be performed within 12 months of the initial biopsy and then every 3 to 4 years until the patient reaches 80 years old.
The University of Toronto (Princess Margaret Cancer Centre-PMCC) (UoTPM) is separate from UoTSB. The AS database at UoTPM began in 2008 under Finelli et al. (16,47-52) and now spans 1992–2017. As of 2013 there were 1,122 patients who met the AS eligibility criteria and were included in the cohort. The eligibility criteria are as follows: PSA ≤10, clinical stage ≤ T2a, Gleason sum ≤6, number of positive cores ≤3, no single core >50% involved and age ≤75 years. Based on interval year of diagnosis, the proportion of eligible patients that were managed with AS was 4.5%, 11%, 48.9% and 35.5% (1991–1999, 2000–2004, 2005–2009 and ≥2010, respectively). The median follow-up on AS was 56 months (IQR, 30–92 months). All of the 539 patients had a follow-up of at least 5 years while 121 patients were followed for >10 years. Overall, 27 patients (2.4%) died, with PCa being the cause of death in 2 patients (0.2%). Metastasis occurred in 7 (0.6%) patients; 5 distant metastases and 2 lymph node metastases (Table 2). Median time from diagnosis to death from all causes was 49 months (IQR, 43–80 months), while median time from diagnosis to development of metastatic disease was 50.5 months (IQR, 6–159 months). Five-year overall survival, cancer-specific survival and metastasis-free survival was 96.8%, 100% and 99.7%, respectively. In the overall cohort (n=1,122), 305 patients underwent definitive treatment. Grade reclassification was the main reason for undergoing treatment. Thirty-six patients (6%) underwent definitive treatment without disease reclassification, while 226 patients did not receive treatment despite demonstrating disease reclassification.
The overall disease reclassification rates at 5 and 10 years were 28% and 40%, and cumulative treatment rates at 5 and 10 years were 21% and 26%. Importantly, following confirmatory biopsy the overall disease reclassification rate decreased to 15% and intervention rate was 12% at median follow-up of 67 months (IQR, 40–102 months). Only 2 patients (0.2%) died of PCa and 7 developed metastatic disease. The surveillance protocol after confirmatory biopsy at PMCC does not include annual biopsy and patients undergo repeat biopsy at 1–3-year intervals. The low mortality and metastasis rate is comparable with other AS cohorts using a similar or stricter AS criteria.
For Memorial Sloan Kettering Cancer Centre (MSKCC) (53) the inclusion criteria included an AJCC (1992) clinical stage ≤ T2a, PSA <10 ng/mL, and low-risk features on initial prostate biopsy: Three or less cores involved, no single core with ≥50% maximum involvement of cancer, and no Gleason grade >3 present in the specimen. In their 2008 work they identified all eligible patients undergoing repeat biopsy from March 2002 to June 2007 (n=104). The results of the repeat biopsies were as follows: 27 (26%) were negative, 59 (57%) had Gleason score ≤6, 17 (16%) had Gleason score 7, one patient had Gleason score 9, 10 (10%) of patients had >3 cores involved on repeat biopsy, and 12 (12%) had ≥50% involvement of at least one core. A total of 28/104 (27%) patients were upgraded and/or upstaged. Treated patients who were upgraded and/or upstaged were more likely to have higher pathologic stage (P=0.003) and grade (P=0.001) at RP than those who were not.
In 2011 (11), MSKCC reported on 238 men who met the AS inclusion criteria for their center. Sixty-one patients progressed during follow-up. The 2- and 5-year progression-free probability was 80% and 60%, respectively. With PSA included in progression criteria, PSA at confirmatory biopsy [hazard ratio (HR), 1.29; 95% CI, 1.14–1.46; P<0.0005] and positive confirmatory biopsy (HR, 1.75; 95% CI, 1.01–3.04; P=0.047) were independent predictors of progression. Of the 61 cases who progressed, 34 failed due to increased PSA, including only 5 with subsequent progression by biopsy criteria. When PSA was excluded from progression criteria, only 32 cases progressed, and 2- and 5-year progression-free probability was 91% and 76%, respectively. Using modified criteria as an end point, positive confirmatory biopsy was the only independent predictor of progression (HR, 3.16; 95% CI, 1.41–7.09; P=0.005).
The Prostate Cancer Research International Active Surveillance (PRIAS) (9) is an international AS study that began in 2006. It includes more than 100 centres in 17 countries worldwide. Eligible patients had clinical stage T1/T2 PCa, PSA ≤10 ng/mL, PSA density <0.2 ng/mL per milliliter, one or two positive biopsy cores, and Gleason score ≤6. PSA was measured every 3–6 months, and volume-based repeat biopsies were scheduled after 1, 4, and 7 years. Reclassification was defined as more than two positive cores or Gleason >6 at repeat biopsy. Recommendation for treatment was triggered by PSA doubling time (PSA-DT) <3 years or reclassification. A total of 2,494 patients were included and followed for a median of 1.6 year. One or more repeat biopsies were performed in 1,480 men, of whom 415 men (28%) showed reclassification. Compliance with the first repeat biopsy was estimated to be 81%. During follow-up, 527 patients (21.1%) underwent active therapy. Active therapy-free survival at 2 years was 77.3%. The strongest predictors for reclassification and switching to definitive treatment were the number of positive cores (two cores compared with one core) and PSA density. The disease-specific survival rate was 100%. The follow-up protocol scheduled PSA measurements every 3 months for the first 2 years and every 6 months thereafter. Repeat biopsies were scheduled after 1, 4, and 7 years; in case of a PSA-DT between 3 and 10 years, yearly repeat biopsies were advised. Volume-dependent biopsies were recommended according to protocol (prostate volume <40 cm3: 8 biopsy cores; 40–60 cm3: 10 biopsy cores; and >60 cm3: 12 biopsy cores). Risk reclassification at repeat biopsy triggered a recommendation for active treatment and was defined as three or more positive biopsy cores and/or Gleason score >6. PSA-DT <3 year was used as a recommendation to trigger intervention only after a minimum of four follow-up visits (i.e., after 1 year of follow-up). In total, 1,885 patients (75.6%) continued on AS, 527 patients (21.1%) underwent active therapy, 43 patients (1.7%) were lost to follow-up, 21 patients (0.8%) switched to watchful waiting because of increasing comorbidity, and 18 patients (0.7%) died of causes other than PCa. The median time to active therapy was 1.2 years (IQR, 1.0–1.6 years), while the median time free from intervention for the rest of the cohort was 1.9 years (IQR, 1.0–3.1 years).
The University of Miami (UoM) (54) cohort was first published in 2007. Inclusion criteria were Gleason score of ≤6, a serum PSA level of ≤15 ng/mL, stage ≤T2, low-volume disease and >12 months of follow-up. The follow-up was rigorous, with PSA tests and a digital rectal examination (DRE) every 3 months for 2 years, and a repeat biopsy 6–12 months after the initial diagnosis and yearly when indicated. Ninety-nine patients met the inclusion criteria; their mean age at diagnosis was 66 years, their mean PSA level was 5.77 ng/mL and the mean follow-up was 45.3 months. On initial repeat biopsy, 63% had no cancer and 34% had a Gleason sum of ≤6. Eight patients were treated (three with hormones; five with curative intent); two had RP (one had pT2c pNO Gleason 7 disease); three had radiotherapy. The probability of remaining treatment-free at 5 years was 85%; no patient died from PCa. The PSA-DT and clinical stage at diagnosis were predictive of progression.
For the John Hopkins (JH) (55) cohort 1,298 men (median age, 66 years) with a median follow-up of 5 years (range, 0.01–18.00 years) contributed 6,766 person-years of follow-up since 1995. Overall, cancer-specific, and metastasis-free survival rates were 93%, 99.9%, and 99.4%, respectively, at 10 years and 69%, 99.9%, and 99.4%, respectively, at 15 years. The cumulative incidence of grade reclassification was 26% at 10 years and was 31% at 15 years; cumulative incidence of curative intervention was 50% at 10 years and was 57% at 15 years. The median treatment-free survival was 8.5 years (range, 0.01–18.00 years).
Initiated in 2002, the Royal Marsden (RM) AS criteria and protocol is as follows: men aged 50–80 years, fit for radical treatment, stage T1/T2 disease, PSA <15 ng/mL, Gleason score ≤3+3, and percent positive biopsy cores ≤50%. Patients were assessed clinically and with PSA at 3-monthly intervals in year 1, at 4-monthly intervals in year 2, and at 6-monthly intervals thereafter. Transrectal ultrasound-guided prostate biopsy was performed after 18–24 months and every 2 years thereafter. At 22 months 73% of patients (n=238) were still on AS and 5% had switched to watchful waiting (12). In 2013, the group reported satisfactory medium-term outcomes for AS in selected men with localized PCa. From the 471 men who were enrolled in AS, approximately 70% avoided treatment within 5 years of diagnosis, the 5-year rate of adverse histology and treatment-free probability was 22% (56). The outcomes of deferred treatment were reported to be comparable to that of immediate treatment.
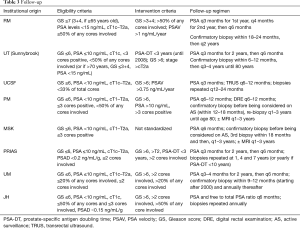
Interestingly the intensity of follow-up protocols described in (Table 3) does not correspond to the degree of strictness of the eligibility criteria across institutions. Even though intuitively a protocol that is more liberal in its entry criteria would be expected to have a more vigorous follow-up protocol to ensure that those men who may be at a higher risk of progression are treated as soon as they progress.
Full table
Application of protocols
Only a limited number of reports have been published that compare real world outcomes of different AS protocols and demonstrate the trade-off of stringent versus more inclusive criteria and risk of future disease progression (48).
In 2016 we applied the various eligibility criteria to a single cohort and found that while there are differences in inclusion criteria for AS, more stringent criteria were not associated with significant improvements in patient outcomes when considering relative risk of Gleason score upgrading, or BCR after treatment. All patients who underwent at least two biopsies were assessed for entry eligibility into the following protocols: UT-PMCC, PRIAS, UCSF, MSK, UM, JH, RM, UT-Sunnybrook Hospital. A cohort of 1,365 men that fulfilled at least 1 of 8 criteria was examined, and 1,085 of these men met the UT-PMCC criteria with a median follow-up of 5 years. There were no significant differences in the rate of BCR or adverse pathology for men who were followed by AS initially and later underwent a RP compared to men who had upfront RP. Interestingly, the rates of reported positive margins were higher for men who qualified for AS under PRIAS and UM criteria compared to JH criteria, but the differences were not statistically significant. The proportion of BCR among the men meeting the different criteria ranged from 6–6.9% (48).
In a similar study (57), the authors compared 11 AS protocols in European men treated with RP at the Martini-Clinic Prostate Cancer Center. There were 3,498 RP patients, from 2005 to 2016, who underwent 10 core biopsies and fulfilled at least 1 of 11 examined AS eligibility definitions. AS eligibility, ineligibility, presence of primary Gleason 4/5, upstage, and combinations thereof at RP, as well as 5-year BFS were assessed. The most and least stringent criteria were the very low-risk National Comprehensive Cancer Network (NCCN) and the Royal Marsden with 18.8% and 96.1% of AS-eligible patients, respectively. Rates of primary Gleason 4/5 at RP, upstaging, or both features, respectively, ranged from 2.3% to 6.7%, 6.1% to 18.2%, and 7.1% to 21.0% for those two AS entry definitions.
The range of individuals deemed AS-ineligible between the NCCN and Royal Marsden AS entry definitions, despite not harboring unfavorable pathology (primary Gleason pattern 4/5, upstage, or both), was 80.3% to 3.7%, 78.3% to 3.4%, and 77.8% to 3.4%, respectively. BFS rates showed narrow variability, with a range of 85.9% to 91.8%. Use of stringent AS entry definitions reduces the number of AS-eligible patients, which is related to a select range in individual entry parameters. Moreover, rates of unfavorable pathology at RP as much as tripled between most and least stringent AS entry definitions. However, less stringent AS entry definitions result in the lowest AS-ineligibility rates. In men without unfavorable pathology BFS rates were virtually invariably high.
Based on these concepts AS should be offered to most men found to have low-risk disease and for whom higher grade tumour is excluded. These men should be monitored carefully and diligently to ensure that high grade disease is detected when present in those who have not transitioned to watchful waiting. Institutions have developed follow-up protocols as described in Table 3 to balance over-testing (repeat biopsy) and the importance of surveillance.
Most protocols recommend that following the initial assessment and confirmatory biopsy, patients should be followed with semi-annual PSA, annual DRE, and repeat biopsy and/or imaging at 3- to 5-year intervals. This interval depends on patients’ underlying risk factors and level of concern. For stable patients with life expectancy less than 5 to 7 years (typically 80 years of age), follow-up should be limited to annual PSA. An additional consideration is patients in whom there may be a lower life expectancy, either because of age or comorbidities. An example might be patients with chronic obstructive pulmonary disease or other serious comorbidity, with a higher-volume Gleason 3+4 or a 4+3 tumor. Although there may be a greater risk of prostate disease progression in such patients, in most cases progression (increasing PSA, for example) is not associated with any disease-related side effects. Consequential progression end points (pain, metastases, and death) remain years away in most such patients.
Trends in Ontario, Canada
Richard et al. (2016) examined the proportion of men with localized PCa being managed by AS in Ontario and assessed the factors associated with its uptake (58). They found that while there has been a steady increase in the uptake of AS between 2002 and 2010, only 18% of men diagnosed with localized PCa were managed by AS during the study period. The decision to adopt AS was influenced by several individual and physician characteristics. The data suggest that there is significant opportunity for more widespread adoption of AS. Management by AS was defined as undergoing a repeat biopsy following the initial diagnostic biopsy before any definitive treatments was instituted. This rate increased to up to 22% when more liberal definitions were used to define AS. Data also demonstrated that over time, AS use has increased by approximately 1% per year to reach the rate of 21% in 2010. When more liberal definitions of AS were used, this rate reached nearly 25% in 2010. This study showed substantial heterogeneity in the adoption of AS across centres and physicians, yet the reasons for this variation are not clear. The variation did not appear to be related to practice size or the type of treating center and the authors postulate that it may be due to lack of clear Ontario- or Canadian-based guidelines on the management of localized PCa as well as the absence of a standardized protocol on how to follow men who opt to be managed by AS.
Trends in USA and Europe
In the United States, Luckenbaugh et al. (2017) (59), as part of the Michigan Urological Surgery Improvement Collaborative (MUSIC), examined the frequency of follow-up PSA testing and prostate biopsy among men treated with AS in the academic and community urology practices. They studied 513 patients who were followed on AS between 2012 and 2013 and had at least 2 years of follow-up. Among the 431 men (84%) who remained on AS for 2 years, 132 (30.6%) underwent follow-up surveillance testing at a frequency that was concordant with NCCN recommendations. At the practice level, the median rate of guideline concordant follow-up was 26.5% (range, 10.0% to 67.5%, P<0.001). Among patients with discordant follow-up, the absence of follow-up biopsy was common and not significantly different across practices (median rate 82.0%, P=0.35). With these results they concluded that among diverse community and academic practices in Michigan, there is wide variation in the proportion of men on AS who meet guideline recommendations for surveillance. These data highlight the need for standardized AS pathways that emphasize the role of repeat surveillance biopsies.
Loeb et al. (60) performed a nationwide, population-based study on the use of AS for localized PCa in Sweden. They found that the use of AS increased in men of all ages from 57% (380 of 665) to 91% (939 of 1,027) for very-low-risk PCa and from 40% (1,159 of 2,895) to 74% (1,951 of 2,644) for low-risk PCa, with the strongest increase occurring from 2011 onward. Among men aged 50 to 59 years, 88% (211 of 240) with very-low-risk and 68% (351 of 518) with low-risk disease chose AS in 2014. Use of AS for intermediate-risk disease remained lower, 19% (561 of 3,030) in 2014. These findings suggest that AS has become the dominant management for low-risk PCa among men in Sweden, with the highest rates yet reported and almost complete uptake for very-low-risk cancer.
Outcomes of AS
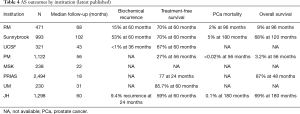
As AS cohorts mature more insight is gained into outcomes of various AS protocols (Table 4). It is generally agreed that death in men on AS occurs most commonly due to causes other than PCa such as cardiovascular disease. In the most mature published cohort (46) with a median follow-up of 9 years, the relative risk for non-PCa death was 10 times that for PCa mortality. Some have argued that AS may lead to anxiety or decreased quality of life from adverse psychological effects; however, published evidence does not show any significant adverse psychological effects in men on AS. Anxiety is common with a diagnosis of PCa, but is not associated with any one given treatment modality. In the Scandinavian trial comparing RP with watchful waiting, there was no difference in psychological functioning, anxiety, or depression between the two groups (61).
Full table
As previously mentioned, the length of follow-up is the greatest limitation of most of prospective AS studies. Median follow-up ranges from less than 1 year to about 8.5 years in the most recent publications. One key study that generated a great deal of concern about conservative management in young patients in Sweden reported that the HR for PCa mortality in patients managed by watchful waiting was low for many years but tripled after 15 years of follow-up (62). It will be 5 to 7 years before the most mature AS cohorts have a median of 15 years of follow-up. Therefore, it is prudent that clinicians be particularly vigilant with this approach as younger men are being offered AS.
Collectively, the published experience with AS from all institutions includes 200 patients followed for more than 15 years. A few of these patients were reported to have had late disease progression, but there is no evidence of a sharp increase in mortality after 15 years. The two extremes among all reviewed cohorts, those practicing most inclusive and restrictive approaches (UoTSB vs. JH, respectively), have reported their respective 15-year outcomes. The comparison is very instructive.
The Toronto group has a more liberal approach, including all low-risk and selected intermediate risk (Gleason 7 or PSA >10) patients (46) and published an actuarial 15-year PCa mortality rate of 5%. Most of the metastatic cases were Gleason 7 at diagnosis. The HR for metastasis at 15 years was 3.75 times greater for intermediate- than low-risk patients. The Gleason 7 patients in particular were at risk; these patients had a 20% or greater metastasis rate at 15 years (46). (Importantly, PSA >10 had very little correlation with likelihood of metastasis). In contrast, the Hopkins group (JH) took a restrictive approach, offering surveillance only to patients who fulfilled the Epstein criteria (Gleason 6 with no more than 2 positive cores, no core >50% involved, and PSA density <0.15). The benefit was a PCa mortality rate of 0.5% at 15 years. The downside was that only 20% of newly diagnosed patients were eligible (vs. 50% in the Toronto cohort). Based on the data summarized in this article, there is an emerging consensus that the appropriate strategy lies between these two extremes. Most Gleason 6 patients are appropriately managed with surveillance [i.e., not just those fulfilling Epstein criteria of Gleason 6 with no more than 2 positive cores, no core >50% involved, and PSA density <0.15 (55)]; however, surveillance should be offered only cautiously to Gleason 7 patients. All of the mature surveillance cohorts reflect the pre-MRI/biomarker experience. Although the results are favorable, it is very likely that the incorporation of these strategies will broaden the indications for surveillance while further reducing the already low rate of metastasis.
Future direction
Looking into the future, MRI will likely play a larger role in AS selection criteria and follow-up, but its utility in this setting is currently under investigation. In 2017 most groups advise that MRI be used selectively and biomarkers be considered investigational. MRI has recently been incorporated into some AS algorithms. It has been shown to be effective in identifying large high-grade cancers with relatively high accuracy. MRI with targeted biopsies of any area of restricted diffusion is likely to significantly enhance the early identification of higher-grade cancer. A PiRads 4 to 5 lesion has been reported to have a 90% positive predictive value for high-grade cancer in an AS cohort (63). It is reasonable to predict that, as data accumulate, both MRI and biomarkers will be increasingly used to enhance patient selection and outcomes.
Conclusions
The array of protocols and guidelines that exist share many similarities and as such it can be argued that using one over another (a more restrictive approach over a more liberal one, or vice versa) results in no significant benefit or detriment for patients. However, there are also some key differences, namely inclusion of men with Gleason 7 disease. When considering any patient for AS it is important to understand the differences between protocols, and review published results to appreciate the impact on follow-up such that a more liberal entry requires more frequent and strict surveillance.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ross HM, Kryvenko ON, Cowan JE, et al. Do adenocarcinomas of the prostate with Gleason score (GS) ≤6 have the potential to metastasize to lymph nodes? Am J Surg Pathol 2012;36:1346-52. [Crossref] [PubMed]
- Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol 2011;185:869-75. [Crossref] [PubMed]
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008;19:175-81. [Crossref] [PubMed]
- Hugosson J, Carlsson S. Overdetection in screening for prostate cancer. Curr Opin Urol 2014;24:256-63. [Crossref] [PubMed]
- Iremashvili V, Pelaez L, Manoharan M, et al. Pathologic prostate cancer characteristics in patients eligible for active surveillance: a head-to-head comparison of contemporary protocols. Eur Urol 2012;62:462-8. [Crossref] [PubMed]
- Mufarrij P, Sankin A, Godoy G, et al. Pathologic outcomes of candidates for active surveillance undergoing radical prostatectomy. Urology 2010;76:689-92. [Crossref] [PubMed]
- Kane CJ, Im R, Amling CL, et al. Outcomes after radical prostatectomy among men who are candidates for active surveillance: results from the SEARCH database. Urology 2010;76:695-700. [Crossref] [PubMed]
- van den Bergh RC, Roemeling S, Roobol MJ, et al. Outcomes of men with screen-detected prostate cancer eligible for active surveillance who were managed expectantly. Eur Urol 2009;55:1-8. [Crossref] [PubMed]
- Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol 2013;63:597-603. [Crossref] [PubMed]
- Dall'Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer 2008;112:2664-70. [Crossref] [PubMed]
- Adamy A, Yee DS, Matsushita K, et al. Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J Urol 2011;185:477-82. [Crossref] [PubMed]
- van As NJ, Norman AR, Thomas K, et al. Predicting the probability of deferred radical treatment for localised prostate cancer managed by active surveillance. Eur Urol 2008;54:1297-305. [Crossref] [PubMed]
- Ouzzane A, Renard-Penna R, Marliere F, et al. Magnetic Resonance Imaging Targeted Biopsy Improves Selection of Patients Considered for Active Surveillance for Clinically Low Risk Prostate Cancer Based on Systematic Biopsies. J Urol 2015;194:350-6. [Crossref] [PubMed]
- Hu JC, Chang E, Natarajan S, et al. Targeted prostate biopsy in select men for active surveillance: do the Epstein criteria still apply? J Urol 2014;192:385-90. [Crossref] [PubMed]
- Inoue LY, Trock BJ, Partin AW, et al. Modeling grade progression in an active surveillance study. Stat Med 2014;33:930-9. [Crossref] [PubMed]
- Komisarenko M, Wong LM, Richard PO, et al. An Increase in Gleason 6 Tumor Volume While on Active Surveillance Portends a Greater Risk of Grade Reclassification with Further Followup. J Urol 2016;195:307-12. [Crossref] [PubMed]
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71:618-29. [Crossref] [PubMed]
- EAU. Guidelines on prostate cancer. Available online: http://uroweb.org/guideline/prostate-cancer/
- Neal RD. NICE prostate cancer clinical guideline: implications for primary care. Br J Gen Pract 2008;58:607-8. [Crossref] [PubMed]
- Graham J, Kirkbride P, Cann K, et al. Prostate cancer: summary of updated NICE guidance. BMJ 2014;348:f7524. [Crossref] [PubMed]
- NICE. Prostate cancer: diagnosis and management. 2014. Available online: https://www.nice.org.uk/guidance/cg175
- Interdisziplinare Leitlinie der Qualitat S3 zur Fruherkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms. 2014. Available online: https://www.urologenportal.de/fileadmin/MDB/PDF/konsultationsfassung-leitlinie-prostatakarzinom.pdf
- Prostaatcarcinoom - Algemeen. Richtlijnen database. 2014. Available online: http://richtlijnendatabase.nl/richtlijn/prostaatcarcinoom/algemeen.html
- KCE. A national clinical practice guideline on the management of localised prostate cancer2013. Available online: https://kce.fgov.be/sites/default/files/atoms/files/KCE_194C_prostate_cancer_0.pdf
- Prostate cancer (Eturauhassyöpä). 2014. Available online: http://www.kaypahoito.fi/web/kh/suositukset/suositus?id=hoi11060
- SESCN. SCAN guideline for active surveillance (deferred radical treatment) of early, low-risk, prostate cancer 2015. Available online: http://www.scan.scot.nhs.uk/Documents/SCAN%20Protocol%20for%20Active%20Surveillance%20of%20Early%20Prostate%20Cancer%20-%2017072009.pdf
- (I+CS) AIoHS. Clinical practice guideline for prostate cancer treatment. Biblioteca de Guias del Practica Clinica del Systema Nacional de Salud 2008. Available online: http://portal.guiasalud.es/emanuales/actualizacion/documentos/manual_actualizacion.pdf
- Morash C, Tey R, Agbassi C, et al. Active surveillance for the management of localized prostate cancer: guideline recommendations. Can Urol Assoc J 2015;9:171-8. [Crossref] [PubMed]
- AHS. Alberta Health Services clinical practice guideline: prostate cancer. 2014. Available online: http://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-gu004-prostate.pdf
- CCNS. Guidelines for the management of prostate cancer. 2006. Available online: http://www.cdha.nshealth.ca/nova-scotia-cancer-care-program-3
- AUA. Guideline for the management of clinically localized prostate cancer: 2007 update. 2007. Available online: https://www.auanet.org/documents/education/clinical-guidance/Prostate-Cancer.pdf
- Carroll PR, Parsons JK, Andriole G, et al. Prostate cancer early detection, version 1.2014. Featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2014;12:1211-9. [Crossref] [PubMed]
- Carroll PR, Parsons JK, Andriole G, et al. NCCN Clinical Practice Guidelines Prostate Cancer Early Detection, Version 2.2015. J Natl Compr Canc Netw 2015;13:1534-61. [Crossref] [PubMed]
- Carroll PR, Parsons JK, Andriole G, et al. NCCN Guidelines Insights: Prostate Cancer Early Detection, Version 2.2016. J Natl Compr Canc Netw 2016;14:509-19. [Crossref] [PubMed]
- (NCCN) TNCCN. NCCN clinical practice guidelines in oncology. 2014; Prostate cancer. Available online: http://www.tri-kobe.org/nccn/guideline/urological/english/prostate.pdf
- Singapore Ministry of Health (NCCS). Guidelines on Management of Prostate Cancer. Annals Academy of Medicine, Singapore. Available online: http://www.annals.edu.sg/pdf/42VolNo4Apr2013/V42N4p190.pdf
- Clinical practice guidelines PSA Testing and Early Management of Test-Detected Prostate Cancer. 2015. Available online: http://wiki.cancer.org.au/australiawiki/index.php?oldid=106555
- Prostate Cancer Taskforce (PCT). Diagnosis and management of prostate cancer in New Zealand men: recommendations from the Prostate Cancer Taskforce. The New Zealand Ministry of Health. 2013. Available online: http://www.prostate.org.nz/documents/diagnosis-management-prostate-cancer-nz-men_(3).pdf
- Bruinsma SM, Bangma CH, Carroll PR, et al. Active surveillance for prostate cancer: a narrative review of clinical guidelines. Nat Rev Urol 2016;13:151-67. [Crossref] [PubMed]
- Enterpise ANSCTA. The AGREE II Instrument 2009. Available online: https://www.agreetrust.org/wp-content/uploads/2013/10/AGREE-II-Users-Manual-and-23-item-Instrument_2009_UPDATE_2013.pdf
- Anwer MA, Al-Fahed OB, Arif SI, et al. Quality assessment of recent evidence-based clinical practice guidelines for management of type 2 diabetes mellitus in adults using the AGREE II instrument. J Eval Clin Pract 2018;24:166-172. [PubMed]
- Welty CJ, Cowan JE, Nguyen H, et al. Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol 2015;193:807-11. [Crossref] [PubMed]
- Klotz L. Active surveillance for prostate cancer: for whom? J Clin Oncol 2005;23:8165-9. [Crossref] [PubMed]
- Choo R, Klotz L, Danjoux C, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol 2002;167:1664-9. [Crossref] [PubMed]
- Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol 2010;28:126-31. [Crossref] [PubMed]
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015;33:272-7. [Crossref] [PubMed]
- Finelli A, Trottier G, Lawrentschuk N, et al. Impact of 5alpha-reductase inhibitors on men followed by active surveillance for prostate cancer. Eur Urol 2011;59:509-14. [Crossref] [PubMed]
- Komisarenko M, Timilshina N, Richard PO, et al. Stricter Active Surveillance Criteria for Prostate Cancer do Not Result in Significantly Better Outcomes: A Comparison of Contemporary Protocols. J Urol 2016;196:1645-50. [Crossref] [PubMed]
- Satkunasivam R, Kulkarni GS, Zlotta AR, et al. Pathological, oncologic and functional outcomes of radical prostatectomy following active surveillance. J Urol 2013;190:91-5. [Crossref] [PubMed]
- Wong LM, Fleshner N, Finelli A. Impact of 5-alpha reductase inhibitors on men followed by active surveillance for prostate cancer: a time-dependent covariate reanalysis. Eur Urol 2013;64:343. [Crossref] [PubMed]
- Wong LM, Toi A, Van der Kwast T, et al. Regular transition zone biopsy during active surveillance for prostate cancer may improve detection of pathological progression. J Urol 2014;192:1088-93. [Crossref] [PubMed]
- Wong LM, Trottier G, Toi A, et al. Should follow-up biopsies for men on active surveillance for prostate cancer be restricted to limited templates? Urology 2013;82:405-9. [Crossref] [PubMed]
- Berglund RK, Masterson TA, Vora KC, et al. Pathological upgrading and up staging with immediate repeat biopsy in patients eligible for active surveillance. J Urol 2008;180:1964-7; discussion 1967-8.
- Soloway MS, Soloway CT, Williams S, et al. Active surveillance; a reasonable management alternative for patients with prostate cancer: the Miami experience. BJU Int 2008;101:165-9. [PubMed]
- Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol 2015;33:3379-85. [Crossref] [PubMed]
- Selvadurai ED, Singhera M, Thomas K, et al. Medium-term outcomes of active surveillance for localised prostate cancer. Eur Urol 2013;64:981-7. [Crossref] [PubMed]
- Leyh-Bannurah SR, Karakiewicz PI, Dell'Oglio P, et al. Comparison of 11 Active Surveillance Protocols in Contemporary European Men Treated With Radical Prostatectomy. Clin Genitourin Cancer 2017. [Epub ahead of print]. [PubMed]
- Richard PO, Alibhai SM, Panzarella T, et al. The uptake of active surveillance for the management of prostate cancer: A population-based analysis. Can Urol Assoc J 2016;10:E342-6. [Crossref] [PubMed]
- Luckenbaugh AN, Auffenberg GB, Hawken SR, et al. Variation in Guideline Concordant Active Surveillance Followup in Diverse Urology Practices. J Urol 2017;197:621-6. [Crossref] [PubMed]
- Loeb S, Folkvaljon Y, Curnyn C, et al. Uptake of Active Surveillance for Very-Low-Risk Prostate Cancer in Sweden. JAMA Oncol 2017;3:1393-8. [Crossref] [PubMed]
- Steineck G, Helgesen F, Adolfsson J, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med 2002;347:790-6. [Crossref] [PubMed]
- Popiolek M, Rider JR, Andren O, et al. Natural history of early, localized prostate cancer: a final report from three decades of follow-up. Eur Urol 2013;63:428-35. [Crossref] [PubMed]
- Vargas HA, Akin O, Afaq A, et al. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol 2012;188:1732-8. [Crossref] [PubMed]


